- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Ataxia oculomotor apraxia-1 (AOA1) is a neurological disorder caused by mutations in the gene (_APTX_) encoding aprataxin1,2. Aprataxin is a member of the histidine triad (HIT)
family of nucleotide hydrolases and transferases3, and inactivating mutations are largely confined to this HIT domain. Aprataxin associates with the DNA repair proteins XRCC1 and XRCC4,
which are partners of DNA ligase III and ligase IV, respectively4,5,6,7, suggestive of a role in DNA repair. Consistent with this, _APTX_-defective cell lines are sensitive to agents that
cause single-strand breaks and exhibit an increased incidence of induced chromosomal aberrations4,5,8. It is not, however, known whether aprataxin has a direct or indirect role in DNA
repair, or what the physiological substrate of aprataxin might be. Here we show, using purified aprataxin protein and extracts derived from either _APTX_-defective chicken DT40 cells or
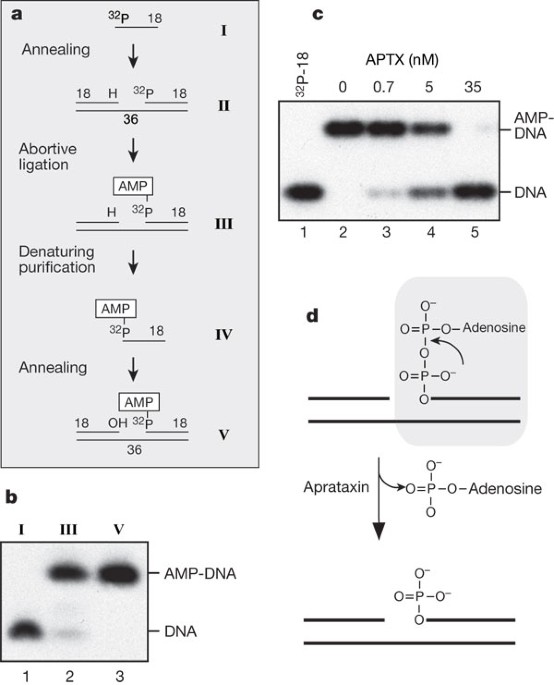
_Aptx_-/- mouse primary neural cells, that aprataxin resolves abortive DNA ligation intermediates. Specifically, aprataxin catalyses the nucleophilic release of adenylate groups covalently
linked to 5′-phosphate termini at single-strand nicks and gaps, resulting in the production of 5′-phosphate termini that can be efficiently rejoined. These data indicate that neurological
disorders associated with _APTX_ mutations may be caused by the gradual accumulation of unrepaired DNA strand breaks resulting from abortive DNA ligation events. Access through your
institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print
issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS PATHOGENIC _ARH3_ MUTATIONS RESULT IN ADP-RIBOSE CHROMATIN SCARS DURING DNA STRAND BREAK REPAIR Article Open access 07 July 2020 XRCC1 PROTECTS TRANSCRIPTION FROM
TOXIC PARP1 ACTIVITY DURING DNA BASE EXCISION REPAIR Article Open access 22 November 2021 NEURONAL ENHANCERS ARE HOTSPOTS FOR DNA SINGLE-STRAND BREAK REPAIR Article 25 March 2021 REFERENCES
* Date, H. et al. Early-onset ataxia with ocular motor apraxia and hypoalbuminemia is caused by mutations in a new HIT superfamily gene. _Nature Genet._ 29, 184–188 (2001) Article CAS
Google Scholar * Moreira, M. C. et al. The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. _Nature Genet._ 29, 189–193 (2001) Article CAS Google
Scholar * Brenner, C. Hint, Fhit, and GalT: Function, structure, evolution and mechanism of three branches of the histidine triad superfamily of nucleotide hydrolases and transferases.
_Biochemistry_ 41, 9003–9014 (2002) Article CAS Google Scholar * Clements, P. M. et al. The ataxia-oculomotor apraxia 1 gene product has a role distinct from ATM and interacts with the
DNA strand break repair proteins XRCC1 and XRCC4. _DNA Repair (Amst.)_ 3, 1493–1502 (2004) Article CAS Google Scholar * Gueven, N. et al. Aprataxin, a novel protein that protects against
genotoxic stress. _Hum. Mol. Genet._ 13, 1081–1093 (2004) Article CAS Google Scholar * Sano, Y. et al. Aprataxin, the causative protein for EAOH is a nuclear protein with a potential role
as a DNA repair protein. _Ann. Neurol._ 55, 241–249 (2004) Article CAS Google Scholar * Luo, H. et al. A new XRCC1-containing complex and its role in cellular survival of methyl
methanesulfonate treatment. _Mol. Cell. Biol._ 24, 8356–8365 (2004) Article CAS Google Scholar * Mosesso, P. et al. The novel human gene aprataxin is directly involved in DNA
single-strand break repair. _Cell. Mol. Life Sci._ 62, 485–491 (2005) Article CAS Google Scholar * Tomkinson, A. E., Vijayakumar, S., Pascal, J. M. & Ellenberger, T. DNA ligases:
structure, reaction mechanism and function. _Chem. Rev._ 106, 687–699 (2006) Article CAS Google Scholar * Kijas, A. W., Harris, J. L., Harris, J. M. & Lavin, M. F. Aprataxin forms a
discrete branch in the HIT (histidine triad) superfamily of proteins with both DNA/RNA binding and nucleotide hydrolase activities. _J. Biol. Chem._ 281, 13939–13948 (2006) Article CAS
Google Scholar * Bieganowski, P. et al. Adenosine monophosphoramidase activity of Hint and Hnt1 supports function of Kin28, Ccl1, and Tfb3. _J. Biol. Chem._ 277, 10852–10860 (2002) Article
CAS Google Scholar * Brenner, C. et al. Crystal structures of Hint demonstrate that histidine triad proteins are GalT-related nucleotide-binding proteins. _Nature Struct. Biol._ 4,
231–238 (1997) Article CAS Google Scholar * Krakowiak, A. et al. Biochemical, crystallographic, and mutagenic characterization of Hint, the AMP-lysine hydrolase, with novel substrates and
inhibitors. _J. Biol. Chem._ 279, 18711–18716 (2004) Article CAS Google Scholar * Barnes, L. D. et al. Fhit, a putative tumor suppressor in humans, is a dinucleoside
5′,5‴-P1,P3-triphosphate hydrolase. _Biochemistry_ 35, 11529–11535 (1996) Article CAS Google Scholar * Lima, C. D., Klein, M. G. & Hendrickson, W. A. Structure-based analysis of
catalysis and substrate definition in the HIT protein family. _Science_ 278, 286–290 (1997) Article CAS Google Scholar * Draganescu, A., Hodawadekar, S. C., Gee, K. R. & Brenner, C.
Fhit-nucleotide specificity probed with novel fluorescent and fluorogenic substrates. _J. Biol. Chem._ 275, 4555–4560 (2000) Article CAS Google Scholar * Ghaemmaghami, S. et al. Global
analysis of protein expression in yeast. _Nature_ 425, 737–741 (2003) Article ADS CAS Google Scholar * Plateau, P., Fromant, M., Schmitter, J. M., Buhler, J. M. & Blanquet, S.
Isolation, characterization, and inactivation of the Apa1 gene encoding yeast diadenosine 5′,5‴-P1,P4-tetraphosphate phosphorylase. _J. Bacteriol._ 171, 6437–6445 (1989) Article CAS Google
Scholar * Henle, E. S. & Linn, S. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. _J. Biol. Chem._ 272, 19095–19098 (1997) Article CAS Google Scholar *
Pogozelski, W. K. & Tullius, T. D. Oxidative strand scission of nucleic acids: routes initiated by hydrogen abstraction from the sugar moiety. _Chem. Rev._ 98, 1089–1108 (1998) Article
CAS Google Scholar * Ward, J. F. in _DNA Damage and Repair_ VOL. 2 (eds Nicoloff, J. A. & Hoekstra, M. F.) 65–85 (Humana, Totowa, New Jersey, 1998) Google Scholar * Loizou, J. I. et
al. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. _Cell_ 117, 17–28 (2004) Article CAS Google Scholar * Whitehouse, C. J. et al. XRCC1 stimulates
human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. _Cell_ 104, 107–117 (2001) Article CAS Google Scholar * Caldecott, K. W. DNA
single-strand break repair and spinocerebellar ataxia. _Cell_ 112, 7–10 (2003) Article CAS Google Scholar * Seidle, H. F., Bieganowski, P. & Brenner, C. Disease-associated mutations
inactivate AMP-lysine hydrolase activity of Aprataxin. _J. Biol. Chem._ 280, 20927–20931 (2005) Article CAS Google Scholar * Barnes, D. E. DNA damage: Air-breaks? _Curr. Biol._ 12,
R262–R264 (2002) Article CAS Google Scholar * Barnes, D. E., Stamp, G., Rosewell, I., Denzel, A. & Lindahl, T. Targeted disruption of the gene encoding DNA ligase IV leads to
lethality in embryonic mice. _Curr. Biol._ 8, 1395–1398 (1998) Article CAS Google Scholar * Gao, Y. et al. A critical role for DNA end-joining proteins in both lymphogenesis and
neurogenesis. _Cell_ 95, 891–902 (1998) Article CAS Google Scholar * Abner, C. W. & McKinnon, P. J. The DNA double-strand break response in the nervous system. _DNA Repair (Amst.)_ 3,
1141–1147 (2004) Article CAS Google Scholar * El Khamisy, S. F. et al. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. _Nature_ 434, 108–113
(2005) Article ADS CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank D. Barnes and T. Lindahl for discussions and the gift of DNA ligase III–XRCC1 complex, and A. Ciccia
for advice and comments. We thank S. Rulten for the gift of recombinant His-tagged aprataxin, and M. Taylor for provision of AOA1 lymphoblastoid cells. This work was supported by Cancer
Research UK (S.C.W.), the EU DNA Repair Consortium (S.C.W.), the Medical Research Council (K.W.C.) and the NIH (P.J.M.). I.A. is supported by a fellowship from the European Molecular Biology
Organisation. Author Contributions I.A. made the initial discovery of DNA–adenylate activity, and I.A. and U.R. were responsible for experimental design and the generation of most of the
experimental data. S.F.E.-K., S.K. and P.M.C. were responsible for the development of vertebrate models and performed some of the experiments. P.J.M. and K.W.C. provided critical resources
and expertise, and contributed to writing the manuscript. S.C.W. was the project leader and produced the final version of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Cancer
Research UK, London Research Institute, Clare Hall Laboratories, South Mimms, Herts, EN6 3LD, UK Ivan Ahel, Ulrich Rass & Stephen C. West * Genome Damage and Stability Centre,
University of Sussex, BN1 9RQ, Falmer, Brighton, UK Sherif F. El-Khamisy, Paula M. Clements & Keith W. Caldecott * Biochemistry Department, Faculty of Pharmacy, Ain Shams University, PO
Box 11566, Cairo, Egypt Sherif F. El-Khamisy * Department of Genetics and Tumor Cell Biology, St. Jude Children's Research Hospital, 332N Lauderdale, Tennessee, 38105, Memphis, USA
Sachin Katyal & Peter J. McKinnon Authors * Ivan Ahel View author publications You can also search for this author inPubMed Google Scholar * Ulrich Rass View author publications You can
also search for this author inPubMed Google Scholar * Sherif F. El-Khamisy View author publications You can also search for this author inPubMed Google Scholar * Sachin Katyal View author
publications You can also search for this author inPubMed Google Scholar * Paula M. Clements View author publications You can also search for this author inPubMed Google Scholar * Peter J.
McKinnon View author publications You can also search for this author inPubMed Google Scholar * Keith W. Caldecott View author publications You can also search for this author inPubMed
Google Scholar * Stephen C. West View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Stephen C. West. ETHICS
DECLARATIONS COMPETING INTERESTS Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY NOTES This file contains Supplementary Methods and Supplementary Figure Legends. (DOC 74 kb) SUPPLEMENTARY FIGURE 1 Reaction mechanism of DNA ligases. (PDF 114 kb)
SUPPLEMENTARY FIGURE 2 Purification of recombinant human Aprataxin. (PDF 257 kb) SUPPLEMENTARY FIGURE 3 Direct measurement of AMP release from the DNA-adenylate intermediate. (PDF 209 kb)
SUPPLEMENTARY FIGURE 4 Analysis of Aprataxin on the DNA ligase-adenylate complex. (PDF 133 kb) SUPPLEMENTARY FIGURE 5 Phylogenetic tree showing that Aprataxin (APTX) forms a distinct branch
of the HIT superfamily. (PDF 111 kb) SUPPLEMENTARY FIGURE 6 Extracts from Aptx-disrupted DT40 cells exhibit reduced ligation activity with the DNA adenylate substrate. (PDF 355 kb)
SUPPLEMENTARY FIGURE 7 Generation and characterization of Aptx-/- mice. (PDF 210 kb) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ahel, I., Rass, U.,
El-Khamisy, S. _et al._ The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. _Nature_ 443, 713–716 (2006). https://doi.org/10.1038/nature05164
Download citation * Received: 07 June 2006 * Accepted: 17 August 2006 * Published: 10 September 2006 * Issue Date: 12 October 2006 * DOI: https://doi.org/10.1038/nature05164 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative