- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
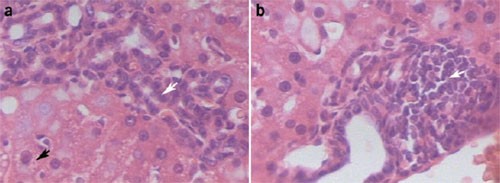
Access through your institution Buy or subscribe We sent a 500-g sample of feed pooled from different bags of the same batch to an external laboratory for mycotoxin analysis. Two different
toxins, aflatoxin B1 (AB1) at 10 ppb and fusarenon-X at 500 ppb, were identified in the feed sample (Table 2). Several mold species constitute the normal microflora of agricultural crops and
products. Molds may produce toxic metabolites called mycotoxins; once formed, mycotoxins persist in the product even if the molds are eliminated. Heat treatment of animal feed during
pelleting is not sufficient to degrade mycotoxins because they are relatively resistant to heat2. Aflatoxins are a family of mycotoxins generated by _Aspergillus flavus_, _A. parasiticus_
and _A. nomius_. Of the 18 varieties of aflatoxins, AB1 and aflatoxin M1 (a metabolic derivative of AB1 present in milk and urine) are the most toxic3. AB1 is metabolized in the liver,
producing highly reactive chemical intermediaries, which bind to DNA and lead to abnormal cell proliferation, mutagenesis and carcinogenesis4,5,6. Additionally, chronic low levels of AB1
cause growth and reproductive problems in cattle and poultry3. Fusarenon-X is a mycotoxin produced by _Fusarium nivale_ and _F. episharia_. We know of no studies describing the effects of
low levels of fusarenon-X in laboratory animals. This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution ADDITIONAL ACCESS
OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support REFERENCES * Servicio Nacional de Sanidad y Calidad Agroalimentaria (National Service
of Health and Quality Food of Argentina), Resolución 617/02. Requisitos, condiciones y procedimientos para la habilitación técnica de laboratorios que posean bioterios de producción,
mantenimiento y local de experimentación. Informe de ensayo de residuos de productos fitosanitarios en matrices vegetales. Boletín Oficial Julio-Agosto 2002. * Joint FAO/WHO Expert Committee
on Food Additives, World Health Organization, International Program on Chemical Safety. _Safety Evaluation of Certain Mycotoxins in Food_. (World Health Organization; Geneva; 2001). *
Gimeno, A. & Martins, M.L.M. _Mycotoxins and Mycotoxicosis in Animals and Humans_ (Special Nutrients; Miami, FL; 2006). Google Scholar * Hussein, H.S. & Brasel, J.M. Toxicity,
metabolism, and impact of mycotoxins on humans and animals. _Toxicology_ 167, 101–134 (2001). Article CAS Google Scholar * Mace, K. et al. Aflatoxin B1-induced DNA adduct formation and
p53 mutations in CYP450-expressing human liver cell lines. _Carcinogenesis_ 18, 1291–1297 (1997). Article CAS Google Scholar * Theumer, M.G. et al. Immunobiological effects of AFB1 and
AFB1-FB1 mixture in experimental subchronic mycotoxicoses in rats. _Toxicology_ 186, 159–170 (2003). Article CAS Google Scholar * Eaton, D.L. & Gallagher, E.P. Mechanisms of aflatoxin
carcinogenesis. _Annu. Rev. Pharmacol. Toxicol._ 34, 135–172 (1994). Article CAS Google Scholar * Feldsine, P., Abeyta, C. & Andrews, W.H. AOAC International methods committee
guidelines for validation of qualitative and quantitative food microbiological official methods of analysis. _J. AOAC Int._ 85, 1187–1200 (2002). CAS PubMed Google Scholar * Trucksess,
M.W. Committee on Natural Toxins and Food Allergens. Mycotoxins. _J. AOAC Int._ 88, 314–324 (2005). CAS PubMed Google Scholar * Anonymous. General considerations for feeding and diet
formulation. in: _Nutrient Requirements of Laboratory Animals_ 4th edn. 3–10 (National Academy of Science; Washington, DC; 1995). * Wangikar, P.B. et al. Effect in rats of simultaneous
prenatal exposure to ochratoxin A and aflatoxin B1. II. Histopathological features of teratological anomalies induced in fetuses. _Birth Defects Res. B Dev. Reprod. Toxicol._ 71, 352–358
(2004). Article CAS Google Scholar * Wangikar, P.B., Dwivedi, P. & Sinha, N. Effect in rats of simultaneous prenatal exposure to ochratoxin A and aflatoxin B1. I. Maternal toxicity
and fetal malformations. _Birth Defects Res. B Dev. Reprod. Toxicol._ 71, 343–351 (2004). Article CAS Google Scholar * Casado, J.M. et al. Experimental subchronic mycotoxicoses in mice:
individual and combined effects of dietary exposure to fumonisins and aflatoxin B1. _Food Chem. Toxicol._ 39, 579–586 (2001). Article CAS Google Scholar * Kelly, W.R. The liver and
biliary system. in: _Pathology of Domestic Animals_ 4th edn. (Jubb, K.V.F., Kennedy, P.C. & Palmer, N., eds.) 339 (Academic; San Diego; 1993). Google Scholar * Hesseltine, C.W.
Conditions leading to mycotoxin contamination of food feeds. in: _Mycotoxins and Other Fungal Related Food Problems: A Symposium_ (Rodricks, J.V., ed.) 1–22 (American Chemical Society;
Washington, DC; 1976). Google Scholar * Waldemarson, A.H. et al. Mycotoxins in laboratory rodent feed. _Lab. Anim._ 39, 230–235 (2005). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We thank Gema Biotech for technical assistance; A. Gimeno, C.C. García Bonelli, M. Cereseto and P. Halperín for their interesting comments; ININFA husbandry staff M.
Voeffray, A. Arín and M.A. Cáceres for their constant commitment to the management of animal facilities; and Dr. E. Adler and Dr. M.C. Rubio for allocating sufficient funds and for their
generous support. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Diagnosis | Mycotoxin poisoning. _Lab Anim_ 37, 154–155 (2008).
https://doi.org/10.1038/laban0408-154 Download citation * Issue Date: April 2008 * DOI: https://doi.org/10.1038/laban0408-154 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative







