- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Viral infection is commonly associated with virus-driven hijacking of host proteins. Here we describe a novel mechanism by which influenza virus affects host cells through the
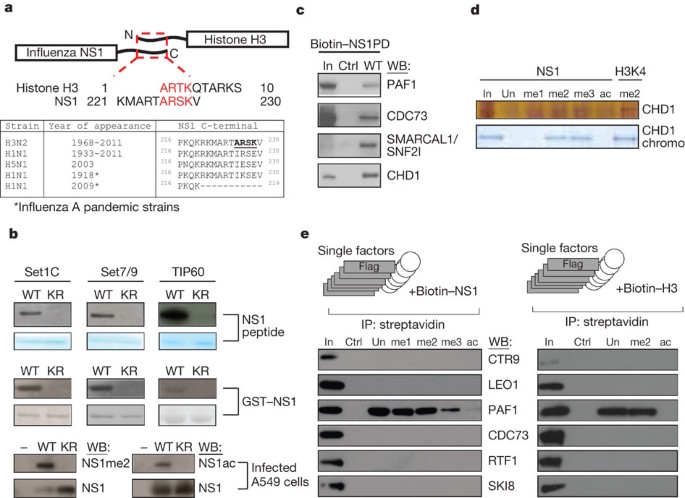
interaction of influenza non-structural protein 1 (NS1) with the infected cell epigenome. We show that the NS1 protein of influenza A H3N2 subtype possesses a histone-like sequence (histone
mimic) that is used by the virus to target the human PAF1 transcription elongation complex (hPAF1C). We demonstrate that binding of NS1 to hPAF1C depends on the NS1 histone mimic and results
in suppression of hPAF1C-mediated transcriptional elongation. Furthermore, human PAF1 has a crucial role in the antiviral response. Loss of hPAF1C binding by NS1 attenuates influenza
infection, whereas hPAF1C deficiency reduces antiviral gene expression and renders cells more susceptible to viruses. We propose that the histone mimic in NS1 enables the influenza virus to
affect inducible gene expression selectively, thus contributing to suppression of the antiviral response. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS SARS-COV-2 DISRUPTS HOST EPIGENETIC
REGULATION VIA HISTONE MIMICRY Article 05 October 2022 NEIL1 BLOCK IFN-Β PRODUCTION AND ENHANCE VRNP FUNCTION TO FACILITATE INFLUENZA A VIRUS PROLIFERATION Article Open access 21 November
2024 THE INFLUENZA VIRUS PB2 PROTEIN EVADES ANTIVIRAL INNATE IMMUNITY BY INHIBITING JAK1/STAT SIGNALLING Article Open access 21 October 2022 REFERENCES * Kornberg, R. D. & Thomas, J. O.
Chromatin structure—oligomers of histones. _Science_ 184, 865–868 (1974) Article ADS CAS Google Scholar * Campos, E. I. & Reinberg, D. Histones: annotating chromatin. _Ann. Rev.
Genet._ 43, 559–599 (2009) Article CAS Google Scholar * Taverna, S. D., Li, H., Ruthenburg, A. J., Allis, C. D. & Patel, D. J. How chromatin-binding modules interpret histone
modifications: lessons from professional pocket pickers. _Nature Struct. Mol. Biol._ 14, 1025–1040 (2007) Article CAS Google Scholar * Kouzarides, T. Chromatin modifications and their
function. _Cell_ 128, 693–705 (2007) Article CAS Google Scholar * Kelly, A. E. et al. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B.
_Science_ 330, 235–239 (2010) Article ADS CAS Google Scholar * Fernandez-Capetillo, O. et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. _Nature Cell Biol._
4, 993–997 (2002) Article CAS Google Scholar * Li, B., Carey, M. & Workman, J. L. The role of chromatin during transcription. _Cell_ 128, 707–719 (2007) Article CAS Google Scholar
* Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. _Nature_ 468, 1067–1073 (2010) Article ADS CAS Google Scholar * Nicodeme, E. et al. Suppression of inflammation
by a synthetic histone mimic. _Nature_ 468, 1119–1123 (2010) Article ADS CAS Google Scholar * Nishiyama, A. et al. Intracellular delivery of acetyl-histone peptides inhibits native
bromodomain-chromatin interactions and impairs mitotic progression. _Febs Lett._ 582, 1501–1507 (2008) Article CAS Google Scholar * Hargreaves, D. C., Horng, T. & Medzhitov, R.
Control of inducible gene expression by signal-dependent transcriptional elongation. _Cell_ 138, 129–145 (2009) Article CAS Google Scholar * Sampath, S. C. et al. Methylation of a histone
mimic within the histone methyltransferase G9a regulates protein complex assembly. _Mol. Cell_ 27, 596–608 (2007) Article CAS Google Scholar * Elde, N. C. & Malik, H. S. The
evolutionary conundrum of pathogen mimicry. _Nature Rev. Microbiol._ 7, 787–797 (2009) Article CAS Google Scholar * Hale, B. G., Randall, R. E., Ortin, J. & Jackson, D. The
multifunctional NS1 protein of influenza A viruses. _J. Gen. Virol._ 89, 2359–2376 (2008) Article CAS Google Scholar * Garcia-Sastre, A. et al. Influenza A virus lacking the NS1 gene
replicates in interferon-deficient systems. _Virology_ 252, 324–330 (1998) Article CAS Google Scholar * Lu, Y., Wambach, M., Katze, M. G. & Krug, R. M. Binding of the influenza-virus
NS1 protein to double-stranded-RNA inhibits the activation of the protein-kinase that phosphorylates the Elf-2 translation initiation-factor. _Virology_ 214, 222–228 (1995) Article CAS
Google Scholar * Gack, M. U. et al. Influenza A Virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. _Cell Host Microbe_ 5, 439–449 (2009)
Article CAS Google Scholar * Pichlmair, A. et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. _Science_ 314, 997–1001 (2006) Article ADS CAS
Google Scholar * Hale, B. G., Jackson, D., Chen, Y. H., Lamb, R. A. & Randall, R. E. Influenza A virus NS1 protein binds p85b and activates phosphatidylinositol-3-kinase signaling.
_Proc. Natl Acad. Sci. USA_ 103, 14194–14199 (2006) Article ADS CAS Google Scholar * Krug, R. M., Yuan, W. M., Noah, D. L. & Latham, A. G. Intracellular warfare between human
influenza viruses and human cells: the roles of the viral NS1 protein. _Virology_ 309, 181–189 (2003) Article CAS Google Scholar * Nemeroff, M. E., Barabino, S. M. L., Li, Y. Z., Keller,
W. & Krug, R. M. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3' end formation of cellular pre-mRNAs. _Mol. Cell_ 1, 991–1000 (1998)
Article CAS Google Scholar * Das, K. et al. Structural basis for suppression of a host antiviral response by influenza A virus. _Proc. Natl Acad. Sci. USA_ 105, 13093–13098 (2008) Article
ADS CAS Google Scholar * Satterly, N. et al. Influenza virus targets the mRNA export machinery and the nuclear pore complex. _Proc. Natl Acad. Sci. USA_ 104, 1853–1858 (2007) Article
ADS CAS Google Scholar * Luger, K., Mader, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. _Nature_
389, 251–260 (1997) Article ADS CAS Google Scholar * Hale, B. G., Barclay, W. S., Randall, R. E. & Russell, R. J. Structure of an avian influenza A virus NS1 protein effector domain.
_Virology_ 378, 1–5 (2008) Article CAS Google Scholar * Xhemalce, B. & Kouzarides, T. A chromodomain switch mediated by histone H3 Lys 4 acetylation regulates heterochromatin
assembly. _Genes Dev._ 24, 647–652 (2010) Article CAS Google Scholar * Becker, P. B. et al. Site-specific acetylation of ISWI by GCN5. _BMC Mol. Biol._ 8, (2007) * Ruthenburg, A. J.,
Allis, C. D. & Wysocka, J. Methylation of lysine 4 on histone H3: Intricacy of writing and reading a single epigenetic mark. _Mol. Cell_ 25, 15–30 (2007) Article CAS Google Scholar *
Wang, P. F. et al. Global analysis of H3K4 methylation defines MLL family member targets and points to a role for MLL1-mediated H3K4 methylation in the regulation of transcriptional
initiation by RNA polymerase II. _Mol. Cell. Biol._ 29, 6074–6085 (2009) Article CAS Google Scholar * Guillemette, B. et al. H3 lysine 4 is acetylated at active gene promoters and is
regulated by H3 lysine 4 methylation. _PLoS Genet._ 7, (2011) Article CAS Google Scholar * Lachner, M., O’Carroll, N., Rea, S., Mechtler, K. & Jenuwein, T. Methylation of histone H3
lysine 9 creates a binding site for HP1 proteins. _Nature_ 410, 116–120 (2001) Article ADS CAS Google Scholar * Shi, X. B. et al. ING2 PHD domain links histone H3 lysine 4 methylation to
active gene repression. _Nature_ 442, 96–99 (2006) Article ADS CAS Google Scholar * Lan, F. et al. Recognition of unmethylated histone H3 lysine 4 links BHC80 to LSD1-mediated gene
repression. _Nature_ 448, 718–722 (2007) Article ADS CAS Google Scholar * Wysocka, J. Identifying novel proteins recognizing histone modifications using peptide pull-down assay.
_Methods_ 40, 339–343 (2006) Article CAS Google Scholar * Sims, R. J. et al. Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem
chromodomains. _J. Biol. Chem._ 280, 41789–41792 (2005) Article CAS Google Scholar * Kim, J., Guermah, M. & Roeder, R. G. The human PAF1 complex acts in chromatin transcription
elongation both independently and cooperatively with SII/TFIIS. _Cell_ 140, 491–503 (2010) Article CAS Google Scholar * Ramirez-Carrozzi, V. R. et al. A unifying model for the selective
regulation of inducible transcription by CpG islands and nucleosome remodeling. _Cell_ 138, 114–128 (2009) Article CAS Google Scholar * Kim, K. Y. & Levin, D. E. Mpk1 MAPK association
with the Paf1 complex blocks Sen1-mediated premature transcription termination. _Cell_ 144, 745–756 (2011) Article CAS Google Scholar * Chen, Y. X. et al. DSIF, the Paf1 complex, and
Tat-SF1 have nonredundant, cooperative roles in RNA polymerase II elongation. _Genes Dev._ 23, 2765–2777 (2009) Article CAS Google Scholar * Jaehning, J. A. The Paf1 complex: platform or
player in RNA polymerase II transcription? _Biochim. Bioiphys. Acta_ 1799, 379–388 (2010) Article CAS Google Scholar * Core, L. J., Waterfall, J. J. & Lis, J. T. Nascent RNA
sequencing reveals widespread pausing and divergent initiation at human promoters. _Science_ 322, 1845–1848 (2008) Article ADS CAS Google Scholar * Min, I. M. et al. Regulating RNA
polymerase pausing and transcription elongation in embryonic stem cells. _Gene Dev._ 25, 742–754 (2011) Article CAS Google Scholar * Mapendano, C. K., Lykke-Andersen, S., Kjems, J.,
Bertrand, E. & Jensen, T. H. Crosstalk between mRNA 3′ end processing and transcription initiation. _Mol. Cell_ 40, 410–422 (2010) Article CAS Google Scholar * Loucaides, E. M. et al.
Nuclear dynamics of influenza A virus ribonucleoproteins revealed by live-cell imaging studies. _Virology_ 394, 154–163 (2009) Article CAS Google Scholar * Engelhardt, O. G., Smith, M.
& Fodor, E. Association of the influenza a virus RNA-dependent RNA polymerase with cellular RNA polymerase II. _J. Virol._ 79, 5812–5818 (2005) Article CAS Google Scholar * Jackson,
D., Hossain, M. J., Hickman, D., Perez, D. R. & Lamb, R. A. A new influenza virus virulence determinant: The NS1 protein four C-terminal residues modulate pathogenicity. _Proc. Natl
Acad. Sci. USA_ 105, 4381–4386 (2008) Article ADS CAS Google Scholar * Strahl, B. D. & Allis, C. D. The language of covalent histone modifications. _Nature_ 403, 41–45 (2000) Article
ADS CAS Google Scholar * Turner, B. M. Histone acetylation and an epigenetic code. _Bioessays_ 22, 836–845 (2000) Article CAS Google Scholar * Jenuwein, T. & Allis, C. D.
Translating the histone code. _Science_ 293, 1074–1080 (2001) Article CAS Google Scholar * Yang, Y. et al. The transmissibility and control of pandemic influenza A (H1N1) virus. _Science_
326, 729–733 (2009) Article ADS CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank P. deGross and A. Rudensky for the mass spectroscopy analysis of the NS1 binding
proteins. A. Rojas Soto, D. Reinberg, M. Dobenecker and T. Zhanyun provided us with recombinant CHD1 (A.R.S., D.R.), recombinant Set7/9 (M.D.) and Set1C (T.Z.). F. Casadio, P. Lewis, O.
Binda, O. Gozani, N. Levenkova, A. Mele, R. Darnell, L. Core, J. Lis and P. Palese gave us valuable technical advice and help with data analysis. We acknowledge the Rockefeller University
Genomics Resource Center for technical support. We thank R. Cadagan, A. Santana, W. Huang, R. Chandramouli and H. Zebronsky for technical assistance, R. Rizzo for help with manuscript
preparation and C. Nathan for discussion. L.M.K. for artwork. B.M is supported by NIH/NIAID K99 Pathway to Independence award (1K99AI095320-01). A.G.-S. is partially supported by NIAID
grants R01AI046954, U19AI083025 and by CRIP (Center for Research in Influenza Pathogenesis), an NIAID funded Center of Excellence for Influenza Research and Surveillance, HHSN266200700010C.
R.G.R. is supported by NIH grant CA129325. J.K. is supported by Charles H. Revson Foundation. I.M. is supported by American Italian Cancer Foundation. J.H. is supported by the Agency for
Science, Technology and Research (A*STAR), Singapore. A.T. is supported by the NIH grant R01AI068058 and by Starr Cancer Consortium. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Laboratory
of Immune Cell Epigenetics and Signaling, The Rockefeller University, 1230 York Avenue, New York, New York 10065, USA, Ivan Marazzi, Jessica S. Y. Ho, Uwe Schaefer, Kate L. Jeffrey &
Alexander Tarakhovsky * Laboratory of Biochemistry and Molecular Biology, The Rockefeller University, 1230 York Avenue, New York, New York 10065, USA, Jaehoon Kim & Robert G. Roeder *
Department of Microbiology, Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1124, New York, New York 10029, USA, Balaji Manicassamy, Randy A. Albrecht, Chris W. Seibert &
Adolfo García-Sastre * Global Health and Infectious Pathogens Institute, Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1124, New York, New York 10029, USA, Balaji
Manicassamy, Randy A. Albrecht & Adolfo García-Sastre * Genomics Resource Center, The Rockefeller University, 1230 York Avenue, New York, New York 10065, USA, Scott Dewell * Epinova DPU,
Immuno-Inflammation Centre of Excellence for Drug Discovery, GlaxoSmithKline, Medicines Research Centre, Gunnels Wood Road, Stevenage SG1 2NY, UK, Rab K. Prinjha & Kevin Lee *
Department of Medicine, Division of Infectious Diseases, Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1124, New York, New York 10029, USA, Adolfo García-Sastre Authors *
Ivan Marazzi View author publications You can also search for this author inPubMed Google Scholar * Jessica S. Y. Ho View author publications You can also search for this author inPubMed
Google Scholar * Jaehoon Kim View author publications You can also search for this author inPubMed Google Scholar * Balaji Manicassamy View author publications You can also search for this
author inPubMed Google Scholar * Scott Dewell View author publications You can also search for this author inPubMed Google Scholar * Randy A. Albrecht View author publications You can also
search for this author inPubMed Google Scholar * Chris W. Seibert View author publications You can also search for this author inPubMed Google Scholar * Uwe Schaefer View author publications
You can also search for this author inPubMed Google Scholar * Kate L. Jeffrey View author publications You can also search for this author inPubMed Google Scholar * Rab K. Prinjha View
author publications You can also search for this author inPubMed Google Scholar * Kevin Lee View author publications You can also search for this author inPubMed Google Scholar * Adolfo
García-Sastre View author publications You can also search for this author inPubMed Google Scholar * Robert G. Roeder View author publications You can also search for this author inPubMed
Google Scholar * Alexander Tarakhovsky View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS I.M. contributed to design, execution, analysis of
the experiments and manuscript preparation. J.S.Y.H. studied the role of PAF1 in viral infection and assisted in manuscript preparation. J.K. and R.R. studied the impact of NS1 on hPAF1C and
transcriptional elongation. B.M., R.A.A. engineered the recombinant influenza viruses and studied viral infectivity. U.S. was involved in gene expression studies. S.D. performed
bioinformatic analysis. C.W.S. generated antibody against viral polymerase. K.L.J. gave technical assistance. R.K.P. and K.L. contributed to manuscript preparation and enabled ChIP-seq and
RNA-seq. A.G.-S. supervised and discussed the work with infectious influenza viruses. A.T. conceived and supervised this study and wrote the final manuscript. CORRESPONDING AUTHORS
Correspondence to Ivan Marazzi or Alexander Tarakhovsky. ETHICS DECLARATIONS COMPETING INTERESTS R.K.P. and K.L. are employees of GlaxoSmithKline. Research support, excluding salaries to the
members of The Rockefeller University, was partially provided by GlaxoSmithKline. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION This file contains Supplementary Figures 1-12,
Supplementary Methods, additional references and full legends for Supplementary Tables 1-8. (PDF 1437 kb) SUPPLEMENTARY TABLE 1 This table shows genes affected by Influenza Infection - see
Supplementary Information file for full legend. (XLS 135 kb) SUPPLEMENTARY TABLE 2 This table contains a list of genes used for the integrated ChIP-seq profile - see Supplementary
Information file for full legend. (XLS 43 kb) SUPPLEMENTARY TABLE 3 This table shows siPAF dependent genes in PR8/∆NS1 infected cells - see Supplementary Information file for full legend.
(XLS 249 kb) SUPPLEMENTARY TABLE 4 This table shows siPAF dependent genes in Influenza (H1N1) infected cells - see Supplementary Information file for full legend. (XLS 656 kb) SUPPLEMENTARY
TABLE 5 This table shows siPAF dependent genes in Influenza (H1N1) infected cells - see Supplementary Information file for full legend. (XLS 596 kb) SUPPLEMENTARY TABLE 6 This table shows
siPAF dependent genes in Poly(I:C) transfected cells - see Supplementary Information file for full legend. (XLS 888 kb) SUPPLEMENTARY TABLE 7 This table shows siPAF dependent genes in IFNβ1
treated cells - see Supplementary Information file for full legend. (XLS 111 kb) SUPPLEMENTARY TABLE 8 This table shows that expression of housekeeping genes are not affected by siPAF
mediated hPAF1 deficiency - see Supplementary Information file for full legend. (XLS 24 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR
FIG. 3 POWERPOINT SLIDE FOR FIG. 4 POWERPOINT SLIDE FOR FIG. 5 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Marazzi, I., Ho, J., Kim, J. _et al._
Suppression of the antiviral response by an influenza histone mimic. _Nature_ 483, 428–433 (2012). https://doi.org/10.1038/nature10892 Download citation * Received: 07 September 2011 *
Accepted: 23 January 2012 * Published: 14 March 2012 * Issue Date: 22 March 2012 * DOI: https://doi.org/10.1038/nature10892 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative