- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
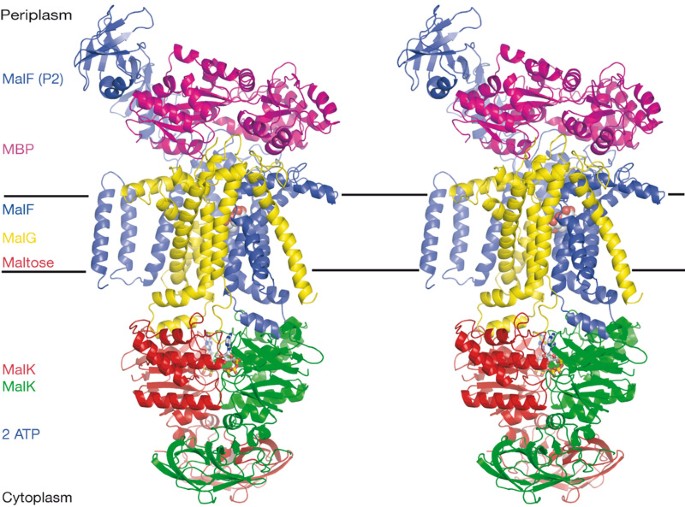
ABSTRACT The maltose uptake system of _Escherichia coli_ is a well-characterized member of the ATP-binding cassette transporter superfamily. Here we present the 2.8-Å crystal structure of
the intact maltose transporter in complex with the maltose-binding protein, maltose and ATP. This structure, stabilized by a mutation that prevents ATP hydrolysis, captures the ATP-binding
cassette dimer in a closed, ATP-bound conformation. Maltose is occluded within a solvent-filled cavity at the interface of the two transmembrane subunits, about halfway into the lipid
bilayer. The binding protein docks onto the entrance of the cavity in an open conformation and serves as a cap to ensure unidirectional translocation of the sugar molecule. These results
provide direct evidence for a concerted mechanism of transport in which solute is transferred from the binding protein to the transmembrane subunits when the cassette dimer closes to
hydrolyse ATP. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full
article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs *
Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS ATP HYDROLYSIS AND NUCLEOTIDE EXIT ENHANCE MALTOSE TRANSLOCATION IN THE MALFGK2E IMPORTER Article Open access 19 May 2021
STRUCTURES OF HUMAN SGLT IN THE OCCLUDED STATE REVEAL CONFORMATIONAL CHANGES DURING SUGAR TRANSPORT Article Open access 22 May 2023 A ROTARY MECHANISM FOR ALLOSTERY IN BACTERIAL HYBRID MALIC
ENZYMES Article Open access 23 February 2021 REFERENCES * Higgins, C. F. ABC transporters: from microorganisms to man. _Annu. Rev. Cell Biol._ 8, 67–113 (1992) Article CAS Google Scholar
* Boos, W. & Lucht, J. M. in _Escherichia coli and Salmonella: Cellular and Molecular Biology_ (eds Neidhardt, F. C. et al.) 1175–1209 (ASM Press, Washington DC, 1996) Google Scholar
* Holland, I. B. & Blight, M. A. ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. _J. Mol. Biol._
293, 381–399 (1999) Article CAS Google Scholar * Walker, J. E., Saraste, M., Runswick, M. J. & Gay, N. J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin,
kinases and other ATP-requiring enzymes and a common nucleotide binding fold. _EMBO J._ 1, 945–951 (1982) Article CAS Google Scholar * Pinkett, H. W., Lee, A. T., Lum, P., Locher, K. P.
& Rees, D. C. An inward-facing conformation of a putative metal-chelate-type ABC transporter. _Science_ 315, 373–377 (2007) Article ADS CAS Google Scholar * Locher, K. P., Lee, A. T.
& Rees, D. C. The _E. _ _coli_ BtuCD structure: a framework for ABC transporter architecture and mechanism. _Science_ 296, 1091–1098 (2002) Article ADS CAS Google Scholar *
Hollenstein, K., Frei, D. C. & Locher, K. P. Structure of an ABC transporter in complex with its binding protein. _Nature_ 446, 213–216 (2007) Article ADS CAS Google Scholar *
Dawson, R. J. & Locher, K. P. Structure of a bacterial multidrug ABC transporter. _Nature_ 443, 180–185 (2006) Article ADS CAS Google Scholar * Ferenci, T. The recognition of
maltodextrins by _Escherichia coli._ . _Eur. J. Biochem._ 108, 613–636 (1980) Article Google Scholar * Raibaud, O., Roa, M., Braun-Breton, C. & Schwartz, M. Structure of the _malB_
region in _Escherichia coli_ K-12. I. Genetic map of the _malK-lamB_ operon. _Mol. Gen. Genet._ 174, 241–248 (1979) Article CAS Google Scholar * Silhavy, T. J. et al. Structure of the
_malB_ region in _Escherichia coli_ K12. II. Genetic map of the malE,F,G operon. _Mol. Gen. Genet._ 174, 249–259 (1979) Article CAS Google Scholar * Davidson, A. L. & Nikaido, H.
Purification and characterization of the membrane-associated components of the maltose transport system from _Escherichia coli_ . _J. Biol. Chem._ 266, 8946–8951 (1991) CAS PubMed Google
Scholar * Davidson, A. L. & Nikaido, H. Overproduction, solubilization, and reconstitution of the maltose transport system from _Escherichia coli_ . _J. Biol. Chem._ 265, 4254–4260
(1990) CAS PubMed Google Scholar * Landmesser, H. et al. Large-scale purification, dissociation and functional reassembly of the maltose ATP-binding cassette transporter (MalFGK(2)) of
_Salmonella typhimurium_ . _Biochim. Biophys. Acta_ 1565, 64–72 (2002) Article CAS Google Scholar * Davidson, A. L., Shuman, H. A. & Nikaido, H. Mechanism of maltose transport in
_Escherichia coli_: Transmembrane signalling by periplasmic binding proteins. _Proc. Natl Acad. Sci. USA_ 89, 2360–2364 (1992) Article ADS CAS Google Scholar * Sharff, A. J., Rodseth, L.
E., Spurlino, J. E. & Quiocho, F. A. Crystallographic evidence of a large ligand-induced hinge-twist motion between the two domains of the maltodextrin binding protein involved in
active transport and chemotaxis. _Biochemistry_ 31, 10657–10663 (1992) Article CAS Google Scholar * Quiocho, F. A., Spurlino, J. C. & Rodseth, L. E. Extensive features of tight
oligosaccharide binding revealed in high-resolution structures of the maltodextrin transport/chemosensory receptor. _Structure_ 5, 997–1015 (1997) Article CAS Google Scholar * Duan, X.
& Quiocho, F. A. Structural evidence for the dominant role of nonpolar interactions in the binding of a transport/chemonsensory receptor to its highly polar ligands. _Biochemistry_ 41,
706–712 (2002) Article CAS Google Scholar * Chen, J., Sharma, S., Quiocho, F. A. & Davidson, A. L. Trapping the transition state of an ATP-binding-cassette transporter: Evidence for a
concerted mechanism of maltose transport. _Proc. Natl Acad. Sci. USA_ 98, 1525–1530 (2001) Article ADS CAS Google Scholar * Chen, J., Lu, G., Lin, J., Davidson, A. L. & Quiocho, F.
A. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. _Mol. Cell_ 12, 651–661 (2003) Article CAS Google Scholar * Lu, G., Westbrooks, J. M., Davidson, A.
L. & Chen, J. ATP hydrolysis is required to reset the ATP-binding cassette dimer into the resting-state conformation. _Proc. Natl Acad. Sci. USA_ 102, 17969–17974 (2005) Article ADS
CAS Google Scholar * Orelle, C., Dalmas, O., Gros, P., Di Pietro, A. & Jault, J. M. The conserved glutamate residue adjacent to the Walker-B motif is the catalytic base for ATP
hydrolysis in the ATP-binding cassette transporter BmrA. _J. Biol. Chem._ 278, 47002–47008 (2003) Article CAS Google Scholar * Moody, J. E., Millen, L., Binns, D., Hunt, J. F. &
Thomas, P. J. Cooperative, ATP-dependent association of the nucleotide binding cassettes during the catalytic cycle of ATP-binding cassette transporters. _J. Biol. Chem._ 277, 21111–21114
(2002) Article CAS Google Scholar * Smith, P. C. et al. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. _Mol. Cell_ 10, 139–149
(2002) Article CAS Google Scholar * Rossmann, M. G. The molecular replacement method. _Acta Crystallogr. A_ 46, 73–82 (1990) Article Google Scholar * Covitz, K.-M. Y., Panagiotidis, C.
H., Reyes, M., Treptow, N. A. & Shuman, H. A. Mutations that alter the transmembrane signalling pathway in an ATP binding cassette (ABC) transporter. _EMBO J._ 13, 1752–1759 (1994)
Article CAS Google Scholar * Dassa, E. & Muir, S. Membrane topology of MalG, an inner membrane protein from the maltose transport system of _Escherichia coli_ . _Mol. Microbiol._ 7,
29–38 (1993) Article CAS Google Scholar * Froshauer, S., Green, G. N., Boyd, D., McGovern, K. & Beckwith, J. Genetic analysis of the membrane insertion and topology of MalF, a
cytoplasmic membrane protein of _Escherichia coli_ . _J. Mol. Biol._ 200, 501–511 (1988) Article CAS Google Scholar * Hvorup, R. N. et al. Asymmetry in the structure of the ABC
transporter binding protein complex BtuCD-BtuF. _Science_ 317, 1387–1390 (2007) Article ADS CAS Google Scholar * Dassa, E. & Hofnung, M. Sequence of gene _malG_ in _E. _ _coli_ K12:
homologies between integral membrane components from binding protein-dependent transport systems. _EMBO J._ 4, 2287–2293 (1985) Article CAS Google Scholar * Saurin, W., Koster, W. &
Dassa, E. Bacterial binding protein-dependent permeases: characterization of distinctive signatures for functionally related integral cytoplasmic membrane proteins. _Mol. Microbiol._ 12,
993–1004 (1994) Article CAS Google Scholar * Busch, W. & Saier, M. H. Jr. The transporter classification (TC) system, 2002. _Crit. Rev. Biochem. Mol. Biol._ 37, 287–337 (2002) Article
CAS Google Scholar * Mourez, M., Hofnung, M. & Dassa, E. Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases
defines an important site of interaction with the ATPase subunits. _EMBO J._ 16, 3066–3077 (1997) Article CAS Google Scholar * Ehrle, R., Pick, C., Ulrich, R., Hofmann, E. & Ehrmann,
M. Characterization of transmembrane domains 6, 7, and 8 of MalF by mutational analysis. _J. Bacteriol._ 178, 2255–2262 (1996) Article CAS Google Scholar * Steinke, A., Grau, S.,
Davidson, A., Hofmann, E. & Ehrmann, M. Characterization of transmembrane segments 3, 4, and 5 of MalF by mutational analysis. _J. Bacteriol._ 183, 375–381 (2001) Article CAS Google
Scholar * Quiocho, F. A. Carbohydrate-binding proteins: tertiary structures and protein–sugar interactions. _Annu. Rev. Biochem._ 55, 287–315 (1986) Article CAS Google Scholar * Shuman,
H. A. Active transport of maltose in _Escherichia coli_ K-12: role of the periplasmic maltose binding protein and evidence for a substrate recognition site in the cytoplasmic membrane. _J.
Biol. Chem._ 257, 5455–5461 (1982) CAS PubMed Google Scholar * Schirmer, T., Keller, T. A., Wang, Y. F. & Rosenbusch, J. P. Structural basis for sugar translocation through maltoporin
channels at 3.1 Å resolution. _Science_ 267, 512–514 (1995) Article ADS CAS Google Scholar * Quiocho, F. A. & Ledvina, P. S. Atomic structure and specificity of bacterial
periplasmic receptors for active transport and chemotaxis: variation of common themes. _Mol. Microbiol._ 20, 17–25 (1996) Article CAS Google Scholar * Miller, D. M., Olson, J. S.,
Pflugrath, J. W. & Quiocho, F. A. Rates of ligand binding to periplasmic proteins involved in bacterial transport and chemotaxis. _J. Biol. Chem._ 258, 13665–13672 (1983) CAS PubMed
Google Scholar * Nelson, B. D. & Traxler, B. Exploring the role of integral membrane proteins in ATP-binding cassette transporters: analysis of a collection of MalG insertion mutants.
_J. Bacteriol._ 180, 2507–2514 (1998) CAS PubMed PubMed Central Google Scholar * Austermuhle, M. I., Hall, J. A., Klug, C. S. & Davidson, A. L. Maltose-binding protein is open in the
catalytic transition state for ATP hydrolysis during maltose transport. _J. Biol. Chem._ 279, 28243–28250 (2004) Article CAS Google Scholar * Fetsch, E. E. & Davidson, A. L.
Vanadate-catalyzed photocleavage of the signature motif of an ATP-binding cassette (ABC) transporter. _Proc. Natl Acad. Sci. USA_ 99, 9685–9690 (2002) Article ADS CAS Google Scholar *
Sharma, S. & Davidson, A. L. Vanadate-induced trapping of nucleotide by the purified maltose transport complex requires ATP hydrolysis. _J. Bacteriol._ 182, 6570–6576 (2000) Article CAS
Google Scholar * Urbatsch, I. L., Sankaran, B., Bhagat, S. & Senior, A. E. Both P-glycoprotein nucleotide-binding sites are catalytically active. _J. Biol. Chem._ 270, 26956–26962
(1995) Article CAS Google Scholar * Urbatsch, I. L., Sankaran, B., Weber, J. & Senior, A. E. P-glycoprotein is stably inhibited by vanadate-induced trapping of nucleotide at a single
catalytic site. _J. Biol. Chem._ 270, 19383–19390 (1995) Article CAS Google Scholar * Jardetzky, O. Simple allosteric model for membrane pumps. _Nature_ 211, 969–970 (1966) Article ADS
CAS Google Scholar * Neuhard, J. & Nygaard, P. in _Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology_ (eds Ingraham, J. L. & Neidhardt, F. C.) 445–473
(ASM Press, Washington DC, 1987) Google Scholar * Davidson, A. L., Laghaeian, S. S. & Mannering, D. E. The maltose transport system of _Escherichia coli_ displays positive cooperativity
in ATP hydrolysis. _J. Biol. Chem._ 271, 4858–4863 (1996) Article CAS Google Scholar * Sun, H. & Nathans, J. Mechanistic studies of ABCR, the ABC transporter in photoreceptor outer
segments responsible for autosomal recessive Stargardt disease. _J. Bioenerg. Biomembr._ 33, 523–530 (2001) Article CAS Google Scholar * Davidson, A. L. & Nikaido, H. Overproduction,
solubilization, and reconstitution of the maltose transport system from _Escherichia coli_ . _J. Biol. Chem._ 265, 4254–4260 (1990) CAS PubMed Google Scholar * Chen, J., Lu, G., Lin, J.,
Davidson, A. L. & Quiocho, F. A. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. _Mol. Cell_ 12, 651–661 (2003) Article CAS Google Scholar * Dean,
D. A., Fikes, J. D., Gehring, K., Bassford, P. J. & Nikaido, H. Active transport of maltose in membrane vesicles obtained from _Escherichia coli_ cells producing tethered maltose-binding
protein. _J. Bacteriol._ 171, 503–510 (1989) Article CAS Google Scholar * Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. _Macro
Crystallogr. A_ 276, 307–326 (1997) Article CAS Google Scholar * Project, C. C. The CCP4 suite: programs for protein crystallography. _Acta Crystallogr. D_ 50, 760–763 (1994) Article
Google Scholar * Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron-density maps and the location of errors in these
models. _Acta Crystallogr. A_ 47, 110–119 (1991) Article Google Scholar * Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. _Acta Crystallogr. D_ 60, 2126–2132
(2004) Article Google Scholar * Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. _Acta Crystallogr. D_ 54, 905–921
(1998) Article CAS Google Scholar * Painter, J. & Merritt, E. A. TLSMD web server for the generation of multi-group TLS models. _J. Appl. Cryst._ 39, 109–111 (2006) Article CAS
Google Scholar * Howlin, B., Butler, S. A., Moss, D. S., Harris, G. W. & Driessen, H. P. C. TLSANL—TLS parameter-analysis program for segmented anisotropic refinement of macromolecular
structures. _J. Appl. Cryst._ 26, 622–624 (1993) Article Google Scholar * Ehrmann, M. & Beckwith, J. Proper insertion of a complex membrane protein in the absence of its amino-terminal
export signal. _J. Biol. Chem._ 266, 16530–16533 (1991) CAS PubMed Google Scholar * DeLano, W.L. The PyMOL Molecular Graphics System (2002) on the World Wide Web 〈http://www.pymol.org〉 *
Kleywegt, G. J. & Jones, T. A. Detection, delineation, measurement and display of cavities in macromolecular structures. _Acta Crystallogr. D_ 50, 178–185 (1994) Article CAS Google
Scholar Download references ACKNOWLEDGEMENTS We thank the beamline staff at the Advanced Photon Source beamline 23-ID for assistance with data collection, and R. MacKinnon, H. Shuman, D.
Yernool and C. Orelle for discussions. This work was supported by NIH grants (J.C., A.L.D. and F.A.Q.), the Welch Foundation (F.A.Q.), the Pew Scholar Program (J.C.) and a postdoctoral
fellowship from American Heart Association (M.L.O). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biological Sciences,, Michael L. Oldham, Dheeraj Khare & Jue Chen *
Department of Chemistry, Purdue University, West Lafayette, Indiana 47907, USA, Amy L. Davidson * Verna and Marrs McLean Department of Biochemistry and Molecular Biology, Baylor College of
Medicine, Houston, Texas 77030, USA, Florante A. Quiocho Authors * Michael L. Oldham View author publications You can also search for this author inPubMed Google Scholar * Dheeraj Khare View
author publications You can also search for this author inPubMed Google Scholar * Florante A. Quiocho View author publications You can also search for this author inPubMed Google Scholar *
Amy L. Davidson View author publications You can also search for this author inPubMed Google Scholar * Jue Chen View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHOR Correspondence to Jue Chen. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION The file contains Supplementary Figures 1-5 and Legends, Supplementary Tables 1-2
and additional references. (PDF 762 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Oldham, M., Khare, D., Quiocho, F. _et al._ Crystal structure of
a catalytic intermediate of the maltose transporter. _Nature_ 450, 515–521 (2007). https://doi.org/10.1038/nature06264 Download citation * Received: 13 August 2007 * Accepted: 17 September
2007 * Issue Date: 22 November 2007 * DOI: https://doi.org/10.1038/nature06264 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative




