- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Kramers developed the theory on how chemical reaction rates are influenced by the viscosity of the medium1,2. At the viscosity of water, the kinetics of unimolecular reactions are
described by diffusion of a Brownian particle over a free-energy barrier separating reactants and products. For reactions in solution this famous theory extended Eyring’s transition state
theory, and is widely applied in physics, chemistry and biology, including to reactions as complex as protein folding3,4. Because the diffusion coefficient of Kramers’ theory is determined
by the dynamics in the sparsely populated region of the barrier top, its properties have not been directly measured for any molecular system. Here we show that the Kramers diffusion
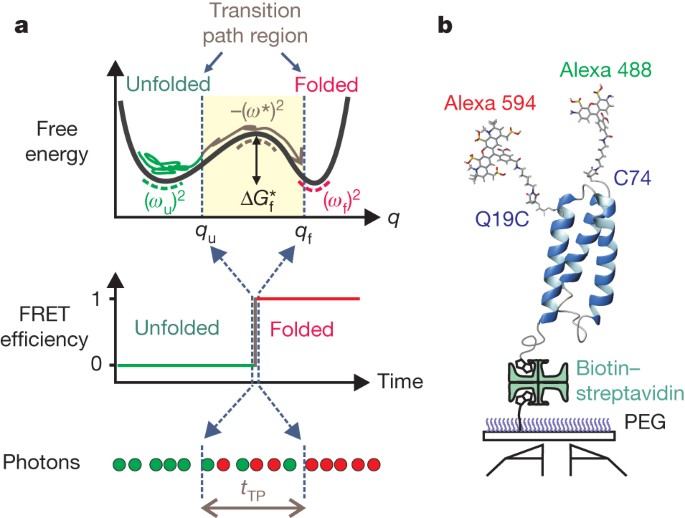
coefficient and free-energy barrier can be characterized by measuring the temperature- and viscosity-dependence of the transition path time for protein folding. The transition path is the
small fraction of an equilibrium trajectory for a single molecule when the free-energy barrier separating two states is actually crossed. Its duration, the transition path time, can now be
determined from photon trajectories for single protein molecules undergoing folding/unfolding transitions5. Our finding of a long transition path time with an unusually small solvent
viscosity dependence suggests that internal friction as well as solvent friction determine the Kramers diffusion coefficient for α-helical proteins, as opposed to a breakdown of his theory,
which occurs for many small-molecule reactions2. It is noteworthy that the new and fundamental information concerning Kramers’ theory and the dynamics of barrier crossings obtained here come
from experiments on a protein rather than a much simpler chemical or physical system. Access through your institution Buy or subscribe This is a preview of subscription content, access via
your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS FLUORESCENCE RESONANCE ENERGY TRANSFER AT THE SINGLE-MOLECULE
LEVEL Article 28 March 2024 TRANSIENT BINDING AND JUMPING DYNAMICS OF P53 ALONG DNA REVEALED BY SUB-MILLISECOND RESOLVED SINGLE-MOLECULE FLUORESCENCE TRACKING Article Open access 13 August
2020 EXTENDING FLUORESCENCE ANISOTROPY TO LARGE COMPLEXES USING REVERSIBLY SWITCHABLE PROTEINS Article Open access 10 October 2022 REFERENCES * Kramers, H. A. Brownian motion in a field of
force and the diffusion model of chemical reactions. _Physica_ 7, 284–304 (1940) Article MathSciNet CAS ADS Google Scholar * Hänggi, P., Talkner, P. & Borkovec, M. Reaction rate
theory; fifty years after Kramers. _Rev. Mod. Phys._ 62, 251–341 (1990) Article MathSciNet ADS Google Scholar * Oliveberg, M. & Wolynes, P. G. The experimental survey of
protein-folding energy landscapes. _Q. Rev. Biophys._ 38, 245–288 (2005) Article CAS Google Scholar * Kubelka, J., Henry, E. R., Cellmer, T., Hofrichter, J. & Eaton, W. A. Chemical,
physical, and theoretical kinetics of an ultrafast folding protein. _Proc. Natl Acad. Sci. USA_ 105, 18655–18662 (2008) Article CAS ADS Google Scholar * Chung, H. S., McHale, K., Louis,
J. M. & Eaton, W. A. Single-molecule fluorescence experiments determine protein folding transition path times. _Science_ 335, 981–984 (2012) Article CAS ADS Google Scholar * Chung,
H. S. et al. Extracting rate coefficients from single-molecule photon trajectories and FRET efficiency histograms for a fast-folding protein. _J. Phys. Chem. A_ 115, 3642–3656 (2011) Article
CAS Google Scholar * Lindorff-Larsen, K., Piana, S., Dror, R. O. & Shaw, D. E. How fast-folding proteins fold. _Science_ 334, 517–520 (2011) Article CAS ADS Google Scholar *
Gopich, I. V. & Szabo, A. Decoding the pattern of photon colors in single-molecule FRET. _J. Phys. Chem. B_ 113, 10965–10973 (2009) Article CAS Google Scholar * Chung, H. S., Louis,
J. M. & Eaton, W. A. Experimental determination of upper bound for transition path times in protein folding from single-molecule photon-by-photon trajectories. _Proc. Natl Acad. Sci.
USA_ 106, 11837–11844 (2009) Article CAS ADS Google Scholar * Chung, H. S., Cellmer, T., Louis, J. M. & Eaton, W. A. Measuring ultrafast protein folding rates from photon-by-photon
analysis of single molecule fluorescence trajectories. _Chem. Phys._ 422, 229–237 (2013) Article CAS Google Scholar * Hummer, G. From transition paths to transition states and rate
coefficients. _J. Chem. Phys._ 120, 516–523 (2004) Article CAS ADS Google Scholar * Socci, N. D., Onuchic, J. N. & Wolynes, P. G. Diffusive dynamics of the reaction coordinate for
protein folding funnels. _J. Chem. Phys._ 104, 5860–5868 (1996) Article CAS ADS Google Scholar * Klimov, D. K. & Thirumalai, D. Viscosity dependence of the folding rates of proteins.
_Phys. Rev. Lett._ 79, 317–320 (1997) Article CAS ADS Google Scholar * Best, R. B. & Hummer, G. Reaction coordinates and rates from transition paths. _Proc. Natl Acad. Sci. USA_
102, 6732–6737 (2005) Article CAS ADS Google Scholar * Cellmer, T., Henry, E. R., Hofrichter, J. & Eaton, W. A. Measuring internal friction of an ultrafast-folding protein. _Proc.
Natl Acad. Sci. USA_ 105, 18320–18325 (2008) Article CAS ADS Google Scholar * Kubelka, J., Hofrichter, J. & Eaton, W. A. The protein folding ‘speed limit’. _Curr. Opin. Struct.
Biol._ 14, 76–88 (2004) Article CAS Google Scholar * Yang, W. Y. & Gruebele, M. Folding at the speed limit. _Nature_ 423, 193–197 (2003) Article CAS ADS Google Scholar * Hummer,
G. & Szabo, A. Free energy surfaces from single-molecule force spectroscopy. _Acc. Chem. Res._ 38, 504–513 (2005) Article CAS Google Scholar * Godoy-Ruiz, R. et al. Estimating
free-energy barrier heights for an ultrafast folding protein from calorimetric and kinetic data. _J. Phys. Chem. B_ 112, 5938–5949 (2008) Article CAS Google Scholar * Portman, J. J.,
Takada, S. & Wolynes, P. G. Microscopic theory of protein folding rates. II. Local reaction coordinates and chain dynamics. _J. Chem. Phys._ 114, 5082–5096 (2001) Article CAS ADS
Google Scholar * Makarov, D. E. Interplay of non-Markov and internal friction effects in the barrier crossing kinetics of biopolymers: insights from an analytically solvable model. _J.
Chem. Phys._ 138, 014102 (2013) Article ADS Google Scholar * Schulz, J. C. F., Schmidt, L., Best, R. B., Dzubiella, J. & Netz, R. R. Peptide chain dynamics in light and heavy water:
zooming in on internal friction. _J. Am. Chem. Soc._ 134, 6273–6279 (2012) Article CAS Google Scholar * Ansari, A., Jones, C. M., Henry, E. R., Hofrichter, J. & Eaton, W. A. The role
of solvent viscosity in the dynamics of protein conformational changes. _Science_ 256, 1796–1798 (1992) Article CAS ADS Google Scholar * Soranno, A. et al. Quantifying internal friction
in unfolded and intrinsically disordered proteins with single-molecule spectroscopy. _Proc. Natl Acad. Sci. USA_ 109, 17800–17806 (2012) Article CAS ADS Google Scholar * Bryngelson, J.
D. & Wolynes, P. G. Intermediates and barrier crossing in a random energy-model (with applications to protein folding). _J. Phys. Chem._ 93, 6902–6915 (1989) Article CAS Google Scholar
* Zagrovic, B. & Pande, V. Solvent viscosity dependence of the folding rate of a small protein: distributed computing study. _J. Comput. Chem._ 24, 1432–1436 (2003) Article CAS
Google Scholar * Sutto, L., Latzer, J., Hegler, J. A., Ferreiro, D. U. & Wolynes, P. G. Consequences of localized frustration for the folding mechanism of the IM7 protein. _Proc. Natl
Acad. Sci. USA_ 104, 19825–19830 (2007) Article CAS ADS Google Scholar * Jas, G. S., Eaton, W. A. & Hofrichter, J. Effect of viscosity on the kinetics of α-helix and β-hairpin
formation. _J. Phys. Chem. B_ 105, 261–272 (2001) Article CAS Google Scholar * Wensley, B. G. et al. Experimental evidence for a frustrated energy landscape in a three-helix-bundle
protein family. _Nature_ 463, 685–688 (2010) Article CAS ADS Google Scholar * Hagen, S. J. Solvent viscosity and friction in protein folding dynamics. _Curr. Protein Pept. Sci._ 11,
385–395 (2010) Article CAS ADS Google Scholar * Merchant, K. A., Best, R. B., Louis, J. M., Gopich, I. V. & Eaton, W. A. Characterizing the unfolded states of proteins using
single-molecule FRET spectroscopy and molecular simulations. _Proc. Natl Acad. Sci. USA_ 104, 1528–1533 (2007) Article CAS ADS Google Scholar * Vogelsang, J. et al. A reducing and
oxidizing system minimizes photobleaching and blinking of fluorescent dyes. _Angew. Chem._ 47, 5465–5469 (2008) Article CAS Google Scholar * Benninger, R. K. P. et al. Quantitative 3D
mapping of fluidic temperatures within microchannel networks using fluorescence lifetime imaging. _Anal. Chem._ 78, 2272–2278 (2006) Article CAS Google Scholar * Viterbi, A. J. Error
bounds for convolution codes and an asymptotically optimum decoding algorithm. _IEEE Trans. Inf. Theory_ 13, 260–269 (1967) Article Google Scholar * Rabiner, L. R. A tutorial on hidden
Markov models and selected applications in speech. _Proc. IEEE_ 77, 257–286 (1989) Article Google Scholar * Best, R. B., Hummer, G. & Eaton, W. A. Native contacts determine protein
folding mechanisms in atomistic simulations. _Proc. Natl Acad. Sci USA_ http://dx.doi.org/10.1073/pnas.1311599110 (in the press) * Zhu, Y. et al. Ultrafast folding of α3D: a _de novo_
designed three-helix bundle protein. _Proc. Natl Acad. Sci. USA_ 100, 15486–15491 (2003) Article CAS ADS Google Scholar * Liu, F. et al. A one-dimensional free energy surface does not
account for two-probe folding kinetics of protein α3D. _J. Chem. Phys._ 130, 061101 (2009) Article ADS Google Scholar Download references ACKNOWLEDGEMENTS We are particularly indebted to
J. M. Louis for the preparation, dye labelling and purification of the protein used in this work, with technical assistance from A. Aniana. We also thank R. Best, G. Hummer and A. Szabo for
discussions and comments on the manuscript, and D.E. Shaw Research for providing access to their molecular dynamics trajectories for the calculations by R. Best. This work was supported by
the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Laboratory of Chemical Physics, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, 20892-0520, Maryland, USA Hoi Sung Chung &
William A. Eaton Authors * Hoi Sung Chung View author publications You can also search for this author inPubMed Google Scholar * William A. Eaton View author publications You can also search
for this author inPubMed Google Scholar CONTRIBUTIONS H.S.C. and W.A.E. designed the research and wrote the manuscript; H.S.C. collected and analysed the experimental data. CORRESPONDING
AUTHORS Correspondence to Hoi Sung Chung or William A. Eaton. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. EXTENDED DATA FIGURES AND TABLES
EXTENDED DATA FIGURE 1 AMINO ACID SEQUENCES OF POLYPEPTIDES CONTAINING PROTEIN Α3D. Dyes were attached to the cysteine residues (red) and a biotin molecule was attached to the lysine residue
(blue) in the AviTag sequence. EXTENDED DATA FIGURE 2 PHOTON TRAJECTORY AND KINETICS MODELS. A, The definition of photon indices and time interval of a photon trajectory with a folding
transition. B, C, Photon trajectories were analysed using the two-state model to determine kinetics parameters (B) or the three-state model to determine the average transition path times
(_t_TP = 1/2_k_S) (C). EXTENDED DATA FIGURE 3 FRET EFFICIENCY HISTOGRAMS OF Α3D IN 2.25 M GDMCL SOLUTION AT DIFFERENT TEMPERATURES. The FRET efficiency histograms were constructed from 1-ms
bins in the trajectories with the mean photon count rate >40 ms−1. Wide and narrow bars are the experimental histograms and the histograms constructed from re-coloured photon
trajectories using the parameters obtained from the maximum likelihood method with the two-state model (Extended Data Table 1), respectively. The agreement between the two histograms
validates the description of α3D as a two-state folder8. The similar ratio of the integral of the folded (high FRET) and the unfolded (low FRET) distributions indicates that the equilibrium
constant is unchanged over the temperature range of the measurement, as shown more precisely in the maximum likelihood analysis. At high temperature and low pH, where the 11 glutamates and 1
aspartate are protonated, more than two states are observed37,38. EXTENDED DATA FIGURE 4 DONOR–ACCEPTOR CROSS-CORRELATION FUNCTIONS AT DIFFERENT TEMPERATURES. Black solid lines are
exponential functions that best fit the data. The fitting parameters are listed in Extended Data Table 1. EXTENDED DATA FIGURE 5 FRET EFFICIENCY HISTOGRAMS OF Α3D AT VARIOUS SOLVENT
VISCOSITIES. The FRET efficiency histograms were constructed from 1-ms bins in the trajectories with the mean photon count rate >50 ms−1 for 2.25 M and 3.2 M GdmCl and from 2-ms bins in
the trajectories with the mean photon count rate >30 ms−1 for 4.6 M, 4.3 M and 3.8 M GdmCl concentrations. At the relative viscosity (_η_/_η_0) 1, 10 and 38, the higher concentration of
GdmCl was used to counteract the stabilization of proteins by glycerol to maintain the ratio of folded to unfolded molecules as close to unity as practically possible. The similar ratio of
the integral of the folded (high FRET) and the unfolded (low FRET) distributions indicates that the equilibrium constant is unchanged at these conditions, as shown more precisely in the
maximum likelihood analysis. EXTENDED DATA FIGURE 6 DONOR–ACCEPTOR CROSS-CORRELATION OF THE SEGMENTS OF THE FLUORESCENCE TRAJECTORIES CORRESPONDING TO THE UNFOLDED STATE (REF. 24). A, Black
solid lines are exponential functions that best fit the data. The fitting parameters are listed in Extended Data Table 2. B, The unfolded state dynamics are slowed approximately linearly by
the solvent viscosity as previously observed at high denaturant concentrations24. The relaxation time at _η_/ _η_0 = 1 (aqueous solution) is too fast to be measured by this method.
POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Chung, H., Eaton, W. Single-molecule fluorescence probes dynamics of barrier crossing. _Nature_ 502, 685–688 (2013). https://doi.org/10.1038/nature12649 Download
citation * Received: 15 May 2013 * Accepted: 12 September 2013 * Published: 23 October 2013 * Issue Date: 31 October 2013 * DOI: https://doi.org/10.1038/nature12649 SHARE THIS ARTICLE Anyone
you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by
the Springer Nature SharedIt content-sharing initiative