- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Access through your institution Buy or subscribe Histiocytic neoplasms are tumors that develop from macrophages and dendritic cells and are also called histiocytes. Histiocytic sarcoma is a
malignant tumor derived from mature histiocytes that are characterized by the expression of one or more histiocytic markers (CD163, CD68 or lysozyme) and the absence of Langerhans cell,
follicular dendritic cell and myeloid cell markers (CD1a, langerin, CD21, CD35, CD33, CD13 and myeloperoxidase).1, 2 Non-Langerhans cell histiocytosis describes a very diverse group of
histiocytic proliferations for which the cells do not meet the criteria for Langerhans cells (CD1a+, Langerin+ and S100+).3 The genetic etiology of these tumors has not been described as
yet, but considering the cell types involved, the presumption is that these tumors derive from a myeloid stem cell. Both histiocytic sarcoma and non-Langerhans cell histiocytosis are very
rare and mostly affect adults. A few pediatric and young adult cases have been described, and in these cases, the histiocytic tumor often co-occurred or developed subsequently to lymphoid
neoplasms such as lymphoma and B-lineage or T-lineage acute lymphoblastic leukemia (T-ALL) (Pagni _et al._,4 Feldman _et al._,5 Kumar _et al._6 and McClure _et al._,7 and references
therein). Hematopoiesis is postulated as the unidirectional maturation of stem cells into lineage-committed cells. However, immunoglobulin and T-cell receptor gene rearrangements (TR
rearrangements) typical for lymphoid cells were also identified in histiocytic tumors (Vos _et al._,2 Pagni _et al._,4 Feldman _et al._,5 Kumar _et al._,6 McClure _et al._7 and Brunner _et
al._,8 and references therein). This apparent lineage switch of lymphoid cells might have occurred through (1) transdifferentiation, defined as the transformation of one cell type into
another without passing an intermediate pluripotent state or progenitor cell type, or (2) de-differentiation, defined as the transformation via an intermediate pluripotent state or
progenitor cell type. Alternatively, these tumors may have developed from a multipotent stem cell that is capable of differentiating into both myeloid and lymphoid precursors.9, 10, 11 In
this letter, we describe a child with three tumors from two different lineages in which genetic analysis shows that the tumors were derived from a common precursor cell showing lineage
plasticity for both lymphoid and myeloid development. We isolated genomic DNA from skin fibroblasts, from a bone marrow sample taken at the time of T-ALL diagnosis and from biopsy specimens
from the non-Langerhans cell histiocytosis and the histiocytic sarcoma, after informed consent and approval by the medical ethics committee/institutional board (CMO, study 2012/271) of the
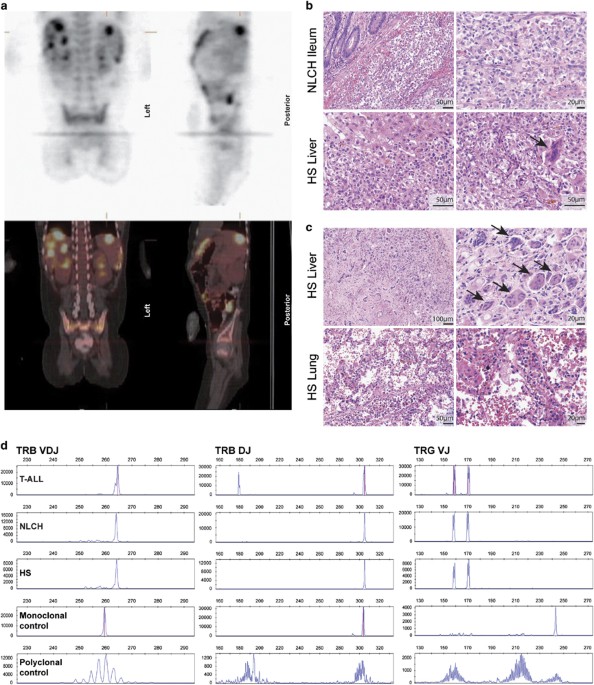
Radboudumc. To investigate the clonal relationship between the malignancies, we determined the TR gene rearrangements (Supplementary Methods). All samples showed a VDJ-rearranged TRB gene,
an incomplete DJ TRB gene rearrangement and two (biallelic) VJ-rearranged TRG genes (Figure 1d). The four clonal rearrangements were of identical size in the three specimens, which suggests
that the three tumors originate from the same precursor cell. This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe
to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF
Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact
customer support REFERENCES * Pileri SA, Grogan TM, Harris NL, Banks P, Campo E, Chan JK _et al_. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to
classification from the International Lymphoma Study Group based on 61 cases. _Histopathology_ 2002; 41: 1–29. Article CAS Google Scholar * Vos JA, Abbondanzo SL, Barekman CL, Andriko JW,
Miettinen M, Aguilera NS . Histiocytic sarcoma: a study of five cases including the histiocyte marker CD163. _Mod Pathol_ 2005; 18: 693–704. Article Google Scholar * Weitzman S, Jaffe R .
Uncommon histiocytic disorders: the non-Langerhans cell histiocytoses. _Pediatr Blood Cancer_ 2005; 45: 256–264. Article Google Scholar * Pagni F, Fazio G, Zannella S, Spinelli M, De
Angelis C, Cusi C _et al_. The role of PAX5 and C/EBP alpha/beta in atypical non-Langerhans cell histiocytic tumor post acute lymphoblastic leukemia. _Leukemia_ 2014; 28: 1377–1379. Article
CAS Google Scholar * Feldman AL, Minniti C, Santi M, Downing JR, Raffeld M, Jaffe ES . Histiocytic sarcoma after acute lymphoblastic leukaemia: a common clonal origin. _Lancet Oncol_
2004; 5: 248–250. Article Google Scholar * Kumar R, Khan SP, Joshi DD, Shaw GR, Ketterling RP, Feldman AL . Pediatric histiocytic sarcoma clonally related to precursor B-cell acute
lymphoblastic leukemia with homozygous deletion of CDKN2A encoding p16INK4A. _Pediatr Blood Cancer_ 2011; 56: 307–310. Article Google Scholar * McClure R, Khoury J, Feldman A, Ketterling R
. Clonal relationship between precursor B-cell acute lymphoblastic leukemia and histiocytic sarcoma: a case report and discussion in the context of similar cases. _Leuk Res_ 2010; 34:
e71–e73. Article Google Scholar * Brunner P, Rufle A, Dirnhofer S, Lohri A, Willi N, Cathomas G _et al_. Follicular lymphoma transformation into histiocytic sarcoma: indications for a
common neoplastic progenitor. _Leukemia_ 2014; 28: 1937–1940. Article CAS Google Scholar * Dorantes-Acosta E, Pelayo R . Lineage switching in acute leukemias: a consequence of stem cell
plasticity? _Bone Marrow Res_ 2012; 2012: 406796. Article Google Scholar * Kawamoto H, Ikawa T, Masuda K, Wada H, Katsura Y . A map for lineage restriction of progenitors during
hematopoiesis: the essence of the myeloid-based model. _Immunol Rev_ 2010; 238: 23–36. Article CAS Google Scholar * Slamova L, Starkova J, Fronkova E, Zaliova M, Reznickova L, van Delft
FW _et al_. CD2-positive B-cell precursor acute lymphoblastic leukemia with an early switch to the monocytic lineage. _Leukemia_ 2014; 28: 609–620. Article CAS Google Scholar * Cobaleda
C, Jochum W, Busslinger M . Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. _Nature_ 2007; 449: 473–477. Article CAS Google Scholar * Xie H, Ye
M, Feng R, Graf T . Stepwise reprogramming of B cells into macrophages. _Cell_ 2004; 117: 663–676. Article CAS Google Scholar * Yu D, Allman D, Goldschmidt MH, Atchison ML, Monroe JG,
Thomas-Tikhonenko A . Oscillation between B-lymphoid and myeloid lineages in Myc-induced hematopoietic tumors following spontaneous silencing/reactivation of the EBF/Pax5 pathway. _Blood_
2003; 101: 1950–1955. Article CAS Google Scholar * Carrasco DR, Fenton T, Sukhdeo K, Protopopova M, Enos M, You MJ _et al_. The PTEN and INK4A/ARF tumor suppressors maintain myelolymphoid
homeostasis and cooperate to constrain histiocytic sarcoma development in humans. _Cancer Cell_ 2006; 9: 379–390. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We
acknowledge Prof Dr Jacques van Dongen and Prof Dr Robert Arceci for their expert advice on the treatment of this patient. We thank Dr Charles Mullighan for helpful discussions and Ingrid
Vogelaar, Christian Gilissen and Eugène Verwiel for technical assistance. We thank the members of the Genomic Disorders Group Nijmegen and the Radboud Genomics Technology Center for their
technical support. EW is a KWF fellow from the Dutch Cancer Society (KUN2012-5366), AH is supported by the Netherlands Organization for Health Research and Development (ZonMW 916-12-095) and
RPK is funded by Stichting Kinderen Kankervrij (KiKa project 150). AUTHOR INFORMATION Author notes * R P Kuiper and D M W M te Loo: These authors contributed equally to this work. AUTHORS
AND AFFILIATIONS * Department of Human Genetics, Radboud University Medical Center and Radboud Institute for Molecular Life Sciences, Nijmegen, The Netherlands E Waanders, E J Kamping, A
Simons, A Hoischen, M C J Jongmans & R P Kuiper * Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands K M Hebeda & P J T A Groenen * Princess Máxima
Center for Pediatric Oncology, De Bilt, The Netherlands P M Hoogerbrugge * Department of Pediatric Oncology, Radboud University Medical Center and Radboud Institute for Molecular Life
Sciences, Nijmegen, The Netherlands F N van Leeuwen & D M W M te Loo Authors * E Waanders View author publications You can also search for this author inPubMed Google Scholar * K M
Hebeda View author publications You can also search for this author inPubMed Google Scholar * E J Kamping View author publications You can also search for this author inPubMed Google Scholar
* P J T A Groenen View author publications You can also search for this author inPubMed Google Scholar * A Simons View author publications You can also search for this author inPubMed
Google Scholar * A Hoischen View author publications You can also search for this author inPubMed Google Scholar * M C J Jongmans View author publications You can also search for this author
inPubMed Google Scholar * P M Hoogerbrugge View author publications You can also search for this author inPubMed Google Scholar * F N van Leeuwen View author publications You can also
search for this author inPubMed Google Scholar * R P Kuiper View author publications You can also search for this author inPubMed Google Scholar * D M W M te Loo View author publications You
can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to D M W M te Loo. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of
interest. ADDITIONAL INFORMATION Supplementary Information accompanies this paper on the Leukemia website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (DOC 629 KB) RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Waanders, E., Hebeda, K., Kamping, E. _et al._ Independent development of lymphoid and histiocytic malignancies from
a shared early precursor. _Leukemia_ 30, 955–958 (2016). https://doi.org/10.1038/leu.2015.193 Download citation * Published: 23 July 2015 * Issue Date: April 2016 * DOI:
https://doi.org/10.1038/leu.2015.193 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative