- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Accurate segregation of chromosomes, essential for the stability of the genome, depends on ‘bi-orientation’—simultaneous attachment of each individual chromosome to both poles of
the mitotic spindle1. On bi-oriented chromosomes, kinetochores (macromolecular complexes that attach the chromosome to the spindle) reside on the opposite sides of the chromosome’s
centromere2. In contrast, sister kinetochores shift towards one side of the centromere on ‘syntelic’ chromosomes that erroneously attach to one spindle pole with both sister kinetochores.
Syntelic attachments often arise during spindle assembly and must be corrected to prevent chromosome loss3. It is assumed that restoration of proper centromere architecture occurs
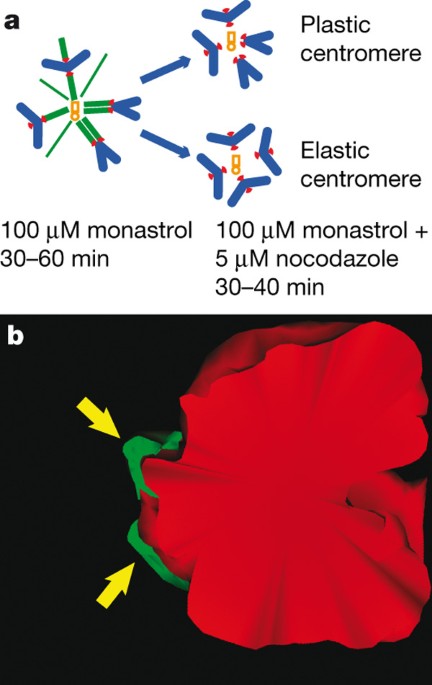
automatically owing to elastic properties of the centromere1,2. Here we test this assumption by combining laser microsurgery and chemical biology assays in cultured mammalian cells. We find
that kinetochores of syntelic chromosomes remain juxtaposed on detachment from spindle microtubules. These findings reveal that correction of syntelic attachments involves an extra step that
has previously been overlooked: external forces must be applied to move sister kinetochores to the opposite sides of the centromere. Furthermore, we demonstrate that the shape of the
centromere is important for spindle assembly, because bipolar spindles do not form in cells lacking centrosomes when multiple chromosomes with juxtaposed kinetochores are present. Thus,
proper architecture of the centromere makes an important contribution to achieving high fidelity of chromosome segregation. Access through your institution Buy or subscribe This is a preview
of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only
$3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS KINETOCHORE- AND
CHROMOSOME-DRIVEN TRANSITION OF MICROTUBULES INTO BUNDLES PROMOTES SPINDLE ASSEMBLY Article Open access 27 November 2022 MECHANISMS UNDERLYING SPINDLE ASSEMBLY AND ROBUSTNESS Article 28
March 2023 PRINCIPLES AND DYNAMICS OF SPINDLE ASSEMBLY CHECKPOINT SIGNALLING Article 24 March 2023 REFERENCES * Nicklas, R. B. How cells get the right chromosomes. _Science_ 275, 632–637
(1997) Article CAS PubMed Google Scholar * Rieder, C. L. & Salmon, E. D. The vertebrate kinetochore and its roles during mitosis. _Trends Cell Biol._ 8, 310–318 (1998) Article CAS
PubMed PubMed Central Google Scholar * Cimini, D. & Degrassi, F. Aneuploidy: a matter of bad connections. _Trends Cell Biol._ 8, 442–451 (2005) Article Google Scholar * Ault, J. G.
& Nicklas, R. B. Tension, microtubule rearrangements, and the proper distribution of chromosomes in mitosis. _Chromosoma_ 98, 33–39 (1989) Article CAS PubMed Google Scholar *
Nicklas, R. B. & Ward, S. C. Elements of error correction in mitosis: microtubule capture, release, and tension. _J. Cell Biol._ 126, 1241–1253 (1994) Article CAS PubMed Google
Scholar * Lampson, M. A., Renduchitala, K., Khodjakov, A. & Kapoor, T. M. Correcting improper chromosome–spindle attachments during cell division. _Nature Cell Biol._ 6, 232–237 (2004)
Article CAS PubMed Google Scholar * Kline-Smith, S. L., Khodjakov, A., Hergert, P. & Walczak, C. E. Depletion of centromeric MCAK leads to chromosome congression and segregation
defects due to improper kinetochore attachments. _Mol. Biol. Cell_ 15, 1146–1159 (2004) Article CAS PubMed PubMed Central Google Scholar * Andrews, P. D. et al. Aurora B regulates MCAK
at the mitotic centromere. _Dev. Cell_ 6, 253–268 (2004) Article CAS PubMed Google Scholar * Sullivan, K. F. & Shelby, R. D. Using time-lapse confocal microscopy for analysis of
centromere dynamics in human cells. _Methods Cell Biol._ 58, 183–202 (1999) Article CAS PubMed Google Scholar * Poirier, M. G. & Marko, J. F. Micromechanical studies of mitotic
chromosomes. _Curr. Top. Dev. Biol._ 55, 75–141 (2003) Article CAS PubMed Google Scholar * Kapoor, T. M., Mayer, T. U., Coughlin, M. L. & Mitchison, T. J. Probing spindle assembly
mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. _J. Cell Biol._ 150, 975–988 (2000) Article CAS PubMed PubMed Central Google Scholar * Khodjakov, A.,
Copenagle, L., Gordon, M. B., Compton, D. A. & Kapoor, T. M. Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. _J. Cell Biol._ 160, 671–683 (2003)
Article CAS PubMed PubMed Central Google Scholar * Khodjakov, A. & Rieder, C. L. The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic
exchange throughout the cell cycle, do not require microtubules. _J. Cell Biol._ 146, 585–596 (1999) Article CAS PubMed PubMed Central Google Scholar * Khodjakov, A., Cole, R. W.,
Oakley, B. R. & Rieder, C. L. Centrosome-independent mitotic spindle formation in vertebrates. _Curr. Biol._ 10, 59–67 (2000) Article CAS PubMed Google Scholar * Khodjakov, A. &
Rieder, C. L. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. _J. Cell Biol._ 153, 237–242 (2001) Article CAS PubMed PubMed
Central Google Scholar * Compton, D. A. Focusing on spindle poles. _J. Cell Sci._ 111, 1477–1481 (1998) Article CAS PubMed Google Scholar * Rieder, C. L. & Alexander, S. P.
Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. _J. Cell Biol._ 110, 81–95 (1990) Article CAS PubMed
Google Scholar * Maiato, H., Rieder, C. L. & Khodjakov, A. Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. _J. Cell Biol._
167, 831–840 (2004) Article CAS PubMed PubMed Central Google Scholar * Tulu, U. S., Fagerstrom, C., Ferenz, N. P. & Wadsworth, P. Molecular requirements for kinetochore-associated
microtubule formation in mammalian cells. _Curr. Biol._ 16, 536–541 (2006) Article CAS PubMed PubMed Central Google Scholar * Indjeian, V. B., Stern, B. M. & Murray, A. W. The
centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. _Science_ 307, 130–133 (2005) Article ADS CAS PubMed Google Scholar * Li, X. & Nicklas, R. B.
Mitotic forces control a cell-cycle checkpoint. _Nature_ 373, 630–632 (1995) Article ADS CAS PubMed Google Scholar * Waters, J. C., Chen, R.-H., Murray, A. W. & Salmon, E. D.
Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. _J. Cell Biol._ 141, 1181–1191 (1998) Article CAS PubMed PubMed Central Google Scholar * Cimini, D.
et al. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. _J. Cell Biol._ 153, 517–528 (2001) Article CAS PubMed PubMed Central
Google Scholar * Rieder, C. L., Cole, R. W., Khodjakov, A. & Sluder, G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal
produced by unattached kinetochores. _J. Cell Biol._ 130, 941–948 (1995) Article CAS PubMed Google Scholar * Rieder, C. L., Schultz, A., Cole, R. & Sluder, G. Anaphase onset in
vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. _J. Cell Biol._ 127, 1301–1310 (1994) Article CAS PubMed Google Scholar
* Cassimeris, L., Rieder, C. L. & Salmon, E. D. Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of
chromosome congression. _J. Cell Sci._ 107, 285–297 (1994) Article PubMed Google Scholar * Kapoor, T. M. et al. Chromosomes can congress to the metaphase plate before biorientation.
_Science_ 311, 388–391 (2006) Article ADS CAS PubMed PubMed Central Google Scholar * Magidson, V., Loncarek, J., Hergert, P., Rieder, C. L. & Khodjakov, A. in _Laser Manipulations
of Cells and Tissues_ (eds Berns, M. W. & Greulich, K. O.) 237–266 (Elsevier, 2007) Book Google Scholar * Mayer, T. U. et al. Small molecule inhibitor of mitotic spindle bipolarity
identified in a phenotype-based screen. _Science_ 286, 971–974 (1999) Article CAS PubMed Google Scholar * La Terra, S. et al. The de novo centriole assembly pathway in HeLa cells: cell
cycle progression and centriole assembly/maturation. _J. Cell Biol._ 168, 713–720 (2005) Article CAS PubMed PubMed Central Google Scholar * Khodjakov, A., Cole, R. W., McEwen, B. F.,
Buttle, K. F. & Rieder, C. L. Chromosome fragments possessing only one kinetochore can congress to the spindle equator. _J. Cell Biol._ 136, 229–240 (1997) Article CAS PubMed PubMed
Central Google Scholar Download references ACKNOWLEDGEMENTS We thank B. McEwen, C. Rieder and V. Magidson for fruitful discussions and critical reading of the manuscript. This work was
supported by grants from the National Institutes of Health grants (to A.K. and T.M.K.). Assembly of our laser microsurgery system was supported in part by a Nikon/MBL fellowship (A.K.). We
acknowledge the use of Wadsworth Centre’s electron microscopy core facility. AUTHOR INFORMATION Author notes * Olga Kisurina-Evgenieva Present address: Present address: Department of
Cytology, Biology Faculty, Moscow State University, Moscow 119991, Russia., Moscow * Jadranka Lončarek and Olga Kisurina-Evgenieva: These authors contributed equally to this work. AUTHORS
AND AFFILIATIONS * Division of Molecular Medicine, New York State Department of Health, Wadsworth Center, Albany, Albany, New York 12201-0509, USA, New York Jadranka Lončarek, Olga
Kisurina-Evgenieva, Tatiana Vinogradova, Polla Hergert, Sabrina La Terra & Alexey Khodjakov * Department of Biomedical Sciences, State University of New York, Albany, New York 12222,
USA, New York Sabrina La Terra & Alexey Khodjakov * Laboratory of Chemistry and Cell Biology, The Rockefeller University, New York, New York 10021, USA , New York Tarun M. Kapoor &
Alexey Khodjakov Authors * Jadranka Lončarek View author publications You can also search for this author inPubMed Google Scholar * Olga Kisurina-Evgenieva View author publications You can
also search for this author inPubMed Google Scholar * Tatiana Vinogradova View author publications You can also search for this author inPubMed Google Scholar * Polla Hergert View author
publications You can also search for this author inPubMed Google Scholar * Sabrina La Terra View author publications You can also search for this author inPubMed Google Scholar * Tarun M.
Kapoor View author publications You can also search for this author inPubMed Google Scholar * Alexey Khodjakov View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHORS Correspondence to Tarun M. Kapoor or Alexey Khodjakov. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES The file contains Supplementary Figures S1-S6 with
Legends. (PDF 2537 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lončarek, J., Kisurina-Evgenieva, O., Vinogradova, T. _et al._ The centromere
geometry essential for keeping mitosis error free is controlled by spindle forces. _Nature_ 450, 745–749 (2007). https://doi.org/10.1038/nature06344 Download citation * Received: 06 July
2007 * Accepted: 03 October 2007 * Published: 29 November 2007 * Issue Date: 29 November 2007 * DOI: https://doi.org/10.1038/nature06344 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative