- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Nucleic acid amplification and quantification _via_ polymerase chain reaction (PCR) is one of the most sensitive and powerful tools for clinical laboratories, precision medicine,
personalized medicine, agricultural science, forensic science and environmental science. Ultrafast multiplex PCR, characterized by low power consumption, compact size and simple operation,
is ideal for timely diagnosis at the point-of-care (POC). Although several fast/ultrafast PCR methods have been proposed, the use of a simple and robust PCR thermal cycler remains
challenging for POC testing. Here, we present an ultrafast photonic PCR method using plasmonic photothermal light-to-heat conversion _via_ photon–electron–phonon coupling. We demonstrate an
efficient photonic heat converter using a thin gold (Au) film due to its plasmon-assisted high optical absorption (approximately 65% at 450 nm, the peak wavelength of heat source
light-emitting diodes (LEDs)). The plasmon-excited Au film is capable of rapidly heating the surrounding solution to over 150 °C within 3 min. Using this method, ultrafast thermal cycling
(30 cycles; heating and cooling rate of 12.79±0.93 °C s−1 and 6.6±0.29 °C s−1, respectively) from 55 °C (temperature of annealing) to 95 °C (temperature of denaturation) is accomplished
within 5 min. Using photonic PCR thermal cycles, we demonstrate here successful nucleic acid (λ-DNA) amplification. Our simple, robust and low cost approach to ultrafast PCR using an
efficient photonic-based heating procedure could be generally integrated into a variety of devices or procedures, including on-chip thermal lysis and heating for isothermal amplifications.
SIMILAR CONTENT BEING VIEWED BY OTHERS RAPID QUANTITATIVE PCR EQUIPMENT USING PHOTOTHERMAL CONVERSION OF AU NANOSHELL Article Open access 16 February 2024 A PLASMONIC GOLD NANOFILM-BASED
MICROFLUIDIC CHIP FOR RAPID AND INEXPENSIVE DROPLET-BASED PHOTONIC PCR Article Open access 02 December 2021 NAPTUNE: NUCLEIC ACIDS AND PROTEIN BIOMARKERS TESTING VIA ULTRA-SENSITIVE
NUCLEASES ESCALATION Article Open access 04 February 2025 INTRODUCTION After its initial invention in 1983 by Kary Mullis, polymerase chain reaction (PCR) has become an essential technique
in the fields of clinical laboratories, agricultural science, environmental science, and forensic science.1,2,3,4,5,6,7,8 PCR requires thermal cycling, or repeated temperature changes
between two or three discrete temperatures to amplify specific nucleic acid target sequences. To achieve such thermal cycling, conventional bench-top thermal cyclers generally use a metal
heating block powered by Peltier elements. Whereas commercial PCR systems have improved heating and cooling rates to reduce amplification time, they are still relatively time-consuming
(typically requiring an hour or more per amplification). This can be attributed to the larger thermal capacitance of a system that requires uniformly heating 96- or 384-well plastic PCR
plates and reaction volumes of several tens of microliters per well. Because fast/ultrafast PCR is highly desirable for applications such as timely diagnosis of infectious diseases, cardiac
diseases, cancer, neurological disorder diseases, and rapid biowarfare and pathogen identification at the point-of-care (POC) level, many academic and industrial groups have worked on
improving PCR systems,9,10,11,12,13,14,15 One commercial PCR system (LightCycler® 2.0, Roche Diagnostics USA, Indianapolis, IN, USA) using air heating/cooling and capillary tubes can perform
30 thermal cycles in 10–60 min, depending on sample volume.10 However, this system is not suitable for POC testing due to its high power consumption (800 W maximum) and heavy weight
(approximately 22 kg). For POC diagnostics for global health care in resource-limited environments, such as in developing countries, a fast/ultrafast PCR system should be portable, robust,
simple, easy to use and characterized by low power consumption through miniaturization and integration. To date, microfluidic-based fast/ultrafast PCR systems have been extensively
investigated to reduce amplification time by decreasing sample sizes as well as by increasing heat transfer rates. Resistive heating with microfabricated thin film heaters is most commonly
used to control the temperature in the static microfluidic-based PCR system, in which the PCR runs in the microfluidic chamber.11,12 However, this method requires a complicated fabrication
process to integrate the thin film heater and resistance temperature detection sensor on the chip. In the case of continuous-flow PCR on a chip, the PCR amplification occurs when the
reaction samples pass thorough three discrete temperature zones.13 This method can produce faster thermal cycling for PCR, but requires an external syringe pump for continuous-flow control
and lacks the ability to perform multiple reactions at the same time. Another approach includes infrared-mediated non-contact selective heating of water droplets (nanoliter sample volume)
for ultrafast thermal cycling using an infrared laser, which harnesses the strong absorbance of water at wavelengths over 1000 nm.14,15 However, droplet formation from the PCR mixture is a
precise process prone to human error, which is a drawback for POC testing. More recently, efforts have been made to utilize the advantages of plasmonic photothermal heating of gold
nanoparticles,16,17 using pulsed or continuous-wave laser excitation for photothermal therapy of cancer18,19 and fast PCR,20 for example. However, this arrangement is not ideal for POC
testing, as it requires not only expensive lasers and detection systems but also lacks reliable gold nanoparticles-based sample preparation. In this paper, we present a novel ultrafast
photonic PCR method that combines the use of a thin Au film as a light-to-heat converter and light-emitting diodes (LEDs) as a heat source. The strong light absorption of the thin Au film
(65%, 120 nm thick) generates heat due to the plasmonic photothermal light-to-heat conversion by photon–electron–phonon coupling at the thin Au film, followed by heating of the surrounding
solution with a maximum temperature of over 150 °C within 3 min. Thirty ultrafast thermal cycles (heating rate of 12.79±0.93 °C s−1 and cooling rate of 6.6±0.29 °C s−1) from 55 °C (point of
annealing) to 95 °C (point of denaturation) are accomplished within 5 min. Using this technique, we successfully demonstrate the amplification of λ-DNA. We propose that our PCR system would
be ideal for POC diagnostics due to its ultrafast thermal cycling capability, multiplex PCR, low power consumption (in the current set-up, up to approximately 3.5 W), low cost and simple
configuration for system level integration. Furthermore, our efficient photonic-based heating procedure could be generally integrated into a variety of devices or procedures, including
on-chip thermal lysis and heating for isothermal amplifications. MATERIALS AND METHODS FABRICATION OF THE THIN AU FILM DEPOSITED POLY(METHYL METHACRYLATE) (PMMA) PCR WELLS The 4-mm-thick
PMMA sheets were cut with a VersaLASER VL-200 laser cutting system (Universal Laser System, Inc., Scottsdale, AZ, USA) to make a reaction well with a 4-mm diameter. The 1.5-mm-thick bottom
PMMA sheet and top reaction wells were bonded together using thermal bonding performed at 84 °C with a pressure of 1.0 t after UV/ozone treatment of the PMMA sheet for 10 min. Thin Au films
of different thicknesses were deposited by electron beam evaporation under a base pressure of 2×10−7 Torr. The thin Au film was passivated with thin poly(dimethylsiloxane) by dropping 2 µL
of poly(dimethylsiloxane) into the well and curing in an oven for 2 h to prevent PCR reaction inhibition by the thin Au film and thermocouple. SIMULATION We used COMSOL Multiphysics software
(Ver. 4.3, COMSOL Inc., Burlington, MA, USA) for electromagnetic simulation. The detailed geometry and material properties for the simulation are shown in Supplementary Fig. S1 and Table
S1. A thin Au film is placed on a PMMA substrate, and then the Au film was covered with water. Different thicknesses (10, 20, 40, 80 and 120 nm) of thin Au film were applied to the
simulation to calculate the absorption of the Au films and subsequent resistive heat generation. The plane wave with an _x_-polarized electric field travels in the positive _z_ direction in
the coordinate system shown in Supplementary Fig. S1. The permittivity of Au used in this study was referred from Johnson and Christy,21 and the permittivities of PMMA and water were 3 and
1.77, respectively. ULTRAFAST PHOTONIC PCR CYCLES LEDs (Luxeon® Rebel, Lumileds, San Jose, CA, USA, royal blue with a peak wavelength of 447.5 nm, 890 mW at 700 mA injection current) were
used for plasmonic photothermal heating of the thin Au film with a Keithley 2400 source meter. To focus the light from the LEDs, a Carclo 20 mm fiber coupling optic (part number: 10356,
Carclo Optics, PA, USA) was employed. The temperature of the solution was monitored and recorded in real time by a type-K insulated thermocouple purchased from OMEGA Engineering (part
number, 5SC-TT-K-40-36) for thermal cycling. Temperature cycling using an LED, 80 mm cooling fan, source meter and thermocouple was controlled through the LabVIEW program. A National
Instruments 9213 16 channel thermocouple module with high speed mode, auto zero and cold junction compensation was used for accurate temperature acquisition from the type-K thermocouple.
PREPARATION OF THE PCR REAGENT AND DNA TEMPLATE The template λ-DNA and Takara Z-TaqTM DNA polymerase (2.5 U µL−1), 10× Z-Taq Buffer (Mg2+ plus, 30 mM) and dNTP Mixture (2.5 mM each) were
purchased from Takara Bio Inc. (Otsu-shi, SHG, Japan). Forward primer (5′-CATCGTCTGCCTGTCATGGGCTGTTAAT-3′) and reverse primer (5′-TCGCCAGCTTCAGTTCTCTGGCATTT-3′) were purchased from
Integrated DNA Technologies, Inc. (Coralville, IA, USA). The reaction to amplify a 98-base pair (bp) λ-DNA target with Z-TaqTM DNA polymerase included 0.5 µL Z-Taq DNA polymerase, 5 µL of
10× Z-Taq Buffer, 4 µL of dNTP mixture, 4.5 µL of 10 µM primers (each) and 10 µL of bovine serum albumin (50 µg) and was brought to 50 µL with PCR-grade water. The final concentration of the
template λ-DNA was 0.1 ng µL−1. The 10 µL of PCR mixture was placed within Au-coated PMMA PCR wells for photonic PCR and then covered with 30 µL of mineral oil to prevent evaporation during
thermal cycling. After amplification, a mixture of 10 µL of PCR product and 10 µL of E-Gel® sample loading buffer (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA) was loaded
onto E-Gel® 2% agarose gels with SYBR SafeTM DNA gel stain (Invitrogen) and run in an E-Gel base (Invitrogen) for 30 min. A 1-Kb DNA ladder was used to confirm the size of product. RESULTS
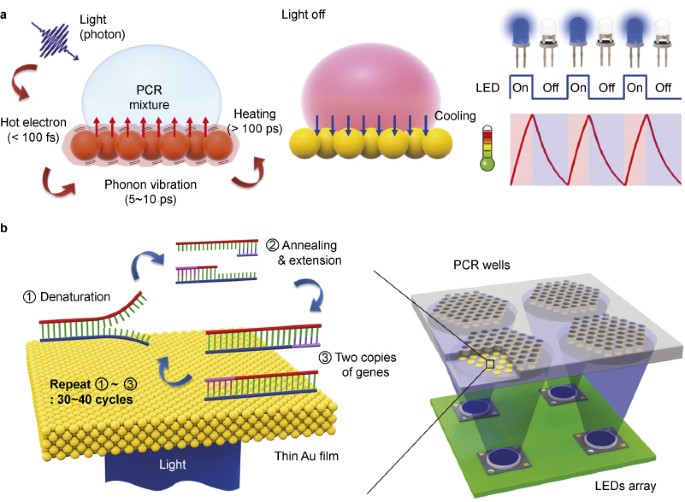
AND DISCUSSION PHOTOTHERMAL LIGHT-TO-HEAT CONVERSION FOR PCR The principle of ultrafast photonic PCR is illustrated in Figure 1a. In considering photon interaction with materials, the
absorption of photons is often treated as heat.22 When the photons from the excitation source reach the surface of the thin Au film, plasmon-assisted strong light absorption can occur. This,
in turn, excites electrons near the surface to higher energy states, generating hot electrons within 100 fs.23,24 These hot electrons can reach a temperature of several thousand degrees
Kelvin due to their small electronic heat capacity. They are also capable of rapidly diffusing throughout the thin Au film, creating a uniform distribution of hot electrons. Rapid heating is
followed by cooling to equilibrium by energy exchange between the hot electrons and the lattice phonons after 5–10 ps. Thus, overall, when thin Au films are illuminated, a large temperature
difference between the hot metal surface and the cooler surrounding solution occurs, resulting in the heating of the surrounding solution in a long time scale over 100 ps. When a light is
turned off, the fast cooling of the heated solution can also be achieved by the heat dissipation through the thin Au film with high thermal conductivity of 317 W m−1 K−1.25 A thin Au film
deposited on a PMMA well is used as a light-to-heat converter, serving as a source of plasmonic photothermal heating for the PCR thermal cycling, as shown in Figure 1b. In addition to
driving multiple PCR reactions with single LEDs, multiple well plates integrated with LED arrays could be used for multiplex PCR by modulating each LED to have unique annealing temperatures
for the various primer designs. This multiple well LED array PCR thermal cycler configuration would be ideal for multiplex ultrafast PCR for POC diagnostics because it could perform multiple
tests at once. We performed a set of electromagnetic simulations to theoretically characterize the plasmonic photothermal light-to-heat conversion of our Au films. We calculated the
electromagnetic field and resistive heat distributions for 10-nm- and 120-nm-thick Au films on a PMMA substrate. As expected from the skin depth, where _ω_ is the angular frequency, _μ_ is
the permeability and _σ_ is the conductivity; the thickness of the thin Au film determines the amount of light to heat conversion. Upon a normal incidence of a 450 nm wavelength light
source, the 10 nm thick Au film transmits an enormous amount of electromagnetic energy (Figure 2a), and the heat conversion energy is saturated along the film depth (Figure 2c). However, the
120-nm thick Au film absorbs most of the incident light (Figure 2b) and subsequently, generates more heat in the Au film by converting light into heat (Figure 2d). Figure 2e shows that an
increase in thickness of thin Au film, in the range of 10–120 nm, corresponds to an increase in the optical absorption. A significant increase of the optical absorption below the 540-nm
wavelength could be attributed to the plasmonic electron resonance of gold.26 As a result, the averaged light-to-heat conversion efficiency over the emission wavelength from each LED
increases with increasing Au film thickness for three different LEDs, as shown in Figure 2f (see Supplementary Fig. S2). It is noteworthy that the blue LEDs with peak emission wavelength of
450 nm show the highest light-to-heat conversion efficiency using the thin Au film. LED-DRIVEN PHOTONIC PCR THERMAL CYCLER The optical absorption spectra of thin Au films with different
thicknesses deposited on PMMA substrates are shown in Figure 3a (see Supplementary Fig. S3). Our simulation results can help us determine when the strongest light absorption occurs, as this
is critical to maximizing photothermal heating. As the thickness of the thin Au film increases, the optical absorption also increases, showing 65 % absorption at the peak wavelength (450 nm)
of the excitation LEDs in the 120-nm-thick Au film. Our photonic PCR thermal cycler uses LEDs as a heating source, PMMA PCR wells deposited with thin Au films, and a lens to focus the
excitation light (see Supplementary Fig. S3). The light from the LEDs is continuous-wave and randomly polarized. Therefore, the efficiency of light-to-heat conversion would be lower in this
case than using a pulsed laser because as electrons are excited to higher energy states, the probability of further excitation decreases. Despite its possibly lower light-to-heat conversion
efficiency than a pulsed light source, however, LEDs require minimal power consumption and are extremely low in cost compared with laser sources, making them an ideal PCR heating source for
POC testing. The component cost of the instruments for our ultrafast photonic PCR thermal cycle (see Supplementary Fig. S3b) could be less than US$100 including the LEDs, focus lens and
driver, if the LabVIEW and data acquisition board for temperature control are integrated into a microcontroller module. The maximum power consumption of an LED is approximately 3.5 W at a 1
A injection current. Figure 3b shows the temperature profiles of 10 µL volumes of solution (here, glycerol was used to show the maximum heating temperatures) upon thin Au films of different
thicknesses at a fixed injection current of 500 mA. The maximum temperatures increased as the thickness of the thin Au film increases from 10 nm to 120 nm due to the increasing optical
absorption. The photothermal heating of the 120-nm-thick Au film was further characterized as a function of the injection current, as shown in Figure 3c, because the heating rate is
determined by the amount of dissipated power (i.e., the injection current of the LEDs). Figure 3d summarizes the temperature of a solution after 3-min heating with Au films of different
thickness and varying injection currents (see Supplementary Table S2). These results clearly indicate that the maximum temperature increases with an increase of Au film thickness to 120 nm
and an increase of injection current to 1 A. Complete PCR thermal cycling, consisting of three representative temperatures (94 °C for denaturation, 60 °C for annealing and 72 °C for
extension), is demonstrated using an LED-driven photonic PCR thermal cycler, as shown in Figure 3e. To prevent evaporation during thermal cycling, 10 µL of PCR buffer was covered with 30 µL
of mineral oil. The averages and standard deviations at each temperature were obtained from the temperature profile, and the results are 94.09±0.17 °C at 94 °C, 59.94±0.13 °C at 60 °C and
72.02±0.12 °C at 72 °C, respectively, showing excellent temperature accuracy and stability. The initial temperature fluctuation before reaching the setting temperature could be further
reduced by optimizing the proportional-integral-derivative controller value in LabVIEW. ULTRAFAST THERMAL CYCLING AND NUCLEIC ACID AMPLIFICATION To determine maximum heating and cooling
rates, a thermal cycle was performed whereby the solution (here, 5 µL of PCR mixture covered with 30 µL of mineral oil) temperature was rapidly cycled between 55 °C and 95 °C, mirroring the
denaturation (95 °C) and annealing (55 °C) temperatures. Figure 4a shows the 30 ultrafast photonic cycles within 5 min. Using the thermal cycling result, heating and cooling rates were
calculated by measuring the temperature difference between successive temperature maxima and minima and then dividing by the time interval between them. The average rates and sample standard
deviations were obtained as shown in Figure 4b. The average heating and cooling rates obtained are 12.79±0.93 °C s−1 and 6.6±0.29 °C s−1, respectively. The amplification of λ-DNA was
performed to verify our photonic PCR method. After running PCR reactions as shown in Figure 4c, the amplicons were visualized by E-Gel® 2% agarose gels with SYBR SafeTM. Lane 1 represents
the 1 kb DNA marker, lanes 2 and 3 are the PCR products from ultrafast photonic PCR with different cycle numbers (94 °C for 0 s, 62 °C for 0 s), and lanes 4 and 5 contain positive controls
produced from standard thermal cycling condition (94 °C for 1 s, 62 °C for 1 s, 60 cycles) by a bench-top thermocycler (Bio-Rad C1000TM Thermal Cycler, Bio-Rad Laboratories Inc., Hercules,
CA, USA). Photonic PCR yielded a single 98-bp major band (lanes 2 and 3), indicating that the λ-DNA was successfully amplified using our ultrafast photonic PCR method. The weak band
intensity from the PCR product amplified by photonic PCR could be attributed to the lower amplification efficiency compared with a traditional bench-top thermal cycler. Currently, only the
thin Au film acts as a two-dimensional photothermal heater, leading to a temperature gradient in the solution and a potentially lower amplification efficiency. This limitation can be
improved by utilizing a three-dimensional substrate in the PCR chamber for uniform photothermal heating of the PCR mixture. Amplification time as well as reagent consumption could be further
reduced, simultaneously improving the efficiency of the PCR reaction by faster molecular diffusion and uniform solution temperature. CONCLUSION In conclusion, we demonstrated a novel
ultrafast photonic PCR through plasmonic photothermal heating of thin Au films driven by LEDs. We designed and fabricated a thin Au film-based light-to-heat converter to heat a PCR solution
over 150 °C by harnessing gold plasmon-assisted high optical absorption. We achieved ultrafast thermal cycling from 55 °C (annealing) to 95 °C (denaturation) within 5 min for 30 cycles with
ultrafast heating (12.79±0.93 °C s−1) and cooling (6.6±0.29 °C s−1) rates. Nucleic acid (λ-DNA) amplification using our ultrafast photonic PCR thermal cycler was successfully demonstrated.
We propose that this simple, robust and low-cost photonic PCR technique with ultrafast thermal cycling capability would be ideal for POC molecular diagnostics, because the photonic PCR
technique can meet the ‘ASSURED’ criteria:27 (i) Affordable: inexpensive system with a LED and lens; (ii) Smaller: compact and light PCR system without a heating block; (iii) Simple step
with disposable PCR chip; (iv) User-friendly interface with an LED driver and display; (v) Rapid and robust PCR without environmental stress; (vi) Equipment-free: consists of only an LED and
microcontroller modules with a cellphone camera; and (vii) Durable in harsh environments and power consumption is low. As the current set-up is based on only one PCR well, future work will
focus on integrating more wells and an LED array to allow high-throughput and multiplexed amplification, as well as optimizing the PCR reaction chamber for uniform heating. REFERENCES *
Heyries KA, Tropini C, VanInsberghe M, Doolin C, Petriv OI _et al_. Megapixel digital PCR. _Nat Methods_ 2011; 8: 649–651. Article Google Scholar * Ottesen EA, Hong JW, Quake SR,
Leadbetter JR . Microfluidic digital PCR enables multigene analysis of individual environmental bacteria. _Science_ 2006; 314: 1464–1467. Article ADS Google Scholar * Barker M . Clever
PCR: more genotyping, smaller volumes. _Nat Methods_ 2010; 7: 351–356. Article Google Scholar * Zhang C, Xing D . Miniaturized PCR chips for nucleic acid amplification and analysis: latest
advances and future trends. _Nucleic Acids Res_ 2007; 35: 4223–4237. Article Google Scholar * Postollec F, Falentin H, Pavan S, Combrisson J, Sohier D . Recent advances in quantitative
PCR (qPCR) applications in food microbiology. _Food Microbiol_ 2011; 28: 848–861. Article Google Scholar * Smith CJ, Osborn AM . Advantages and limitations of quantitative PCR
(Q-PCR)-based approaches in microbial ecology. _FEMS Microbiol Ecol_ 2009; 67: 6–20. Article Google Scholar * Girones R, Ferrús MA, Alonso JL, Rodriguez-Manzano J, Calgua B _et al_.
Molecular detection of pathogens in water—the pros and cons of molecular techniques. _Water Res_ 2010; 44: 4325–4339. Article Google Scholar * Horsman KM, Bienvenue JM, Blasier KR, Landers
JP . Forensic DNA analysis on microfluidic devices: a review. _J Forensic Sci_ 2007; 52: 784–799. Article Google Scholar * Chang CM, Chang WH, Wang CH, Wang JH, Mai JD _et al_. Nucleic
acid amplification using microfluidic systems. _Lab Chip_ 2013; 13: 1225. Article Google Scholar * Lyon E, Wittwer CT . LightCycler technology in molecular diagnostics. _J Mol Diagn_ 2009;
11: 93–101. Article Google Scholar * Liu P, Li X, Greenspoon SA, Scherer JR, Mathies RA . Integrated DNA purification, PCR, sample cleanup, and capillary electrophoresis microchip for
forensic human identification. _Lab Chip_ 2011; 11: 1041–1048. Article Google Scholar * Lagally ET, Emrich CA, Mathies RA . Fully integrated PCR-capillary electrophoresis microsystem for
DNA analysis. _Lab Chip_ 2001; 1: 102–107. Article Google Scholar * Kopp MU, Mello AJ, Manz A . Chemical amplification: continuous-flow PCR on a chip. _Science_ 1998; 280: 1046. Article
ADS Google Scholar * Kim H, Vishniakou S, Faris GW . Petri dish PCR: laser-heated reactions in nanoliter droplet arrays. _Lab Chip_ 2009; 9: 1230–1235. Article Google Scholar * Terazono
H, Hattori A, Takei H, Takeda K, Yasuda K . Development of 1480 nm photothermal high-speed real-time polymerase chain reaction system for rapid nucleotide recognition. _J J Appl Phys_ 2008;
47: 5212–5216. Article ADS Google Scholar * Baffou G, Quidant R . Thermo-plasmonics: using metallic nanostructures as nano-sources of heat. _Laser Photonics Rev_ 2013; 7: 171–187. Article
ADS Google Scholar * Webb JA, Bardhan R . Emerging advances in nanomedicine with engineered gold nanostructures. _Nanoscale_ 2014; 6: 2502. Article ADS Google Scholar * Huang X,
El-Sayed IH, Qian W, El-Sayed MA . Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. _J Am Chem Soc_ 2006; 128: 2115–2120. Article Google
Scholar * Jaque D, Maestro LM, Rosal B, Haro-Gonzalez P, Benayas A _et al_. Nanoparticles for photothermal therapies. _Nanoscale_ 2014; 6: 9494–9530. Article ADS Google Scholar * Roche
PJR, Beitel LK, Khan R, Lumbroso R, Najih M _et al_. Demonstration of a plasmonic thermocycler for the amplification of human androgen receptor DNA. _Analyst_ 2012; 137: 4475–4481. Article
ADS Google Scholar * Johnson PB, Christy RW . Optical constants of noble metals. _Phys Rev B_ 1972; 6: 4370–4379. Article ADS Google Scholar * Carey VP, Chen G, Grigoropoulos C, Kaviany
M, Majumdar A . A review of heat transfer physics. _Nanoscale Microscale Thermophys Eng_ 2008; 12: 1–60. Article ADS Google Scholar * Inouye H, Tanaka K . Ultrafast dynamics of
nonequilibrium electrons in a gold nanoparticle system. _Phys Rev B_ 1998; 57: 11334–11340. Article ADS Google Scholar * Clavero C . Plasmon-induced hot-electron generation at
nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. _Nat Photonics_ 2014; 8: 95–103. Article ADS Google Scholar * Bergman TL, Lavine AS, Incropera FP, DeWitt
DP . _Fundamentals of Heat and Mass Transfer_. 7th ed. New York: John Wiley & Sons; 2011. Google Scholar * Bréchignac C, Houdy P, Lahmani M . _Nanomaterials and Nanochemistry_.
Berlin/New York: Springer; 2007. p208–209. Book Google Scholar * Peeling RW, Mabey D . Point-of-care tests for diagnosing infections in the developing world. _Clin Microbiol Infect_ 2010;
16: 1062–1069. Article Google Scholar Download references ACKNOWLEDGEMENTS This work was supported in part by a grant from the Bill & Melinda Gates Foundation (Global Health Grant:
OPP1028785) and in part by the Global Research Lab Program (2013-050616) through the National Research Foundation of Korea funded by the Ministry of Science, ICT (Information and
Communication Technologies) and Future Planning. We thank Karthik R. Prasad and Nusrat J. Molla for helping with the LabVIEW programming for the photonic PCR thermal cycler. _NOTE: ACCEPTED
ARTICLE PREVIEW ONLINE 29 JUNE 2015_ AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Bioengineering, University of California, Berkeley, 94720, CA, USA Jun Ho Son, Byungrae Cho,
SoonGweon Hong, Sang Hun Lee, Ori Hoxha, Amanda J Haack & Luke P Lee * Berkeley Sensor and Actuator Center, University of California, Berkeley, 94720, CA, USA Jun Ho Son, Byungrae Cho,
SoonGweon Hong, Sang Hun Lee & Luke P Lee * Department of Electrical Engineering and Computer Sciences, University of California, Berkeley, 94720, CA, USA Luke P Lee * Biophysics
Graduate Program, University of California, Berkeley, 94720, CA, USA Luke P Lee Authors * Jun Ho Son View author publications You can also search for this author inPubMed Google Scholar *
Byungrae Cho View author publications You can also search for this author inPubMed Google Scholar * SoonGweon Hong View author publications You can also search for this author inPubMed
Google Scholar * Sang Hun Lee View author publications You can also search for this author inPubMed Google Scholar * Ori Hoxha View author publications You can also search for this author
inPubMed Google Scholar * Amanda J Haack View author publications You can also search for this author inPubMed Google Scholar * Luke P Lee View author publications You can also search for
this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Luke P Lee. ADDITIONAL INFORMATION Note: Supplementary Information for this article can be found on the _Light:
Science & Applications'_ website . SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (DOCX 64445 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons
Attribution 4.0 Unported License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the
credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of
this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Son, J., Cho, B., Hong, S. _et al._ Ultrafast photonic PCR.
_Light Sci Appl_ 4, e280 (2015). https://doi.org/10.1038/lsa.2015.53 Download citation * Received: 17 November 2014 * Revised: 21 January 2015 * Accepted: 28 January 2015 * Published: 31
July 2015 * Issue Date: July 2015 * DOI: https://doi.org/10.1038/lsa.2015.53 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * genomics *
light-emitting diodes (LEDs) * molecular diagnostics * personalized medicine * plasmonics * point-of-care (POC) diagnostics * polymerase chain reaction (PCR)






