- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Thymic stromal lymphopoietin (TSLP) has multifaceted immunological functions ranging from maintenance of tolerance to induction of disease. Two human transcript variants of TSLP are
described: a long form (variant 1; lfTSLP) consisting of four exons and an alternative, short form (variant 2; sfTSLP) that lacks two exons compared with variant 1. SfTSLP has not been
described at the protein level or functionally studied. Here, we demonstrate that the human sfTSLP is the predominant form of TSLP, constitutively expressed at the mRNA and protein level in
keratinocytes of oral mucosa and skin and in salivary glands, is released in saliva, and is not regulated in the same manner as the long form. Compared with lfTSLP, sfTSLP exhibits a
markedly stronger antibacterial activity. Synthetic sfTSLP did not activate signal transducer and activator of transcription 5 (STAT5) signaling in CD1c+ dendritic cells nor interfered with
STAT5 activation by lfTSLP. SfTSLP may, therefore, act as an antimicrobial peptide in the oral cavity and on the skin to create a defense barrier that aids in the control of both commensal
and pathogenic microbes. The results show that the two translational products of the _TSLP_ gene have a different expression and different biological properties, and emphasize the importance
of analyzing the two TSLP isoforms separately. SIMILAR CONTENT BEING VIEWED BY OTHERS ALTERNATIVE SPLICING OF MR1 REGULATES ANTIGEN PRESENTATION TO MAIT CELLS Article Open access 22
September 2020 TRANSCRIPTOMIC DIVERSITY IN HUMAN MEDULLARY THYMIC EPITHELIAL CELLS Article Open access 02 August 2022 K2P18.1 TRANSLATES T CELL RECEPTOR SIGNALS INTO THYMIC REGULATORY T CELL
DEVELOPMENT Article Open access 26 October 2021 INTRODUCTION Thymic stromal lymphopoietin (TSLP) is a cytokine first identified in a mouse model as a B-cell growth factor produced by a
thymic stromal cell line.1 TSLP is produced by multiple cell types including epithelial and dendritic cells (DCs).2, 3 Diverse immune cells can express the TSLP receptor and respond to TSLP,
including mast cells, DCs, and T cells.2, 4, 5 TSLP may have dual immunoregulatory roles. On the one hand, it has been implicated in the pathogenesis of several disorders, including
allergic and asthmatic diseases, autoimmunity, and cancer.6 On the other hand, TSLP has been associated with protective and tolerogenic functions. For example, human DCs conditioned with
TSLP have been shown to augment intestinal epithelial cell-mediated IgA2 class switching through the induction of a proliferation-induced ligand, indicating a protective role of TSLP in the
intestine.7 Further, _in vitro_ studies have suggested a role for TSLP in the generation of tolerogenic DCs that induce the differentiation of regulatory T cells both in the intestine and in
the thymus.8, 9, 10 Finally, TSLP has been shown to exert antimicrobial activity, primarily mediated by the C-terminal region of the protein.11 Two transcript variants of human TSLP are
described: a long form of TSLP (lfTSLP, variant 1) and an alternative, short form (sfTSLP, variant 2) consisting of four and two exons, respectively. LfTSLP has a sequence of 159 amino acids
(aa) corresponding to a calculated molecular weight (MW) of 18.1 kDa, but the N-terminal amino acids 1–28 form a signal peptide, leaving a chain with a calculated MW of 15 kDa, which
corresponds to the MW of commercially available recombinant lfTSLP produced in prokaryotic cells. SfTSLP uses a downstream in-frame start codon compared with variant 1. The encoded variant 2
is shorter at the N terminus and fully overlaps the amino-acid sequence of variant 1 in the C-terminal region (National Center for Biotechnology Information, National Library of Medicine,
Bethesda, MD). The sequence of sfTSLP shows two potential starting methionines that can give rise to either a 63 or a 60 aa peptide (G3XAM8 and Q96AU7, respectively; http://www.uniprot.org),
with calculated MW of 7.4 and 7.1 kDa, respectively. The N-terminal sequence of sfTSLP contains a potential N-terminal 20 aa signal peptide (PSORT; http://psort.hgc.jp), and if this peptide
is left out, the calculated size of the remaining sfTSLP peptide lies at 5.2 kDa. With a few exceptions,12 most studies do not distinguish between these TSLP variants, neither at the mRNA
nor at the protein level, and most authors collectively designate these variants as “TSLP.” Only the lfTSLP variant has previously been described at the protein level, and thus functional
studies of TSLP have been restricted to the investigation of the effects of the full-length TSLP protein. In this study, we wanted to assess whether sfTSLP is translated, map the expression
at the mRNA and protein level _in situ_, examine the regulation, and test the potential for antimicrobial activity. Focusing on barrier surfaces including the oral mucosa and the skin, we
found that sfTSLP is the predominant form of TSLP expressed in keratinocytes at steady state, both at the mRNA and protein levels. SfTSLP was constitutively expressed and only slightly
upregulated in response to inflammatory factors, in marked contrast to lfTSLP. SfTSLP showed antimicrobial activity and the effect was markedly stronger than that of lfTSLP. SfTSLP was
expressed in salivary glands and detected in saliva, whereas lfTSLP was not. Therefore, a function of sfTSLP may be to act as an antimicrobial peptide (AMP) at barrier surfaces. RESULTS
TRANSCRIPTION AND TRANSLATION OF SFTSLP To investigate the mRNA expression of the two variants of human TSLP separately, we used variant-specific primers recognizing sfTSLP and lfTSLP,
respectively. Lysates were made from whole oral mucosal biopsies and cultures of primary oral keratinocytes, and mRNA expression was determined by real-time polymerase chain reaction. We
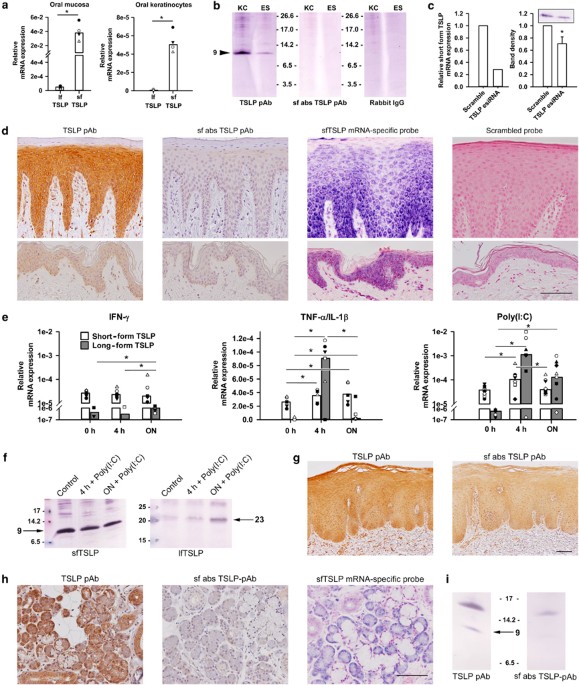
found that sfTSLP mRNA was the predominant form of TSLP expressed in oral mucosa and primary oral keratinocytes (Figure 1a). The same pattern was seen in the skin (D Khnykin _et al._,
unpublished data). The finding of constitutive sfTSLP mRNA expression prompted us to investigate whether sfTSLP was also expressed at the protein level because tissue expression of sfTSLP
protein has not previously been reported. Antibodies prepared with recombinant full-length TSLP as an immunogen recognize both isoforms (Supplementary Figure S1 online, left) because the
sfTSLP protein sequence completely overlaps the lfTSLP sequence in the C-terminal region. Hence, production of sfTSLP-specific antibodies is not possible and we therefore chose an indirect
approach: sfTSLP reactivity was removed from polyclonal anti-full-length TSLP antibody, by incubating the antibody with sfTSLP coupled to CNBr-activated Sepharose 4B beads. Thus, an
sfTSLP-absorbed product that only reacted with lfTSLP was obtained, which allowed us to compare the total content of TSLP protein (long and short combined) with lfTSLP-specific activity
(Supplementary Figure S1, right). After sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blotting of lysates of oral mucosal epithelial sheets and primary oral
epithelial cells, immunoreactivity was detected at 9 kDa with the unabsorbed antibody preparation (Figure 1b, left), which were not visible after incubation with the sfTSLP-absorbed antibody
preparation (Figure 1b, middle). This indicated the presence of sfTSLP in the samples. In accordance with the mRNA results, no lfTSLP protein was detected in either primary oral
keratinocytes or oral mucosal epithelial sheets because no band was seen at the expected position of about 23 kDa13 (compare with Figure 1f, right). For additional validation of the
specificity of the 9 kDa band, cultured oral keratinocytes were transfected with endoribonuclease-prepared siRNA (esiRNA) targeting full-length TSLP mRNA, to reduce the level of sfTSLP mRNA
(Figure 1c, left). Lysates from the transfected cells showed a significantly weakened 9 kDa peptide band as compared with that obtained with scrambled controls (Figure 1c, right), confirming
that this band corresponds to sfTSLP. The band detected at 9 kDa diverges from the possible calculated sizes of 7.4 and 7.1 kDa, or 5.2 kDa without potential leader sequence, of the
amino-acid sequence of sfTSLP. This shift is probably due to posttranslational modifications (PTMs) of sfTSLP. Having demonstrated the protein expression of sfTSLP in western blots, we next
carried out TSLP isoform-specific staining by immunohistochemistry. By comparing stainings of sections of oral mucosa and skin with the sfTSLP-absorbed antibody preparation and the
unabsorbed anti-TSLP antibody, we found that sfTSLP protein was expressed in all epithelial cell layers of clinically healthy oral epithelium and skin epidermis, while no lfTSLP was seen
(Figure 1d). SfTSLP mRNA expression was detected at the same sites with _in situ_ hybridization (Figure 1d). We also investigated isoform-specific expression of TSLP in oral mucosal lesions
induced by the use of the smokeless tobacco “snus.” In these lesions, lfTSLP peptide was found to be upregulated in the epithelial cells (Figure 1g). This constituted a positive staining
control for the present immunochemical staining method for lfTSLP protein. To investigate how the two TSLP variants are regulated under inflammatory conditions, cultured oral keratinocytes
were stimulated with proinflammatory factors, including interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α)/interleukin-1β (IL-1β) and polyinosinic-polycytidylic acid (poly(I:C)). In such
cells, lfTSLP mRNA was upregulated after 4 h exposure to TNF-α/IL-1β and poly(I:C), and after 24 h exposure to all stimuli (Figure 1e). Poly(I:C) and TNF-α/IL-1β stimulation also upregulated
sfTSLP mRNA after 4 h (_P_<0.05), but when compared with lfTSLP, the changes were modest. At the protein level, lfTSLP was upregulated after 24 h in response to poly(I:C), whereas
upregulation of sfTSLP protein was not detected (Figure 1f). SFTSLP EXERTS POTENT ANTIMICROBIAL ACTIVITY AND DOES NOT INDUCE STAT5 SIGNALING Full-length recombinant TSLP displays
antimicrobial activity against bacteria and yeasts.11 We therefore examined whether the antimicrobial activity was retained in the sfTSLP peptide and compared it with the activity of lfTSLP,
using a panel consisting of diverse bacterial and fungal species. SfTSLP (60 or 63 aa) or lfTSLP were added to microbial suspensions for 2 h and then plated and incubated overnight.
Colonies were counted the next day and colony-forming units per ml were determined. The results showed that sfTSLP exerted potent antimicrobial activity against all the tested species
(Figure 2a). Addition of polyclonal anti-TSLP antibody to sfTSLP (ab47943; Abcam), before it was incubated with _Streptococcus mitis_, reduced the antimicrobial activity by about half,
showing that the reduction in colony-forming units per ml was specifically due to the action of sfTSLP (data not shown). Dose–response curves using _S. mitis_ showed that the effect of
sfTSLP was stronger than that obtained by the well-characterized AMP LL-37 (Figure 2b). LfTSLP induces STAT5 signaling in DCs.6 Neither 60- nor 63 aa sfTSLP induced this activation in
poly(I:C)-stimulated CD1c+ DCs, nor interfered with STAT5 activation by lfTSLP (Figure 2c). SFTSLP IS PRODUCED IN SALIVARY GLANDS AND IS RELEASED IN SALIVA Having demonstrated an
antimicrobial role of sfTSLP, we investigated the expression of the peptide in salivary glands and whether it can be detected in saliva. There are three major pairs of salivary glands (the
parotid, submandibular, and sublingual glands), in addition to the minor salivary glands scattered in the submucosa throughout the oral and oropharyngeal cavities. By comparing
immunochemical stainings carried out with the unabsorbed anti-TSLP antibody with the sfTSLP-absorbed antibody preparations, we found that sfTSLP was expressed in all four gland types,
whereas lfTSLP was not detected (Figure 1h and Supplementary Figure S2). In the submandibular gland, in which the acini are both mucous and serous, sfTSLP was expressed in both acini and
ducts (including intercalated, striated, and collecting ducts) (Figure 1h). Production of sfTSLP mRNA was detected by _in situ_ hybridization with an sfTSLP-specific probe (Figure 1h and
Supplementary Figure S2) and supported the observation of sfTSLP protein expression. In the ducts and serous and mucous acini of all glands, both sfTSLP protein and mRNA were detected in
varying degrees (Supplementary Figure S2). We finally investigated the presence of sfTSLP in salivary secretions. Whole, unstimulated saliva was taken from healthy volunteers and subjected
to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blotting, and a band was detected at 9 kDa (Figure 1i) with the unabsorbed anti-TSLP antibody, which was not visible
with the sfTSLP-absorbed antibody, indicating the presence of sfTSLP in saliva. DISCUSSION The epithelial barrier is the first line of defense against microbial invaders and foreign
antigens. TSLP has been shown to be produced by epithelial cells and to promote immune responses that can be protective or detrimental to the host.2, 14 Most studies have, however, not taken
into account that there are two variants of TSLP, but one investigation of a panel of human tissues showed that sfTSLP mRNA was constitutively found at many body sites, whereas lfTSLP mRNA
was not seen.12 The present study shows that sfTSLP mRNA is the predominant form of TSLP expressed in oral mucosa and primary oral keratinocytes, which is in parallel with previous
observations at other body sites.12 Expression of sfTSLP peptide was detected by western blotting of lysates from oral mucosal epithelial sheets and primary oral epithelial cells as a band
at 9 kDa, of which the identity was confirmed by esiRNA knockdown in oral keratinocyte cultures. When the entire gene sequence coding for sfTSLP is translated into amino-acid sequence, the
possible calculated sizes of sfTSLP are 7.4 or 7.1 kDa, or 5.2 kDa when a potential signal peptide is left out of the equation. No specific bands were detected at any of those positions. The
shift to 9 kDa is likely to be due to PTMs of sfTSLP, possibly glycosylation, because the sfTSLP amino-acid sequence contains a potential glycosylation site15 and PTM, probably
glycosylation, is seen for lfTSLP, which has a calculated molecular size of 15 kDa but appears at 23 kDa in western blotting.13 _In situ_ examination by immunohistochemistry and _in situ_
hybridization concurred with the quantitative real-time PCR and western blotting results: constitutive expression of sfTSLP was seen, while no lfTSLP was detected. As TSLP expression has
been shown to be increased in airway smooth muscle cells following exposure to cigarette smoke extract,16 we investigated the isoform-specific expression of TSLP in oral mucosal lesions
induced by the use of another toxic tobacco derivative that is applied to the oral mucosa by users, the smokeless tobacco “snus.” LfTSLP peptide was found to be upregulated in the epithelial
cells of these biopsies. This thus accorded with the observations in cigarette smoke extract-exposed airway tissue16 and in addition constituted a positive staining control for our
immunochemical staining method for lfTSLP protein. Our findings, showing that the sfTSLP transcript is expressed at the protein level and is constitutive in certain types of cells, may
necessitate revisiting of parts of the literature on TSLP. Most of the available antibody preparations against TSLP have unspecified variant specificity and may recognize both isoforms, and
as long as it is unknown which isoform is detected, the interpretation of results remains highly imprecise. Stimulation of cultured oral keratinocytes with proinflammatory factors, including
IFN-γ, TNF-α/IL-1β, and poly(I:C), showed significant upregulation of lfTSLP mRNA, while only slight changes were seen for sfTSLP. This is in accordance with earlier studies that showed
constitutive sfTSLP but no lfTSLP mRNA expression in human bronchial epithelial cells, and upregulation of lfTSLP but not sfTSLP mRNA after exposure to poly(I:C).12 The observation that
sfTSLP is not regulated to the same degree as lfTSLP in oral keratinocytes in response to inflammatory stimuli indicates that sfTSLP serves purposes other than lfTSLP. The body surfaces are
in permanent contact with an array of diverse microorganisms and are colonized by a complex microbiota. Despite these permanent threats, the epithelium is mostly able to shield the body from
microbial invasion. One of the defense strategies used by the epithelia at mucosal and skin surfaces is the production of AMPs.17 Common features for many AMPs are cationicity and α-helical
confirmation,18 characteristics that also apply to TSLP.11 Peptides derived from full-length TSLP display antimicrobial activity against bacteria and yeasts, with the antimicrobial effect
located in a 34 aa peptide spanning the C-terminal region of full-length TSLP.11 We therefore examined whether the antimicrobial activity was retained in the sfTSLP peptide and compared it
with the activity of lfTSLP, using a panel consisting of diverse bacterial species: _S. mitis_ and _Staphylococcus epidermidis_ are components of the commensal flora of the oral cavity19 and
the skin,20 respectively, _Enterococcus faecalis_21 and _Escherichia coli_22 are found within the commensal flora of the gastrointestinal tract, and _Bacillus cereus_ is a Gram-positive
spore-forming bacterial species able to cause food poisoning and opportunistic infections.23 We found that synthetic sfTSLP exerted potent antimicrobial activity against all the tested
bacterial species. The effect was markedly stronger than that obtained with lfTSLP, which in our test system showed lower antimicrobial activity against _S. mitis_, _E. coli_, and _B.
cereus_ as compared with sfTSLP, and no detectable activity against _E. faecalis_ and _S. epidermidis_. This means that the antimicrobial activity was retained within the sfTSLP peptide and
was not dependent on the presence of complete full-length peptide. LfTSLP can be cleaved by _Staphylococcus aureus_ protease, among others in a C-terminal 34 aa fragment, displaying a strong
antimicrobial activity.11 It is conceivable that bacterial proteases also can degrade sfTSLP to this shorter fragment, or that, in fact, the naturally occurring form of this fragment
corresponds to sfTSLP. The surfaces of the oral cavity are covered by secretions produced by the salivary glands. Saliva represents an important ingredient in oral homeostasis through its
participation in functions such as lubrication, digestion, wound healing, and antimicrobial defense. Several AMPs are expressed and secreted by salivary glands, including LL-37, and α- and
β-defensins, and contribute to the protection of oral mucosal surfaces.24 SfTSLP peptide and mRNA were detected by immunohistochemistry and _in situ_ hybridization, respectively, in parotid,
submandibular, sublingual, and labial salivary glands. By western blotting, sfTSLP peptide was detected in saliva at 9 kDa, a size similar to that obtained after processing lysates from
cultured oral keratinocytes. This is different from the 7.1 and 7.4 kDa sizes of the peptides that were used to examine the antimicrobial function of sfTSLP, and therefore salivary sfTSLP
also may have undergone PTM. PTM can affect functionality of proteins and the sfTSLP in saliva may, therefore, not necessarily have the same effect as the synthetic peptide used in the
antimicrobial assays. This hampers the interpretation of the function of—in this case—sfTSLP, as it does for lfTSLP, where this issue also is unexplored: most _in vitro_ research on lfTSLP
has used 15 kDa recombinant lfTSLP produced in prokaryotes, while the naturally occurring TSLP in human cells or tissues appears to have an estimated MW of 22–24 kDa, as detected in the
present and other studies.13, 25, 26 The effect of PTM on the function of both sfTSLP and lfTSLP, therefore, deserves further investigation, but the present observations still indicate that
sfTSLP may act as an AMP in the oral cavity and skin to create a defense barrier that aids in the control of both commensal and pathogenic microbes. Whether levels of sfTSLP can reach active
antimicrobial concentrations is, similar to other AMPs detected in saliva, difficult to determine because saliva is a highly complex secretion that contains an array of other AMPs that
interfere with specific antimicrobial assays.24 In summary, the current study shows that sfTSLP is the predominant form of TSLP, widely and constitutively expressed at the protein level in
oral and skin keratinocytes and in salivary glands, is present in saliva, and has antimicrobial activity. The function of sfTSLP in the oral cavity and in the skin may be to control
microbial invaders. Of note, the fact that sfTSLP is transcribed and translated makes it mandatory to analyze the two TSLP variants separately. METHODS TSLP PEPTIDES AND ANTI-TSLP
ANTIBODIES. Full-length (132 aa; sequence: MYDFTNCDFEKIKAAYLSTISKDLITYMSG-TKSTEFNNTVSCSNRPHCLTEIQSLTFNPTAGCASLAKEMFAMKTKAALAIWCPGYSETQINATQAMKKRRKRKVTTNKCLEQVSQLQGLWRRFNRPLLKQQ) recombinant
TSLP was obtained from Peprotech (Rocky Hill, NJ). Synthetic sfTSLP peptides (63 aa: MFAMKTKAALAIWCPGYSETQINATQAMKKRRKRKVTTNKCLEQVSQLQGLWRRFNRPLLKQQ and 60 aa:
MKTKAALAIWCPGYSETQINATQAMKKRRKRKVTT-NKCLEQVSQLQGLWRRFNRPLLKQQ) were custom-prepared by ProteoGenix SAS (Schiltigheim, France). Antibodies reactive with TSLP were ab47943 (Abcam, Cambridge,
MA; 0.5 μg ml−1), nos. 4021 and 4023 (both from Pro-Sci, Poway, CA). LfTSLP-specific antibody preparations were made by absorbing ab47943 or no. 4021 with 60 aa sfTSLP peptide as follows.
CNBr-activated Sepharose 4B beads (Sigma-Aldrich, St Louis, MO) were prepared and coupled to 60 aa sfTSLP protein (ProteoGenix SAS) following the manufacturer’s protocol. Twenty micrograms
of sfTSLP protein was used per 250 mg beads. SfTSLP-coupled beads were suspended in 1% bovine serum albumin in phosphate-buffered saline (PBS) with NaN3 and incubated with rabbit polyclonal
anti-TSLP antibody (ab47943 or no. 4021) at 4 °C overnight. Supernatant, consisting of lfTSLP-specific antibodies, was collected. The preparation recognized lfTSLP but not sfTSLP
(Supplementary Figure S1). BIOPSY MATERIAL AND COLLECTION OF SALIVA. Oral mucosal biopsies were obtained from healthy volunteers undergoing third molar tooth extraction in private oral
surgery clinics (see Acknowledgments). Biopsies from leukoplakia at oral sites with that were chronically exposed to the smokeless tobacco “snus” were obtained as part of histological
diagnosis. Biopsies of parotid salivary glands were collected from healthy volunteers undergoing orthognatic surgery by retromandibular approach, and biopsies of submandibular glands and
sublingual salivary glands were collected from patients undergoing surgery, including removal of the entire or parts of the glands, at the Oslo University Hospital, Oslo, Norway. Labial
glands were collected from healthy volunteers by excision at the Department of Oral Surgery and Oral Medicine, Faculty of Dentistry, University of Oslo, Norway or at the Oslo University
Hospital, Oslo, Norway. Whole resting saliva was collected in sterile plastic tubes during a 5 min period from healthy volunteers recruited at the Department of Oral Biology, Faculty of
Dentistry, University of Oslo, Norway. The study was carried out according to the Helsinki Declaration’s principles for biomedical research and was approved by the Ethical Committee of
Health (REK SØR, Norway). Written informed consent was obtained from all donors. CELL CULTURE. Primary oral keratinocytes were isolated from oral mucosal biopsies according to an established
protocol.27 The epithelial sheet was removed from the connective tissue of the biopsy after dispase treatment (4 °C, overnight) and cut into small pieces, which were then treated with
trypsin-EDTA (Invitrogen, Paisley, UK) for 7 min at 37 °C. The treatment was stopped by the addition of fetal bovine serum (Cambrex, Verviers, Belgium). Cells were cultured in keratinocyte
serum-free medium (17005; Gibco-BRL, Gaithersburg, MD) supplemented with 1 ng ml−1 human recombinant epidermal growth factor, 25 μg ml−1 bovine pituitary extract (both from Gibco-BRL) and 1%
penicillin, 1% streptomycin, and 0.25 mg ml−1 amphotericin B (Lonza, Basel, Switzerland) in 25-cm2 cell-culture flasks (Nalge Nunc, Rochester, NY). Keratinocytes were passaged 1:4 by
trypsinization. Cells were maintained in a humidified atmosphere of 5% CO2 in air at 37 °C. Cell counting was carried out with a Coulter Counter Z2 (Beckman Coulter, Fullerton, CA). Cells
were used between second and fifth passages. Cells (4 × 105 for stimulation experiments or 3 × 105 for esiRNA experiment per well) were plated in 3 ml of keratinocyte serum-free medium, in
6-well plates. Stimulation experiments were performed at approximately 80% confluency and esiRNA experiments were performed at approximately 50% confluency. Cells were stimulated with either
50 ng ml−1 TNF-α, 10 ng ml−1 IL-1β, 10 ng ml−1 IFN-γ (all from Peprotech), or 10 μg ml−1 poly(I:C) (Sigma-Aldrich). The wells were washed with ice-cold PBS before harvesting. For western
blotting, cells were harvested by scraping in Iscove’s modified Dulbecco’s medium (Sigma-Aldrich) containing 10% FBS, centrifuged, and washed once in ice-cold PBS before lysis with 0.5%
NP-40 (Millipore, Merck Chemicals, Nottingham, UK) in PBS supplemented with protease inhibitor cocktail and EDTA (Pierce Biotechnology, Rockford, IL) for 30 min on ice. Lysates were
centrifuged at 16,000 _g_ and the supernatants were used for further analyses. For RNA extraction, cells were lysed directly in 350 μl RLT buffer containing β-mercaptoethanol (Qiagen,
Valencia, CA) in the well. RNA was extracted from oral mucosal biopsies by cutting biopsies into small pieces, adding 350 μl of RLT buffer containing β-mercaptoethanol and vigorous shaking
in a tissue blender. Lysates were stored at −20°C (protein) or at −80°C (RNA) until further processing. DOT BLOTTING AND WESTERN BLOTTING. Dot blots were prepared by spotting equimolar
amounts of lfTSP (100 ng) and sfTSLP (50 ng) peptides on prewetted nitrocellulose membranes (Bio-Rad, Hercules, CA), after which the membranes were soaked in 4% bovine serum albumin for 1 h
at room temperature. Antibodies were applied for 2 h at room temperature. The secondary antibody used was goat anti-rabbit IgG conjugated to alkaline phosphatase (Jackson ImmunoResearch
Laboratories, West Grove, PA). Spots were visualized using the 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate (Sigma-Aldrich). Protein lysates were mixed 1:3 with
sample buffer containing dithiothreitol and β-mercaptoethanol (for lfTSLP detection) or 1:1 Tricine Sample Buffer (Bio-Rad) with 2% β-mercaptoethanol (for sfTSLP detection) and boiled for 5
min. Whole saliva was mixed 1:1 with sample buffer, and boiled for 10 min. Concentration of protein in the lysates was determined using a protein assay (Bio-Rad) with γ-globulin as a
standard protein. Thirty-five micrograms of protein lysate or 20 μl of saliva sample was loaded onto 4–20% (for lfTSLP detection) or 10–20% precast polyacrylamide gradient gels (Bio-Rad)
(for sfTSLP detection) and subjected to sodium dodecyl sulfate-gel electrophoresis. Proteins were transferred to nitrocellulose membranes (Bio-Rad) (50 min; for lfTSLP detection) or
polyvinylidene difluoride membranes (Bio-Rad) (15 min; for sfTSLP detection) and incubated in Protein Free Blocking buffer (Pierce Biotechnology) or 4% bovine serum albumin for 1 h at room
temperature. LfTSLP and sfTSLP protein were detected using rabbit polyclonal anti-TSLP (no. 4021 (ProSci, Poway, CA; 1 μg ml−1) or ab47943 (Abcam; 0.5 μg ml−1)), as indicated in the figures.
The secondary antibody used was goat anti-rabbit IgG conjugated to alkaline phosphatase (Jackson IRL). Reactive bands were visualized using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue
tetrazolium substrate (Sigma-Aldrich). Densitometry was applied to some of the bands obtained after western blotting, by scanning the blotting membranes and using the gel analysis function
in ImageJ (National Institute of Health, Bethesda, MD). IMMUNOHISTOCHEMISTRY. Biopsies were fixed in 4% buffered formaldehyde for 24 h, dehydrated, and embedded in paraffin. Sections (4 μm)
were dewaxed, rehydrated, and quenched for endogenous peroxidase with 0.3% H2O2 in methanol. Heat-induced epitope retrieval was performed in a 0.05% citraconic anhydride solution (pH 7.4) in
distilled water in a pressure cooker (Biocare Medical, Concord, CA) at 100 °C for 15 min. Sections were cooled, equilibrated in PBS, and blocked with 5% normal rabbit serum before overnight
incubation at 4°C with rabbit anti-human TSLP antibody, as indicated in the figures. As visualization systems, we used biotinylated goat anti-rabbit IgG antibody (Vector Laboratories,
Burlingame, CA), followed by horseradish peroxidase-conjugated avidin–biotin complex (Vector Laboratories). Immunohistochemical staining was visualized using 3,3-diaminobenzidine
(Sigma-Aldrich) as substrate, and hematoxylin was applied for counterstaining. Pictures were obtained using a Nikon Eclipse 90i microscope (Nikon Europe, Amsterdam, The Netherlands) equipped
with a Plan Fluor 20 × NA 0.50 objective (Figure 1d and Supplementary Figure S2), a Plan Fluor 10 × NA 0.30 objective (Figure 1g), and a Nikon DS-Ri1 digital camera on the DS-L2 interface
(Nikon, Tokyo, Japan). Images were captured with the NIS-Elements F software (Nikon; version 3.22.00) at a resolution of 3,840 × 3,072 pixels per manual exposure with fixed shutter time,
gain amplification, and illumination and at room temperature. Pictures were taken in one session, ensuring identical microscope, and camera settings. The images were opened in Adobe
Photoshop (CS5, version 12.0.4) and mounted as displayed in the figures. Then, a separate layer was created that was used to adjust brightness and color balance on all photographs in the
figure at the same time, to ensure that all adjustments for a given figure were made on all pictures collectively. ESIRNA KNOCKDOWN. Cultured oral keratinocytes were transfected with esiRNA
against TSLP (EHU075571) or scramble esiRNA (both from Sigma-Aldrich) with INTERFERin Transfection Reagent (Polyplus, Illkirch, France) according to the manufacturer’s instructions.
Transfection reactions were performed in extract-free keratinocyte serum-free medium (Gibco-BRL). Cell media were changed to extract-containing media 20 h after transfection. Cells were
harvested 24 h after transfection for mRNA analysis and 48 h after transfection for protein analysis. REAL-TIME PCR. Total RNA was extracted from cell or biopsy lysates by the use of columns
(RNeasy; Qiagen), according to the manufacturer’s instructions. Quantity and purity of the RNA was measured using an ND-2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Four
hundred nanograms of RNA was reverse transcribed into cDNA using a Reverse Transcription Core Kit (RT-RTCK-05; Eurogentec, Seraing, Belgium) with random nonamers as RT primers and a Moloney
murine leukemia virus reverse transcriptase enzyme (Eurogentec). Duplicate samples in Fast EvaGreen qPCR Master Mix (Biotium, Hayward, CA) were run on the MX4000 instrument (Stratagene, La
Jolla, CA). The levels of TSLP mRNA were normalized with regard to the levels of the glyceraldehyde 3-phosphate dehydrogenase mRNA (ΔCt method) and were calculated and displayed as 2−ΔCt
values. For the esiRNA knockdown experiment, the level of sfTSLP mRNA was normalized with regard to E-cadherin. Primer sequences are given in Supplementary Table S1 (Sigma-Aldrich). DNA
amplification was performed using the thermal cycling profile of initial denaturation at 95°C for 10 min followed by 40 cycles of amplification (denaturation at 95°C for 15 s and annealing
and elongation for 1 min at 60°C). Fluorescence data were collected during the annealing stage of amplification. _IN SITU_ HYBRIDIZATION. Chromogen _in situ_ hybridization was carried out on
formaldehyde-fixed, paraffin-embedded human tissues using RiboMap and BlueMap Kits (Ventana Medical Systems, Illkirch, France) in the Ventana Discovery environment. A double digoxin-labeled
LNA mRNA detection probe for sfTSLP was used (5′-TCATAGGCGGCAAAGTTTACGA-3′; Exiqon, Vedbaek, Denmark). The probe was hybridized at 52 °C and amplified by using antidigoxin antibodies
(Jackson IRL). Scramble probe was used as a control (Exiqon). ANTIBACTERIAL ASSAY. Overnight bacterial cultures were spun down at 5,000 _g_ for 10 min, resuspended in 10 mM sodium phosphate
buffer, and diluted to prepare 1 ml of bacterial suspension OD600 nm=0.350, corresponding to 106 per ml of _S. mitis_ cells. This estimate was also used for the other bacteria. The OD600 nm
0.350 suspension was diluted 1:20 to get working suspensions (5 × 105 per ml). Two hundred microliters of each bacterial working suspension was applied to wells in a 96-well plate giving 1 ×
105 cells per well. Bacterial cultures were incubated with concentrations of sfTSLP, lfTSLP, and LL-37 as indicated in Figure 2a, b or without test substance, for 2 h at 37°C in air
supplemented with 5% CO2. At the end of the incubation, serial dilutions of each bacterial suspension (25 μl) were plated on agar plates. After overnight incubation at 37°C, the number of
colony-forming units per ml was determined. ANALYSIS OF STAT5 PHOSPHORYLATION. STAT5 phosphorylation in response to sfTSLP and lfTSLP was examined in blood-derived CD1c+ myeloid DCs. CD1c+
DCs were isolated from fresh buffy coats using positive selection with magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and incubated with poly(I:C) for 24 h at 37°C in 5% CO2,
to induce high levels of TSLP receptor expression.28 The next day, DCs were treated with lfTSLP or sfTSLP for 15 min, or preincubated for 15 min with sfTSLP before incubation with lfTSLP for
15 min, and subsequently fixed in 1.5% paraformaldehyde. The DCs were permeabilized in methanol before incubation with monoclonal rabbit antibodies to pSTAT5 (Tyr649) (Cell Signaling
Technology, Danvers, MA), followed by Alexa 488-conjugated goat anti-rabbit secondary antibodies (Life Technologies, Grand Island, NY). Cells were analyzed on a BD LSRFortessa (BD
Biosciences, Franklin Lakes, NJ). STATISTICAL ANALYSIS. The paired _t_-test and repeated-measures analysis of variance on ranks (Friedman) with subsequent pairwise multiple comparison
(Student–Newman–Keuls) were used to test differences between groups, using the Sigmaplot v 12.0 software (Systat Software, San Jose, CA). _P_-values of <0.05 were considered statistically
significant. REFERENCES * Friend, S.L., Hosier, S., Nelson, A., Foxworthe, D., Williams, D.E. & Farr, A. A thymic stromal cell line supports _in vitro_ development of surface IgM+ B
cells and produces a novel growth factor affecting B and T lineage cells. _Exp. Hematol._ 22, 321–328 (1994). CAS PubMed Google Scholar * Soumelis, V. _et al_. Human epithelial cells
trigger dendritic cell mediated allergic inflammation by producing TSLP. _Nat. Immunol._ 3, 673–680 (2002). Article CAS Google Scholar * Kashyap, M., Rochman, Y., Spolski, R., Samsel, L.
& Leonard, W.J Thymic stromal lymphopoietin is produced by dendritic cells. _J. Immunol._ 187, 1207–1211 (2011). Article CAS Google Scholar * Allakhverdi, Z. _et al_. Thymic stromal
lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. _J. Exp. Med._ 204, 253–258 (2007). Article CAS
Google Scholar * Rochman, I., Watanabe, N., Arima, K., Liu, Y.J. & Leonard, W.J Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. _J.
Immunol._ 178, 6720–6724 (2007). Article CAS Google Scholar * Ziegler, S.F., Roan, F., Bell, B.D., Stoklasek, T.A., Kitajima, M. & Han, H. The biology of thymic stromal lymphopoietin
(TSLP). _Adv. Pharmacol._ 66, 129–155 (2013). Article CAS Google Scholar * He, B. _et al_. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching byinducing
epithelial-cell secretion of the cytokine APRIL. _Immunity_ 26, 812–826 (2007). Article CAS Google Scholar * Watanabe, N. _et al_. Hassall’s corpuscles instruct dendritic cells to induce
CD4+CD25+ regulatory T cells in human thymus. _Nature_ 436, 1181–1185 (2005). Article CAS Google Scholar * Iliev, I.D. _et al_. Human intestinal epithelial cells promote the
differentiation of tolerogenic dendritic cells. _Gut_ 58, 1481–1489 (2009). Article CAS Google Scholar * Hanabuchi, S. _et al_. Thymic stromal lymphopoietin-activated plasmacytoid
dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. _J. Immunol._ 184, 2999–3007 (2010). Article CAS Google Scholar * Sonesson, A. _et al_. Thymic stromal
lymphopoietin exerts antimicrobial activities. _Exp. Dermatol._ 20, 1004–1010 (2011). Article CAS Google Scholar * Harada, M. _et al_. Functional analysis of the thymic stromal
lymphopoietin variants in human bronchial epithelial cells. _Am. J. Respir. Cell. Mol. Biol._ 40, 368–374 (2009). Article CAS Google Scholar * Reche, P.A. _et al_. Human thymic stromal
lymphopoietin preferentially stimulates myeloid cells. _J. Immunol._ 167, 336–343 (2001). Article CAS Google Scholar * Rimoldi, M. _et al_. Intestinal immune homeostasis is regulated by
the crosstalk between epithelial cells and dendritic cells. _Nat. Immunol._ 6, 507–514 (2005). Article CAS Google Scholar * Quentmeier, H. _et al_. Cloning of human thymic stromal
lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. _Leukemia_ 15, 1286–1292 (2001). Article CAS Google Scholar * Smelter, D.F., Sathish, V., Thompson, M.A., Pabelick,
C.M., Vassallo, R. & Prakash, Y.S Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. _J. Immunol._ 185, 3035–3040 (2010). Article CAS Google Scholar
* Harder, J., Schröder, J.M. & Gläser, R The skin surface as antimicrobial barrier: present concepts and future outlooks. _Exp. Dermatol._ 22, 1–5 (2013). Article CAS Google Scholar *
De Smet, K. & Contreras, R Human antimicrobial peptides: defensins, cathelicidins and histatins. _Biotechnol. Lett._ 27, 1337–1347 (2005). Article CAS Google Scholar * Aas, J.A.,
Paster, B.J., Stokes, L.N., Olsen, I. & Dewhirst, F.E Defining the normal bacterial flora of the oral cavity. _J. Clin. Microbiol._ 43, 5721–5732 (2005). Article Google Scholar * Lai,
Y. _et al_. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. _J. Invest. Dermatol._ 130,
2211–2221 (2010). Article CAS Google Scholar * Kropec, A., Hufnagel, M., Zimmermann, K. & Huebner, J _In vitro_ assessment of the host response against _Enterococcus faecalis_ used in
probiotic preparations. _Infection_ 33, 377–379 (2005). Article CAS Google Scholar * Alteri, C.J. & Mobley, H.L _Escherichia coli_ physiology and metabolism dictates adaptation to
diverse host microenvironments. _Curr. Opin. Microbiol._ 15, 3–9 (2012). Article CAS Google Scholar * Tran, S.L. _et al_. Haemolysin II is a _Bacillus cereus_ virulence factor that
induces apoptosis of macrophages. _Cell. Microbiol._ 13, 92–108 (2011). Article CAS Google Scholar * Dale, B.A. & Fredericks, L.P Antimicrobial peptides in the oral environment:
expression and function in health and disease. _Curr. Issues Mol. Biol._ 7, 119–133 (2005). CAS PubMed PubMed Central Google Scholar * Nomura, K. _et al_. Regulation of interleukin-33
and thymic stromal lymphopoietin in human nasal fibroblasts by proinflammatory cytokines. _Laryngoscope_ 122, 1185–1192 (2012). Article CAS Google Scholar * Hung, T.J. _et al_. shRNA for
thymic stromal lymphopoietin: a novel therapeutic approach for pulmonary fibrosis. _J. Cell. Sci. Ther._ 4, 144 (2013). Google Scholar * Storesund, T., Hayashi, K., Kolltveit, K.M., Bryne,
M. & Schenck, K. Salivary trefoil factor 3 enhances migration of oral keratinocytes. _Eur. J. Oral Sci._ 116, 135–140 (2008). Article CAS Google Scholar * Lu, N., Wang, Y.H., Wang,
Y.H., Arima, K., Hanabuchi, S. & Liu, Y.J. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. _J. Exp. Med._ 206, 2111–2119 (2009). Article CAS
Google Scholar Download references ACKNOWLEDGEMENTS We thank oral surgery practitioners Dr Karatsaidis (Galleri Oslo Klinikken), Dr Hals (Tannlegene i Bogstadveien 51, Oslo, Norway), and Dr
Li (ENT Division, Oslo University Hospital) for providing biopsy material. We also thank H. Weidemann and C. Khuu, Department of Oral Biology, Dental Faculty, University of Oslo, Norway,
for excellent technical assistance. The study was funded by the University of Oslo, Oslo, Norway. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Oral Biology, Dental Faculty,
University of Oslo, Oslo, Norway L Bjerkan, O Schreurs, S A Engen, I JS Blix & K Schenck * Centre for Immune Regulation and Department of Pathology, Medical Faculty, University of Oslo,
Oslo, Norway F L Jahnsen & E S Baekkevold * Department of Periodontology, Dental Faculty, University of Oslo, Oslo, Norway I JS Blix Authors * L Bjerkan View author publications You can
also search for this author inPubMed Google Scholar * O Schreurs View author publications You can also search for this author inPubMed Google Scholar * S A Engen View author publications You
can also search for this author inPubMed Google Scholar * F L Jahnsen View author publications You can also search for this author inPubMed Google Scholar * E S Baekkevold View author
publications You can also search for this author inPubMed Google Scholar * I JS Blix View author publications You can also search for this author inPubMed Google Scholar * K Schenck View
author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to K Schenck. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare
no conflict of interest. ADDITIONAL INFORMATION SUPPLEMENTARY MATERIAL is linked to the online version of the paper SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (DOC 793 KB)
POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bjerkan, L., Schreurs, O.,
Engen, S. _et al._ The short form of TSLP is constitutively translated in human keratinocytes and has characteristics of an antimicrobial peptide. _Mucosal Immunol_ 8, 49–56 (2015).
https://doi.org/10.1038/mi.2014.41 Download citation * Received: 31 October 2013 * Accepted: 18 April 2014 * Published: 21 May 2014 * Issue Date: January 2015 * DOI:
https://doi.org/10.1038/mi.2014.41 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








