- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Adiponectin secreted from adipocytes binds to adiponectin receptors AdipoR1 and AdipoR2, and exerts antidiabetic effects via activation of AMPK and PPAR-α pathways, respectively.
Levels of adiponectin in plasma are reduced in obesity, which causes insulin resistance and type 2 diabetes. Thus, orally active small molecules that bind to and activate AdipoR1 and AdipoR2
could ameliorate obesity-related diseases such as type 2 diabetes. Here we report the identification of orally active synthetic small-molecule AdipoR agonists. One of these compounds,
AdipoR agonist (AdipoRon), bound to both AdipoR1 and AdipoR2 _in vitro_. AdipoRon showed very similar effects to adiponectin in muscle and liver, such as activation of AMPK and PPAR-α
pathways, and ameliorated insulin resistance and glucose intolerance in mice fed a high-fat diet, which was completely obliterated in AdipoR1 and AdipoR2 double-knockout mice. Moreover,
AdipoRon ameliorated diabetes of genetically obese rodent model _db/db_ mice, and prolonged the shortened lifespan of _db/db_ mice on a high-fat diet. Thus, orally active AdipoR agonists
such as AdipoRon are a promising therapeutic approach for the treatment of obesity-related diseases such as type 2 diabetes. Access through your institution Buy or subscribe This is a
preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per
year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during
checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ANNEXIN A1 BINDS PDZ
AND LIM DOMAIN 7 TO INHIBIT ADIPOGENESIS AND PREVENT OBESITY Article Open access 23 August 2024 ANNONACEOUS ACETOGENINS MIMIC AA005 TARGETS MITOCHONDRIAL TRIFUNCTIONAL ENZYME ALPHA SUBUNIT
TO TREAT OBESITY IN MALE MICE Article Open access 22 October 2024 IMPROVEMENT OF OBESITY-ASSOCIATED DISORDERS BY A SMALL-MOLECULE DRUG TARGETING MITOCHONDRIA OF ADIPOSE TISSUE MACROPHAGES
Article Open access 04 January 2021 REFERENCES * Gesta, S., Tseng, Y. H. & Kahn, C. R. Developmental origin of fat: tracking obesity to its source. _Cell_ 131, 242–256 (2007) Article
CAS Google Scholar * Olefsky, J. M. & Glass, C. K. Macrophages, inflammation, and insulin resistance. _Annu. Rev. Physiol._ 72, 219–246 (2010) Article CAS Google Scholar * Osler, M.
E. & Zierath, J. R. Adenosine 5′-monophosphate-activated protein kinase regulation of fatty acid oxidation in skeletal muscle. _Endocrinology_ 149, 935–941 (2008) Article CAS Google
Scholar * LeRoith, D. & Accili, D. Mechanisms of disease: using genetically altered mice to study concepts of type 2 diabetes. _Nature Clin. Pract. Endocrinol. Metab._ 4, 164–172 (2008)
Article CAS Google Scholar * Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. A novel serum protein similar to C1q, produced exclusively in adipocytes. _J.
Biol. Chem._ 270, 26746–26749 (1995) Article CAS Google Scholar * Hu, E., Liang, P. & Spiegelman, B. M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. _J. Biol.
Chem._ 271, 10697–10703 (1996) Article CAS Google Scholar * Maeda, K. et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose most abundant gene
transcript 1). _Biochem. Biophys. Res. Commun._ 221, 286–289 (1996) Article CAS Google Scholar * Nakano, Y., Tobe, T., Choi-Miura, N. H., Mazda, T. & Tomita, M. Isolation and
characterization of GBP28, a novel gelatin-binding protein purified from human plasma. _J. Biochem._ 120, 803–812 (1996) Article CAS Google Scholar * Hotta, K. et al. Plasma
concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. _Arterioscler. Thromb. Vasc. Biol._ 20, 1595–1599 (2000) Article CAS Google Scholar *
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. _Nature Med._ 7, 941–946 (2001) Article CAS Google Scholar
* Berg, A. H., Combs, T. P., Du, X., Brownlee, M. & Scherer, P. E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. _Nature Med._ 7, 947–953 (2001) Article CAS
Google Scholar * Fruebis, J. et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice.
_Proc. Natl Acad. Sci. USA_ 98, 2005–2010 (2001) Article ADS CAS Google Scholar * Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating
AMP-activated protein kinase. _Nature Med._ 8, 1288–1295 (2002) Article CAS Google Scholar * Tomas, E. et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular
domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. _Proc. Natl Acad. Sci. USA_ 99, 16309–16313 (2002) Article ADS CAS Google Scholar * Kahn, B. B.,
Alquier, T., Carling, D. & Hardie, D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. _Cell Metab._ 1, 15–25 (2005) Article
CAS Google Scholar * Kersten, S., Desvergne, B. & Wahli, W. Roles of PPARs in health and disease. _Nature_ 405, 421–424 (2000) Article ADS CAS Google Scholar * Yamauchi, T. et al.
Globular adiponectin protected ob/ob mice from diabetes and apoE deficient mice from atherosclerosis. _J. Biol. Chem._ 278, 2461–2468 (2003) Article CAS Google Scholar * Yamauchi, T. et
al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. _Nature_ 423, 762–769 (2003) Article ADS CAS Google Scholar * Wess, J. G-protein-coupled receptors:
molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. _FASEB J._ 11, 346–354 (1997) Article CAS Google Scholar * Yamauchi, T. et al. Targeted
disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. _Nature Med._ 13, 332–339 (2007) Article CAS Google Scholar * Iwabu, M. et al.
Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. _Nature_ 464, 1313–1319 (2010) Article ADS CAS Google Scholar * Richter, E. A. & Ruderman, N. B. AMPK
and the biochemistry of exercise: implications for human health and disease. _Biochem. J._ 418, 261–275 (2009) Article CAS Google Scholar * Wu, Z. et al. Mechanisms controlling
mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. _Cell_ 98, 115–124 (1999) Article CAS Google Scholar * Handschin, C. & Spiegelman, B. M. The role
of exercise and PGC1α in inflammation and chronic disease. _Nature_ 454, 463–469 (2008) Article ADS CAS Google Scholar * Cantó, C. et al. AMPK regulates energy expenditure by modulating
NAD+ metabolism and SIRT1 activity. _Nature_ 458, 1056–1060 (2009) Article ADS Google Scholar * Paffenbarger, R. S., Jr et al. The association of changes in physical-activity level and
other lifestyle characteristics with mortality among men. _N. Engl. J. Med._ 328, 538–545 (1993) Article Google Scholar * Open Innovation Center for Drug Discovery
http://www.ocdd.u-tokyo.ac.jp/library_e.html (The University of Tokyo, 2012) * Hawley, S. A. et al. Characterization of the AMP-activated protein kinase kinase from rat liver and
identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. _J. Biol. Chem._ 271, 27879–27887 (1996) Article CAS Google Scholar * Mootha, V.
K. et al. Errα and Gabpa/b specify PGC-1α-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. _Proc. Natl Acad. Sci. USA_ 101, 6570–6575 (2004) Article
ADS CAS Google Scholar * Berchtold, M. W. et al. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. _Physiol. Rev._ 80, 1215–1265 (2000)
Article CAS Google Scholar * Shulman, G. I. Cellular mechanisms of insulin resistance. _J. Clin. Invest._ 106, 171–176 (2000) Article CAS Google Scholar * Brownlee, M. Biochemistry and
molecular cell biology of diabetic complications. _Nature_ 414, 813–820 (2001) Article ADS CAS Google Scholar * Lochhead, P. A. et al. 5-aminoimidazole-4-carboxamide riboside mimics the
effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. _Diabetes_ 49, 896–903 (2000) Article CAS Google Scholar * Hotamisligil, G. S.,
Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. _Science_ 259, 87–91 (1993) Article ADS CAS
Google Scholar * Wellen, K. E. & Hotamisligil, G. S. Inflammation, stress, and diabetes. _J. Clin. Invest._ 115, 1111–1119 (2005) Article CAS Google Scholar * Weisberg, S. P. et al.
Obesity is associated with macrophage accumulation in adipose tissue. _J. Clin. Invest._ 112, 1796–1808 (2003) Article CAS Google Scholar * Xu, H. et al. Chronic inflammation in fat plays
a crucial role in the development of obesity-related insulin resistance. _J. Clin. Invest._ 112, 1821–1830 (2003) Article CAS Google Scholar * Lumeng, C. N., Bodzin, J. L. & Saltiel,
A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. _J. Clin. Invest._ 117, 175–184 (2007) Article CAS Google Scholar * Zhang, H. M. et al. Geldanamycin
derivative ameliorates high fat diet-induced renal failure in diabetes. _PLoS ONE_ 7, e32746 (2012) Article ADS CAS Google Scholar * Li, S., Shin, H. J., Ding, E. L. & van Dam, R. M.
Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. _J. Am. Med. Assoc._ 302, 179–188 (2009) Article CAS Google Scholar * Pischon, T. et al. Plasma
adiponectin levels and risk of myocardial infarction in men. _J. Am. Med. Assoc._ 291, 1730–1737 (2004) Article CAS Google Scholar * Pajvani, U. B. et al. Complex distribution, not
absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. _J. Biol. Chem._ 279, 12152–12162 (2004) Article CAS Google Scholar * Luo,
Z., Saha, A. K., Xiang, X. & Ruderman, N. B. AMPK, the metabolic syndrome and cancer. _Trends Pharmacol. Sci._ 26, 69–76 (2005) Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We thank N. Kubota, K. Hara, I. Takamoto, Y. Hada, T. Kobori, H. Umematsu, S. Odawara, T. Aoyama, Y. Jing, S. Wei, K. Soeda and H. Waki for technical help and support; and
K. Miyata, Y. Nishibaba, M. Yuasa and A. Hayashi for technical assistance and support. This work was supported by a Grant-in-aid for Scientific Research (S) (20229008, 25221307) (to T.K.),
Grant-in-aid for Young Scientists (A) (23689048) (to M.I.), Targeted Proteins Research Program (to T.K.), the Global COE Research Program (to T.K.), Translational Systems Biology and
Medicine Initiative (to T.K.) and Translational Research Network Program (to M.O.-I.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Funding Program for
Next Generation World-Leading Researchers (NEXT Program) (to T.Y.) from Cabinet Office, Government of Japan. AUTHOR INFORMATION Author notes * Miki Okada-Iwabu, Toshimasa Yamauchi and Masato
Iwabu: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Diabetes and Metabolic Diseases, Graduate School of Medicine, The University of Tokyo, Tokyo
113-0033, Japan Miki Okada-Iwabu, Toshimasa Yamauchi, Masato Iwabu, Ken-ichi Hamagami, Koichi Matsuda, Mamiko Yamaguchi, Kohjiro Ueki & Takashi Kadowaki * Department of Integrated
Molecular Science on Metabolic Diseases, 22nd Century Medical and Research Center, The University of Tokyo, Tokyo 113-0033, Japan Miki Okada-Iwabu, Toshimasa Yamauchi, Masato Iwabu &
Takashi Kadowaki * Department of Molecular Medicinal Sciences on Metabolic Regulation, 22nd Century Medical and Research Center, The University of Tokyo, Tokyo 113-0033, Japan Miki
Okada-Iwabu, Toshimasa Yamauchi & Takashi Kadowaki * RIKEN Systems and Structural Biology Center, Tsurumi, Yokohama 230-0045, Japan, Teruki Honma, Hiroaki Tanabe, Tomomi Kimura-Someya,
Mikako Shirouzu, Akiko Tanaka & Shigeyuki Yokoyama * Graduate School of Comprehensive Human Sciences, University of Tsukuba, Tsukuba 305-8577, Japan Hitomi Ogata & Kumpei Tokuyama *
Open Innovation Center for Drug Discovery, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, Tetsuo Nagano & Akiko Tanaka * Graduate School of Science, The
University of Tokyo, Bunkyo-ku, Tokyo 113-0033, Japan, Shigeyuki Yokoyama Authors * Miki Okada-Iwabu View author publications You can also search for this author inPubMed Google Scholar *
Toshimasa Yamauchi View author publications You can also search for this author inPubMed Google Scholar * Masato Iwabu View author publications You can also search for this author inPubMed
Google Scholar * Teruki Honma View author publications You can also search for this author inPubMed Google Scholar * Ken-ichi Hamagami View author publications You can also search for this
author inPubMed Google Scholar * Koichi Matsuda View author publications You can also search for this author inPubMed Google Scholar * Mamiko Yamaguchi View author publications You can also
search for this author inPubMed Google Scholar * Hiroaki Tanabe View author publications You can also search for this author inPubMed Google Scholar * Tomomi Kimura-Someya View author
publications You can also search for this author inPubMed Google Scholar * Mikako Shirouzu View author publications You can also search for this author inPubMed Google Scholar * Hitomi Ogata
View author publications You can also search for this author inPubMed Google Scholar * Kumpei Tokuyama View author publications You can also search for this author inPubMed Google Scholar *
Kohjiro Ueki View author publications You can also search for this author inPubMed Google Scholar * Tetsuo Nagano View author publications You can also search for this author inPubMed
Google Scholar * Akiko Tanaka View author publications You can also search for this author inPubMed Google Scholar * Shigeyuki Yokoyama View author publications You can also search for this
author inPubMed Google Scholar * Takashi Kadowaki View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.O.-I., M.I., T.Y., T.H., K.-i.-H.,
K.M., M.Y., H.T., T.K-S., M.S., H.O., K.T. and A.T. performed experiments. T.K., T.Y., M.O.-I. and M.I. conceived the study. T.K., A.T., T.Y. and S.Y. supervised the study. T.Y., T.K.,
M.O.-I. and M.I. wrote the paper. All authors interpreted data. CORRESPONDING AUTHORS Correspondence to Toshimasa Yamauchi or Takashi Kadowaki. ETHICS DECLARATIONS COMPETING INTERESTS The
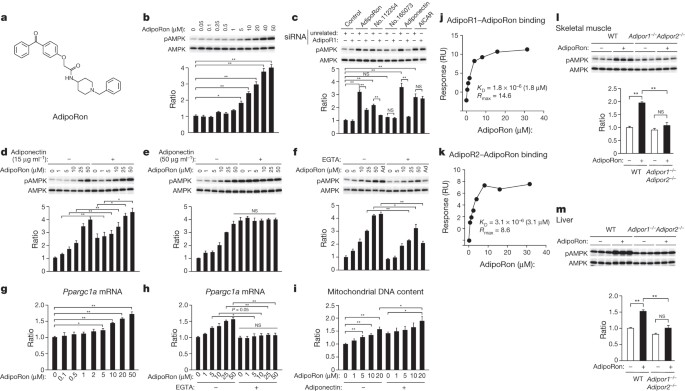
authors declare no competing financial interests. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIGURE 1 PHOSPHORYLATION OF AMPK IN C2C12 MYOTUBES. Phosphorylation of AMPK normalized to the
amount of AMPK in C2C12 myotubes treated for 5 min with 15 µg ml−1 adiponectin or the indicated small-molecule compounds (10 μM). #, AdipoRon; ##, no. 112254; ###, no. 165073. EXTENDED DATA
FIGURE 2 DISTRIBUTION CURVES SHOWING _Z_ SCORES. A, Distribution curve showing _Z_ scores representing AMPK activity for all compounds tested in C2C12 myotubes shown in Extended Data Table
1 and Extended Data Fig. 1. The dashed line indicates the _Z_ score cut-off for compounds scored as hits, which showed higher activity than 80% of that seen with adiponectin. B, Distribution
curve showing _Z_ scores representing AdipoR dependency of AMPK activation for 39 compounds tested in C2C12 myotubes shown in Extended Data Table 2. Indicated are the location of AdipoRon,
another hit (no. 112254), and non-hit (no. 165073). EXTENDED DATA FIGURE 3 THE EFFECT OF ADIPORON ON COMPLEX I ACTIVITY, AND EXPRESSION OF _ADIPOR1_ AND _ADIPOR2_ MRNA IN C2C12 MYOTUBES
TRANSFECTED WITH THE INDICATED SIRNA DUPLEX. A, Complex I activities were measured with the indicated concentrations of rotenone or AdipoRon. B, C, _Adipor1_ (B) and _Adipor2_ (C) mRNA
levels were analysed by RT–qPCR. All values are presented as mean ± s.e.m. A, _n_ = 3–7; B, C, _n_ = 3 each; *_P_ < 0.05 and **_P_ < 0.01 compared to control or unrelated siRNA cells.
NS, not significant. EXTENDED DATA FIGURE 4 ADIPORON BINDING TO ADIPOR1 AND ADIPOR2. A–D, Binding and Scatchard analyses of [3H]AdipoRon to primary hepatocytes from wild-type (A),
_Adipor2_−/− knockout (B), _Adipor1_−/− knockout (C) and _Adipor1_−/− _Adipor2_−/− double-knockout (D) mice. E–H, Concentration-dependent competitive [3H]AdipoRon binding to primary
hepatocytes from wild-type (E), _Adipor2_−/− knockout (F), _Adipor1_−/− knockout (G) and _Adipor1_−/− _Adipor2_−/− double-knockout (H) mice. Binding analyses were performed using the
indicated concentrations of AdipoRon. c.p.m., counts per minute. EXTENDED DATA FIGURE 5 RAW DATA OF FIG. 2 AND TIME COURSE OF GLUCOSE-LOWERING EFFECT OF ADIPORON. A–M, Raw data of Fig. 2a
(A), Fig. 2d, left (B, C), Fig. 2d, right (D, E), Fig. 2e, left (F, G), Fig. 2e, right (H, I), Fig. 2g, left (J, K) and Fig. 2g, right (L, M). N, Time course of glucose-lowering effect of
AdipoRon. Data are calculated from data in Fig. 4a. The glucose-lowering effect of AdipoRon was obtained by the following equation and expressed as %: (vehicle plasma glucose − AdipoRon
plasma glucose)/vehicle plasma glucose. All values are presented as mean ± s.e.m. EXTENDED DATA FIGURE 6 THE EFFECTS OF COMPOUNDS 112254 AND 165073 ON INSULIN RESISTANCE AND GLUCOSE
INTOLERANCE VIA ADIPOR. A, B, Chemical structures of compounds 112254 (A) and 165073 (B). C–J, Plasma glucose (C left, D left, F, G left, H left, J), plasma insulin (C right, D right, G
right, H right) and insulin resistance index (E, I) during oral glucose tolerance test (OGTT) (1.0 g glucose per kg body weight) (C, D, G, H) or during insulin tolerance test (ITT) (0.5 U
insulin per kg body weight) (F, J), in wild-type and _Adipor1_−/− _Adipor2_−/− double-knockout mice, treated with or without compounds 112254 or 165073 (50 mg per kg body weight). All values
are presented as mean ± s.e.m. C–F, _n_ = 10 each; G–J, _n_ = 7 each from 2, 3 independent experiments, *_P_ < 0.05 and **_P_ < 0.01 compared to control or as indicated. NS, not
significant. EXTENDED DATA FIGURE 7 THE EFFECTS OF ADIPORON ON GLUCOSE METABOLISM IN _ADIPOR1_−/−, _ADIPOR2_−/− AND _ADIPOR1_−/− _ADIPOR2_−/− MICE. A, Triglyceride content (A) and TBARS (B)
in skeletal muscle from wild-type or _Adipor1_−/− _Adipor2_−/− double-knockout mice treated with or without AdipoRon (50 mg per kg body weight). C–G, The effects of AdipoRon on glucose
metabolism in _Adipor1_−/−, _Adipor2_−/− and _Adipor1_−/− _Adipor2_−/− mice. Plasma glucose (C–F, left panels), plasma insulin (C–F, right panels) and insulin resistance index (G) during
oral glucose tolerance test (OGTT) (1.0 g glucose per kg body weight). All values are presented as mean ± s.e.m. A–D, F, _n_ = 10 each; E, _n_ = 7 each; G, _n_ = 7–10; *_P_ < 0.05 and
**_P_ < 0.01 compared to vehicle mice. NS, not significant. EXTENDED DATA FIGURE 8 CHEMICAL STRUCTURES AND ADIPOR DEPENDENCY OF AMPK ACTIVATION. A–D, Chemical structures of AdipoRon (A),
compound 168198 (B), compound 112254 (C) and compound 103694 (D). Within the 1-benzyl 4-substituted 6-membered cyclic amine moiety, the cyclic amine moiety is surrounded by a dashed red
circle, and the aromatic ring is surrounded by a light green circle. Cyan and dark green circles surround the carbonyl group and the terminal aromatic ring, respectively, located on the
opposite side from the benzyl cyclic amine. E, Phosphorylation and amount of AMPK in C2C12 myotubes treated for 5 min with the indicated small-molecule compounds. Phosphorylation and amount
of AMPK in C2C12 myotubes, treated for 5 min with the indicated small-molecule compounds (10 μM) (% relative to adiponectin). F, AdipoR dependency of AMPK activation. Phosphorylation and
amount of AMPK in C2C12 myotubes and transfected with or without the AdipoR1 siRNA duplex, treated for 5 min with the indicated small molecule. AdipoR-dependency ratios were obtained by the
following equation: 100 − (ratio for those transfected with the AdipoR1 siRNA duplex/ratio for those transfected without the AdipoR1 siRNA duplex) × 100 (%). SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION This file contains Supplementary Results, Text and Data and additional references. (PDF 719 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR
FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 POWERPOINT SLIDE FOR FIG. 6 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE
THIS ARTICLE Okada-Iwabu, M., Yamauchi, T., Iwabu, M. _et al._ A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. _Nature_ 503, 493–499 (2013).
https://doi.org/10.1038/nature12656 Download citation * Received: 06 June 2012 * Accepted: 10 September 2013 * Published: 30 October 2013 * Issue Date: 28 November 2013 * DOI:
https://doi.org/10.1038/nature12656 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative