- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
The BDNF gene Val66Met single-nucleotide polymorphism is carried by ~0.55% of Sub-Saharan Africans, 19.9% of Europeans and 43.6% of Asians, but may be carried by up to 72% of some Sub-Asian
population groups.1 The switch from a guanine to adenine nucleotide at position 196 within the pro-region of the BDNF gene causes a Valine (Val) to Methionine (Met) amino-acid residue
substitution at codon 66 (Val66Met), resulting in diminished activity-dependent secretion of BDNF at the synapse.2 The BDNF gene Val66Met polymorphism has been implicated as a modifier of
hippocampal function and is a putative locus of risk for anxiety and affective disorders such as post-traumatic stress disorder (PTSD) and major depression.3 However, the literature
suggesting that the loss of function 66Met variant is risk conferring for these disorders is inconsistent; and in some cases is even contradictory by suggesting that the wild-type 66Val
allele provides risk as well.3 Likewise, a growing number of human studies have also failed to replicate the hippocampal deficits associated with the 66Met allele as reported by early
studies, whereas meta-analyses have suggested that effect sizes of the Val66Met polymorphism on both cognition4 and hippocampal structure5 may only be small – if they exist at all. Many
explanations for these discrepant results have been suggested, such as a lack of power, regression towards the mean or biased sampling.3 Another account is that discrepant data may be the
result of failing to control for complex gene–environment interactions, which may determine or unmask BDNF phenotypes. We hypothesized that stress, specifically the action of glucocorticoid
stress hormones acting on the glucocorticoid receptor (GR), may be one such factor that interacts with the BDNF Val66Met polymorphism to determine hippocampus-dependent behavior. To test
this hypothesis, we modeled the long-term effects of chronic stress exposure in a novel mouse model that has been genetically modified to express a humanized BDNF (hBDNF) coding transcript
via endogenous mouse promoters that has yet to be behaviorally phenotyped. Specifically, this mouse model has the Val66Met polymorphism knocked-in including an extended sequence of 11
nucleotides across a 274-bp region that humanizes the coding exon of the rodent _Bdnf_ gene.6 To model stress, we used a chronic corticosterone (CORT) exposure paradigm to specifically
induce GR signaling without the confounding effects of other physiological components of the stress response axis. Exposure to CORT was time locked to a developmental period coinciding with
late adolescence (weeks 6–8), with the behavioral phenotyping of hBDNFVal66Met mice occurring in adulthood (weeks 11–12) so to probe the long-term behavioral adaptation to mid-developmental
GR recruitment.7 Tests of hippocampal function were the primary measures of the current study given that this is the primary phenotype of the Val66Met polymorphism and is a key component of
the pathophysiology of both anxiety and affective disorders. Further details of our experimental design, the genetic construct of our hBDNFVal66Met mouse model and methodology can be found
in the Supplementary Material. To assess emotionally salient behavior, fear conditioning was used as a non-spatial, amygdala- and hippocampus-gated, memory paradigm. On day one, mice were
conditioned using three tone-shock pairings, before being returned to their conditioning context 24 h later and to a novel context 48 h later to assess contextual and tone fear memory,
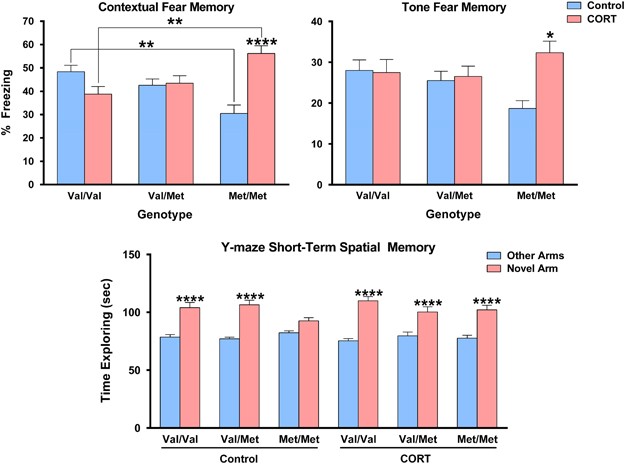
respectively. A significant genotype × treatment interaction was observed for hippocampus-dependent contextual fear memory when analyzing males (_F_(2,76)=7.0, _P_=0.0016) and females
(_F_(2,79)=14.14, _P_<0.0001) separately as well as when combined (_F_(2,161)=16.19, _P_<0.0001). As this interaction occurred independent of sex, the combined dataset was used for
between-group comparisons so to increase power of detecting diminutive effect sizes as predicted by meta-analyses. _Post hoc_ analyses of this dataset revealed that hBDNFMet/Met mice had
significantly worse contextual fear memory relative to wild-type hBDNFVal/Val mice at baseline (_P_<0.01), however following the chronic CORT treatment this pattern was reversed with
hBDNFMet/Met mice having significantly better contextual fear memory than mice carrying the hBDNFVal/Val genotype (_P_<0.01). _Post hoc_ comparisons also revealed that the CORT-treated
hBDNFMet/Met mice had significantly improved contextual fear memory than hBDNFMet/Met mice allocated to vehicle treatment (_P_<0.0001). For tone-elicited fear memory, none of the main
effects reached significance, however, a significant genotype × treatment interaction, following the same direction as that reported for contextual fear memory, was also observed amongst the
sex-collapsed data set (_F_(2, 161)=4.779, _P_=0.0096). The only comparison to reach significance was the enhanced tone-elicited fear memory of CORT-treated hBDNFMet/Met mice relative to
hBDNFMet/Met controls (_P_<0.05). These results highlight that the chronic activation of glucocorticoid receptors during late adolescence potentiates the fear circuitry in adulthood
according to hBDNFVal66Met genotype (see Figure 1). We next examined short-term spatial memory using the Y-maze as an alternative test of hippocampus-dependent memory function that is
independent of the fear circuitry. Briefly, mice were allowed to explore two open arms of a three-arm maze for 10 min. One hour later, mice were returned to the maze but allowed to explore
all three arms, with intact spatial memory being quantified by exploration time of the previously blocked ‘novel’ arm. There was no significant main effect or interaction comprising sex on
time spent in the novel or other arms, so males and females were once more analyzed together to increase power. A significant interaction between the factors of ‘Group’ and ‘Arm’
(_F_(2,160)=5.2, _P_=0.0065) was detected. Within-group analysis revealed that hBDNFVal/Val (_P_<0.0001) and hBDNFVal/Met (_P_<0.0001) mice showed a highly significant preference for
exploring the novel arm, relative to the other arms, indicating intact short-term spatial memory performance. On the other hand, hBDNFMet/Met mice showed a lack of preference for exploring
the novel arm relative to the other arms, suggesting that the short-term spatial memory of these mice is subtly disrupted at baseline. Although, the chronic CORT treatment had no effect on
Y-maze performance of hBDNFVal/Val and hBDNFVal/Met mice, the disrupted short-term spatial memory of hBDNFMet/Met mice was rescued by CORT treatment (_P_<0.0001) to levels consistent with
hBDNFVal/Val controls. Further experimentation determined that this effect was not the result of altered anxiety-related exploratory drive (see Supplementary Material). This subtle Y-Maze
result confirms the specificity of late adolescent glucocorticoid signaling as a long-term modifier of hippocampus-dependent behavior in hBDNFVal66Met mice. The implications for the novel
data reported here is that it is the first to provide experimental evidence that a history of glucocorticoid signaling during adolescence, a bottom-up model of chronic stress, determines
adult hippocampus-dependent memory function according to BDNF Val66Met genotype. Outside of already identified sampling factors (e.g., underpowered clinical studies),3 these results suggest
that discrepant clinical data on the topic of hippocampus-dependent memory function may be explained by a failure to stratify samples for stressful life events, in that the 66Met allele may
be associated with poor memory function at baseline but may recover to the levels similar to, if not better than, controls following a history of stress. In particular, it appears although
this effect occurs via the innate susceptibility of Met/Met homozygotes to CORT due to an increase in the expression of glucocorticoid receptors in the dorsal hippocampus during adolescence
(see Supplementary Figure 2), which has long-lasting effects on hippocampus-dependent behavior into adulthood. The data reported here could be interpreted as a protective mechanism of stress
in 66Met carriers; whereby, in the absence of glucocorticoid exposure during late adolescence the brain may be more vulnerable to stress in adulthood. However, the clinical literature would
suggest that the data reported here is more likely to represent an ‘undesirable gain of function’ than a ‘positive adaptation’. Specifically, risk of depression has been selectively linked
to the 66Met allele in females with a history of childhood stress,8 while a history of adverse events interacts with 66Met genotype to increase rumination.9 In non-humanized 66Met mice
exposed to acute stress, there is also an increase in depression-related behavioral markers such as learned helplessness on the forced-swim test.10 Ultimately, the enhanced memory of fear –
while possibly related to depressive disorders – is likely to hold more relevance to anxiety disorders such as PTSD where stress is a requisite factor in the pathogenesis of the disorder.
Although the Val66Met polymorphism has been only briefly investigated in PTSD patients, there is evidence that the 66Met allele is carried with a two- to threefold higher frequency in
‘probable’ PTSD probands11 and confers resistance to exposure therapy.12 Further, the 66Met allele has been associated with the persistence of fear in an extinction-learning paradigm in both
man and mouse.13 Adding to this literature, our data suggest that there is a long-term effect of glucocorticoids in 66Met carriers that potentiates the fear circuitry into adulthood, which
may increase susceptibility to trauma, events with negative emotional valence and related psychopathology. Although clinical studies are required to confirm that the phenotype described here
replicates in humans, the current data provides the first evidence for a long-term glucocorticoid-mediated ‘switch’ of hippocampus-dependent behavior in the hBDNFVal66Met mouse, as well as
a theoretical framework from which to resolve a putative role of BDNF in anxiety and affective disorders. REFERENCES * Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner S _et
al_. _Mol Psychiatry_ 2009; 15: 810–815. Article Google Scholar * Chen Z-Y, Jing D, Bath KG, Ieraci A, Khan T, Siao C-J _et al_. _Science_ 2006; 314: 140–143. Article CAS Google Scholar
* Notaras M, Hill R, van den Buuse M _Mol Psychiatry_ 2015; 20: 916–930. Article CAS Google Scholar * Mandelman SD, Grigorenko EL . _Genes Brain Behav_ 2012; 11: 127–136. Article CAS
Google Scholar * Molendijk ML, Bus BA, Spinhoven P, Kaimatzoglou A, Voshaar RCO, Penninx BW _et al_. _Am J Med Genet B Neuropsychiatr Genet_ 2012; 159: 731–740. Article CAS Google Scholar
* Cao L, Dhilla A, Mukai J, Blazeski R, Lodovichi C, Mason C _et al_. _Curr Biol_ 2007; 17: 911–921. Article CAS Google Scholar * Klug M, Hill R, Choy K, Kyrios M, Hannan A, van den
Buuse M . _Neurobiol Dis_ 2012; 46: 722–731. Article CAS Google Scholar * Lavebratt C, Åberg E, Sjöholm LK, Forsell Y . _J Affect Disord_ 2010; 125: 249–255. Article CAS Google Scholar
* Clasen PC, Wells TT, Knopik VS, McGeary JE, Beevers CG . _Genes Brain Behav_ 2011; 10: 740–746. Article CAS Google Scholar * Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY . _J
Neurosci_ 2012; 32: 4092–4101. Article CAS Google Scholar * Zhang L, Benedek D, Fullerton C, Forsten R, Naifeh J, Li X _et al_. _Mol Psychiatry_ 2013; 19: 8–10. Article Google Scholar *
Felmingham KL, Dobson-Stone C, Schofield PR, Quirk GJ, Bryant RA . _Biol Psychiatry_ 2013; 73: 1059–1063. Article CAS Google Scholar * Soliman F, Glatt CE, Bath KG, Levita L, Jones RM,
Pattwell SS _et al_. _Science_ 2010; 327: 863–866. Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Behavioural Neuroscience Laboratory, Florey
Institute of Neuroscience and Mental Health, Melbourne, VIC, Australia M Notaras & M van den Buuse * Psychoneuroendocrinology Laboratory, Florey Institute of Neuroscience and Mental
Health, Melbourne, VIC, Australia M Notaras & R Hill * Departments of Biophysics and Neuroscience, Columbia University, New York, NY, USA J A Gogos * School of Psychology and Public
Health, La Trobe University, Melbourne, VIC, Australia M van den Buuse Authors * M Notaras View author publications You can also search for this author inPubMed Google Scholar * R Hill View
author publications You can also search for this author inPubMed Google Scholar * J A Gogos View author publications You can also search for this author inPubMed Google Scholar * M van den
Buuse View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to M van den Buuse. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on the Molecular Psychiatry website SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION (DOC 5073 KB) SUPPLEMENTARY FIGURE 1 (JPG 94 KB) SUPPLEMENTARY FIGURE 2 (JPG 162 KB) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 RIGHTS AND PERMISSIONS This work is licensed
under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to
reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Notaras, M.,
Hill, R., Gogos, J. _et al._ BDNF Val66Met genotype determines hippocampus-dependent behavior via sensitivity to glucocorticoid signaling. _Mol Psychiatry_ 21, 730–732 (2016).
https://doi.org/10.1038/mp.2015.152 Download citation * Published: 06 October 2015 * Issue Date: June 2016 * DOI: https://doi.org/10.1038/mp.2015.152 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative