- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT HT61 and chlorhexidine (CHX) are both putative membrane-active antimicrobials, which non-specifically target the anionic lipids abundant in bacterial membranes. In model systems,
the ability of these antimicrobials to partition into lipid monolayers and increase the permeability of lipid bilayers is dependent upon the presence and proportion of anionic lipids such as
phosphatidylglycerol. Despite their apparent similarity in membrane affinity, we have found that HT61 and CHX differ in the extent to which they affect membrane integrity. HT61 was found to
be capable of severely disrupting the lipid bilayer, resulting in lysis of _Staphylococcus aureus_ membranes and the release of ATP from protoplasts. CHX, by contrast, does not disrupt the
lipid bilayer to a sufficiently large degree to result in lysis of the membrane or release of ATP from _S. aureus_ protoplasts. This suggests that although antimicrobials that interact with
the membrane often have a common target, the action they have on the membrane may differ widely and may not be the primary mode of action of the antimicrobial. SIMILAR CONTENT BEING VIEWED
BY OTHERS LATERAL MEMBRANE ORGANIZATION AS TARGET OF AN ANTIMICROBIAL PEPTIDOMIMETIC COMPOUND Article Open access 07 July 2023 OUTER MEMBRANE AND PHOSPHOLIPID COMPOSITION OF THE TARGET
MEMBRANE AFFECT THE ANTIMICROBIAL POTENTIAL OF FIRST- AND SECOND-GENERATION LIPOPHOSPHONOXINS Article Open access 17 May 2021 CHEMICALLY DIVERSE ANTIMICROBIAL PEPTIDES INDUCE
HYPERPOLARIZATION OF THE _E. COLI_ MEMBRANE Article Open access 05 October 2024 INTRODUCTION Bacterial membranes constitute a good target for antimicrobial action as their integrity is
essential for the survival of both multiplying and non-multiplying bacteria. The presence of anionic lipids such as phosphatidylglycerol (PG) and the zwitterionic phosphatidylethanolamine,1
which are not usually found on the outer surface of mammalian cells, represents the basis for the selectivity of antimicrobials for these bacterial membrane targets.2 Indeed, several
cationic membrane-active antimicrobials have been shown to exhibit non-specific affinity for anionic lipids.3, 4, 5, 6 The disruption to the cytoplasmic membrane leads to increased
permeability, depolarization, leakage of intracellular components and cell death.1, 3, 7, 8, 9, 10 However, the ability for membrane-active antimicrobials to interact with the membrane also
depends on the physiochemical properties of a lipid bilayer, which can alter due to the properties of the different constituent lipids, including lipid charge, head group size, tail chain
length and degree of saturation11, 12 and directly affect the lipid packing and overall charge of the membrane.11, 12 For example, the cytoplasmic membrane of _Staphylococcus aureus_ is
fairly unique in that it contains negligible amounts of zwitterionic phospholipids, but instead is comprised of a mixture of PG, cardiolipin (CL) and the cationic lipid
lysyl-phosphatidylglycerol (lysyl-PG).13, 14 Changes in the ratios of these lipids within the cytoplasmic membrane can result in a degree of resistance to membrane-active antimicrobials.15,
16, 17, 18, 19 The abundance of PG within the _S. aureus_ membrane means that cationic membrane-acting antimicrobials are particularly active towards this pathogen.3, 7, 8, 9, 20, 21 There
are a number of marketed and developmental membrane-active antimicrobials including daptomycin,10 telavancin8 and chlorhexidine (CHX).3, 7 These membrane-active antimicrobials have rapid
bactericidal activity and have been shown to facilitate perturbation of cytoplasmic membranes, as daptomycin does.22 Many of these antimicrobials that are also active against the cytoplasmic
membrane are known to be active against non-multiplying bacteria,3, 21, 23 referred to as persister cells, which have been implicated as the cause of recurrent infections which most
conventional antimicrobials that inhibit specific metabolic processes are not active against.24, 25 It has been observed for a number of membrane-active antimicrobials that it is difficult
to produce bacteria resistant to these antimicrobials _in vitro_, a phenomenon which is attributed to their rapid bactericidal activity and their non-specificity due to the complexity of the
lipid bilayer.26, 27 This apparent lack of resistance has led to membrane-active antimicrobials being an attractive prospect for future drug development. However, resistant strains of _S.
aureus_ and _Enterococci_ have been described for the membrane-active antibiotic, daptomycin,15, 17, 26, 28 and there is also intrinsic resistance to cationic membrane-active antimicrobials
seen in Gram-negative bacteria due to the outer membrane and Gram-positive bacteria that lack a high abundance of anionic lipids in the cytoplasmic membrane.29 The mode of action of CHX has
long been suggested to be the disruption of the membrane,3, 7 however this is only at low concentrations and following relatively long exposure30 and at higher concentrations CHX is
bactericidal and causes the precipitation of the cytoplasm31 meaning the primary mode of action may actually be difficult to pinpoint. Both HT61 and CHX are active against methicillin
sensitive and resistant _S. aureus_ membranes32 and unlike membrane-active antimicrobial peptides, are small molecules. A recent study into the mode of action of HT61, which is currently in
efficacy clinical trials, highlighted that these two compounds resulted in a similar degree of membrane depolarization and release of ATP from _S. aureus_,3 despite a difference in the
structures of the compounds. However some differences between the two compounds were evident, although CHX has a lower minimum inhibitory concentration toward _S. aureus_ (minimum inhibitory
concentration 4 μg ml−1) than HT61 (8 μg ml−1) it was clear that HT61 was more active toward the bacterial membranes than CHX.3 Therefore, the effect that HT61 and CHX have on the membrane,
and the overall mode of action, may in fact be quite different. In this study, using a mixture of microbiological and biophysical methods, we compared the action of HT61 and CHX and the
extent of which they interact with and affect natural _S. aureus_ plasma membranes and simple _S. aureus_-mimetic model membranes, in order to gain a greater insight into how these two
antimicrobials actually interact with lipid bilayers. Our model _S. aureus_ membrane systems required a substitute for the alkali-labile lysyl-PG13 and therefore contained the more stable
cationic lipid 1-palmitoyl-2-oleoyl-_sn_-glycero-3-ethylphosphocholine (POePC) in various mixtures with 1-palmitoyl-2-oleoyl-_sn_-glycero-3-phospho-(1'-_rac_-glycerol) (POPG), to
simulate the natural lipid mixtures in the model monolayers and bilayers. MATERIALS AND METHODS MATERIALS CHX diacetate (Sigma, Poole, Dorset, UK) and HT61 mesylate (Helperby Therapeutics,
London, UK) were both used as supplied and diluted in ultrapure water obtained from a Milli-Q 16 ultrapure water system (Merck Millipore, Billerica, MA, USA) with a specific resistivity of
18.2 MΩ cm−1 to a stock concertation of 10 g l−1. Penicillin G (Sigma) was diluted to a stock concentration of 2 g l−1 in ultrapure water. Brain heart infusion and nutrient broth were
obtained from Oxoid (Basingstoke, Hampshire, UK). Chloroform, 5(6)-carboxyfluorescein, HEPES, glucose, PBS, Tris base, acetic acid, sucrose, sodium chloride, sodium deoxycholate, lysozyme,
lysostaphin, dimethyl sulfoxide, bovine pancreatic DNase and Triton X-100 were all obtained from Sigma Aldrich (Poole, Dorset, UK). Propidium iodide (PI) and SYTO 9 were obtained from
Invitrogen (Paisley, UK). The lipids POPG and POePC were obtained from Avanti Polar Lipids (Alabaster, AL, USA). BACTERICIDAL ASSAY A culture of Oxford _S. aureus_ (NCTC 6571) was grown at
37 °C in nutrient broth at 100 r.p.m. for 18 h and then centrifuged at 10 000 × _g_ for 10 min, the pellet was resuspended in 1 ml of HEPES buffer (5 mM HEPES, 5 mM glucose, pH 7.2) and
diluted to 0.2 OD600. Separately, CHX, HT61, penicillin G or ultrapure water (negative control) were added to 1 ml of the culture to give final concentrations of 16 μg ml−1, and incubated at
25 °C for 20 min. PI and SYTO 9 from the LIVE/DEAD BacLight Bacterial Viability Kit (Invitrogen, Paisley, UK) were mixed together in equal amounts, 3 μl of this dye mixture was added to the
treated culture and incubated in the dark for 15 min. Using a microscope slide, 5 μl of this sample was added under a cover slip and fixed using lacquer. The cells were visualized using a
Zeiss Axiovert 200M inverted microscope (Zeiss, Cambridge, UK) using the specific emission/excitation wavelengths required for each dye; 488/506 nm (SYTO 9) and 538/619 nm (PI). PROTOPLAST
PREPARATION A 200 ml culture of _S. aureus_ was grown for 18 h at 37 °C, shaken continuously at 100 r.p.m. in brain heart infusion broth. The culture was diluted in brain heart infusion
broth to an OD660 of 0.66, and 200 ml of the culture was centrifuged at 10 000 × _g_ for 20 min at 4 °C, washed once in 50 mM Tris-acetic acid (hypotonic buffer) and resuspended in 10 ml of
1 m sucrose, 50 mM Tris-acetic acid (hypertonic buffer) to prevent lysis of the protoplasts due to osmotic pressure. Using 1 ml of the culture, 200 μg ml−1 lysozyme, 100 μg ml−1 lysostaphin
and 25 μg ml−1 bovine pancreatic DNase were added and incubated for 1 h at 37 °C, shaken at 100 r.p.m.. The samples were centrifuged at 10 000 _g_ for 10 min and washed with hypertonic
buffer prior to use in the protoplast lysis and ATP release assays. A Gram stain was performed on the protoplasts to confirm that they were lacking a cell wall. PROTOPLAST LYSIS ASSAY
Protoplasts were diluted in hypertonic buffer to an OD600 of between 0.22 and 0.23, 270 μl of which was added to a 96-well microplate and the OD at 600 nm was measured on a GloMax+
microplate reader (Promega, Southampton, UK) to prevent cell death due to UV exposure. Using a separate 96-well microplate, the OD600 of 270 μl of the hypertonic buffer was measured to give
the background reading at time zero. To the same plate, 270 μl of hypertonic buffer, 30 μl of CHX, HT61 and penicillin G (final concentration of 16 μg ml−1) and Triton X-100 (5% v/v) were
added separately and the OD600 was measured to give background readings. To a separate well, 30 μl of ultrapure water was also added to serve as a negative control. To the 270 μl of
protoplast culture, 30 μl of each HT61, CHX and Triton X-100 were added in triplicate and incubated at 25 °C for 20 min before the OD600 of each well was measured using a GloMax+ microplate
reader. ATP RELEASE FROM PROTOPLASTS Release of ATP from protoplasts was used as an indication of change in loss of membrane integrity. CHX, HT61 and penicillin G were added to 0.5 ml 0.2
OD600 protoplast samples to give final concentrations of 16 μg ml−1 and Triton X-100 added separately to give a final concentration of 5% (v/v) and the amount of ATP released from
protoplasts was analyzed as previously described.3 LIPID MONOLAYER PARTITIONING The ability of the drugs to partition into _S. aureus_ membranes was assessed using biomimetic synthetic lipid
monolayers with molar ratios of 45:55, 40:60 and 35:65 of POePC and POPG (Avanti Lipids, Alabaster, AL, USA). This was performed as previously described.3 The surface pressure was continual
recorded for up to 1600s and the maximum change in surface pressure over this time was recorded to directly compare the degree of partitioning into the monolayer. LIPOSOME PREPARATION POePC
and POPG were combined at molar ratios of 35:65, 40:60 and 45:55, to create _S. aureus_ lipid membrane mimetic liposomes. The lipid mixtures with a total mass of 10 mg ml−1 were dissolved
in chloroform in order to form a thin film by evaporating the solvent under vacuum using a Rotorvapor RII (Buchi, Flawil, Switzerland) and an N820 FT.18 Laboport diaphragm pump (KNF,
Cambridge, UK). To each resultant lipid film, 2.5 ml of a 40 mM 5(6) carboxyfluorescein dye solution (osmolality 140 mOsmol Kg−1) was added and vortex mixed for 30 seconds to produce a
turbid liposome dispersion, before being sonicated using a Labsonic L ultrasonicator (Sartorius BBI Systems GmbH, Melsungen, Germany) until the dispersion became clear, after which it was
allowed to anneal at 25 °C for at least 20 min. Unentrapped dye was separated from the liposomes using a PD-10 size-exclusion column (GE Healthcare, Little Chalfont, UK) which was
equilibrated with three 5 ml washes with 70 mM sodium chloride (osmolality 140 mOsmol Kg−1) and 1 ml of the liposome mixture was eluted through the column and washed through with 5 × 1 ml
aliquots of 70 mM sodium chloride and the eluted fractions were collected, fraction 4 was used in the dye release assay. DYE RELEASE FROM LIPOSOMES Drug-induced release of carboxyfluorescein
from liposomes indicates an ability to severely disrupt or lyse the lipid bilayer. In each sample, 50 μl of liposomes were added to 2350 μl of 70 mM sodium chloride in a 10 × 10 mm
pathlength optical polystyrene macro fluorescence cuvette (Kartell Labware, Milan, Italy), fluorescence was monitored using a Cary Eclipse fluorimeter (Varian Ltd, Walton-on-Thames, UK)
using excitation and emission wavelengths of 490 and 510 nm, respectively, with excitation and emission slit widths of 2.5 nm. After 50 s, 100 μl of either HT61 or CHX, diluted in 70 mM
sodium chloride, was injected (to give a final concentration of 9 μg ml−1) and data were collected for another 500 s. After a total time of 550 s, 100 μl of a 5% w/v sodium deoxycholate
solution was added to lyse any intact liposomes. Using the maximum fluorescence intensity following addition of sodium deoxycholate, the percentage dye release caused by the antimicrobials
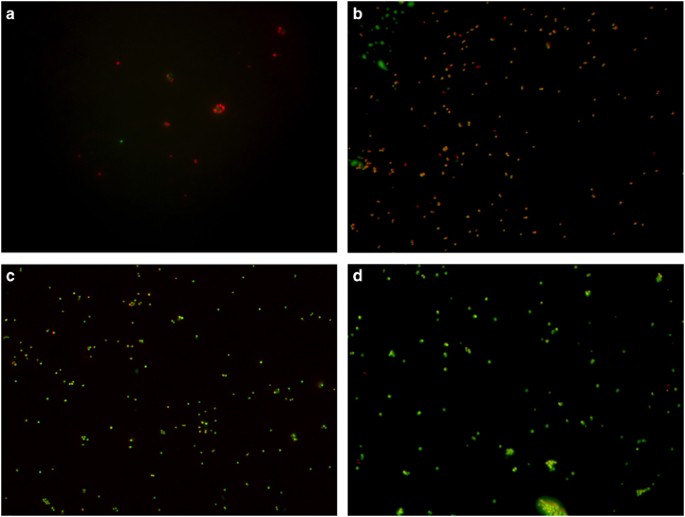
was calculated. An increase in fluorescence intensity over time indicated disruption to the bilayer of the liposome caused by the antimicrobial. RESULTS BACTERICIDAL ASSAY The BacLight assay
employs two nucleic acid dyes to detect the loss of membrane integrity. SYTO 9 can pass across the membrane to enter living cells and intercalate with DNA, emitting a green fluorescence,
whereas PI can only enter cells and intercalate with DNA with a damaged membrane, emitting a red fluorescence.8, 9, 33, 34 Therefore, penicillin G (Figure 1c), a cell wall synthesis
inhibitor added at concentrations eight times the minimum inhibitory concentration3, 35, 36 did not damage or increase membrane permeability as penicillin G mediated lysis should not occur
during the time scale of this experiment, resulting in the visible green fluorescence of SYTO 9. It is likely that CHX (Figure 1b) and HT61 (Figure 1a) both disrupt the membranes of _S.
aureus_ to a certain degree at a concentration of 16 μg ml−1 to allow the influx of PI, but there were also a number of cells that remained with intact membranes, so clearly this effect is
not comprehensive. Interestingly, the orange fluorescence seen following CHX exposure is due to both SYTO 9 and PI binding to the nucleic acid, indicating that although CHX causes some
degree of damage to the membranes, it is to a lesser extent than HT61 as PI is unable to fully displace or quench SYTO 9.33, 34 The few visible cells present in the sample treated with HT61,
in contrast to the large number of cells present following CHX exposure, could indicate a potential lytic activity, but this could not be determined from this data set alone. PROTOPLAST
LYSIS AND ATP RELEASE Direct membrane damage, rather than a by-product of inhibition of cell wall synthesis, can be measured through the lysis of bacterial protoplasts and is a good
indicator of the extent of solubilization of the membrane.20, 37 Penicillin G treatment was able to lyse a small percentage of protoplasts (Figure 2) which is likely to be due to localized
osmotic effect following the addition of the antimicrobial to the sample. However, penicillin G could not induce release of ATP (Figure 3) during the time frame of the assay indicating it
has no direct lytic activity. As expected, the detergent Triton X-100, which can solubilize membranes, was capable of significant lysis (95%) and of inducing the release of 94% of ATP from
the protoplasts. Despite being able to increase the permeability of _S. aureus_ membranes, CHX did not lyse the protoplast membranes or induce release of ATP (Figures 2 and 3), suggesting
that CHX is not capable of lysing _S. aureus_ membranes. In contrast, HT61 did have a solubilising effect on protoplast membranes, but much reduced compared with Triton X-100, only lysing
~25% (Figure 2) and inducing the release of approximately 20% of ATP (Figure 3). With respect to statistical analysis, performed using the paired two-sample _t_-test function in Microsoft
Excel (2016),38 which is relevant for small data sets if normality of the data is assumed,39 a significant difference was found between the degree of protoplast lysis (_P_=0.03) and ATP
release data (_P_=0.03) elicited by HT61 and CHX. Thus, HT61 demonstrated a significantly greater ability to lyse the protoplasts than CHX, suggesting an obvious difference in the effect of
the two antimicrobials on the _S. aureus_ membrane and the amount of resulting damage. It is clear from these results that the modes of action of CHX and HT61 deviate in terms of the degree
of damage they can cause to the _S. aureus_ plasma membrane. LIPID MONOLAYER PARTITIONING To examine the membrane activities of both HT61 and CHX in more detail, we investigated the
interaction and partitioning into air/liquid interface monolayers containing three different molar ratios of POePC and POPG, 35:65, 40:60 and 45:55 to mimic the _S. aureus_ membrane
composition under different conditions. The cationic antimicrobials HT61 and CHX both noticeably interact electrostatically with the anionic lipids within the monolayer, as is evident by the
direct correlation between the increase in maximum change in surface pressure and increase in %PG in the monolayer (Figure 4). Interestingly, the affinity to the monolayer and the
subsequent partitioning by HT61 and CHX are also very similar, resulting in maximum changes in surface pressure of 15.8 and 14.6 mN m−1, respectively, in monolayers with 65% PG content
(Figure 4). The interaction and partitioning of HT61 and CHX was reduced as the %PG content decreased, resulting in the lowest maximum changes in surface pressure of 4.8 and 6.4 mN m−1,
respectively, at 55% PG. The degree of interactions of HT61 and CHX with the monolayer are of a comparable magnitude and, using the paired two-sample _t_-test function in Microsoft Excel
(2016),38 overall there is no significant difference between the action of the two antimicrobials on the 65% PG monolayer (_P_=0.21). In the case of the 60% PG monolayer, there is a small
increase in HT61 partitioning, over that of CHX, which is statistically significant (_P_=0.01). The reverse of this results was observed for the 55% PG monolayer, whereby CHX demonstrated a
small but significant (_P_=0.005) increase in partitioning over that of HT61. These fluctuations suggest that the two antimicrobials may undergo a similar initial interaction with the
monolayer, resulting in a broadly comparable degree of partitioning into the monolayer. DYE RELEASE FROM SYNTHETIC LIPOSOMES HT61 and CHX’s ability to disrupt the lipid bilayer sufficiently
to cause leakage, was investigated using liposomes composed of the same molar ratios of POePC and POPG (35:65, 40:60 and 45:55) as were used to assess lipid monolayer partitioning. Despite
the fact that both HT61 and CHX were capable of interacting and partitioning into a lipid monolayer to the same degree, as well as increasing the permeability of the cytoplasmic membrane in
protoplasts, only HT61 demonstrated lytic activity towards the liposomes (Figure 5). In line with the protoplast lysis assay, HT61 was capable of disrupting the lipid bilayer to a large
enough degree to result in the release of 19% of the entrapped carboxyfluorescein from liposomes containing 65% PG (Figure 5). As previously shown in the lipid partitioning assay, HT61’s
activity against the bilayer was reduced as the content of %PG within the bilayer also deceased, only inducing the release of 13% and 16% of the entrapped dye at 55% and 60% PG, respectively
(Figure 5). CHX’s activity on the lipid bilayer was much reduced compared with that of HT61. At the highest %PG, CHX could only induce 4% carboxyfluorescein leakage from the liposomes, a
quarter of what was released following challenge with HT61 (Figure 5). This slight affect CHX had on the 65% PG lipid bilayers was further attenuated when the %PG within the bilayer was also
reduced, resulting in the release of 1 and 2% entrapped dye from liposomes containing 55% and 60% PG, respectively (Figure 5). DISCUSSION In recent years, a number of antimicrobials that
target and disrupt the bacterial cytoplasmic membrane have been investigated1, 7, 8, 9, 10, 23 with several, such as daptomycin,10 coming to market and being routinely used to treat invasive
bacterial infections. HT61 and CHX have both previously been shown to be active against the bacterial cytoplasmic membrane,3 non-specifically targeting anionic lipids, disrupting the
membrane to a degree that results in the release of the constituents of the cytoplasm, such as ATP and K+.3, 7 However, it is clear from a previous study into the mode of action of HT613
there is a noticeable difference in membrane activity of the two antimicrobials, as their minimum inhibitory concentrations and relative effects on _S. aureus_ membranes did not correlate as
expected. The fact that both HT61 and CHX interact with the cytoplasmic membrane is not in dispute, this study and studies before it quite clearly show that HT61 and CHX electrostatically
interact with anionic lipids that are present in the membrane, resulting in bilayer partitioning and increased permeability. This interaction increases as the content of the anionic lipid
increases, confirming the specificity of the antimicrobials toward bacterial membranes. This type of activity is characteristic of many different membrane-active antimicrobials, such as
daptomycin.8, 9, 10 However, what is less apparent is whether this measurable membrane interaction is the primary mode of action of both HT61 and CHX, as has previously been proposed.3 In
the experiments we have conducted, the liposomes and protoplasts used were dispersed in isosmotic buffers to prevent osmotic lysis prior to challenge with the antimicrobials. This meant that
any ATP release or lysis of _S. aureus_ protoplasts, or release of carboxyfluorescein from liposomes could only be due to a disruptive lytic effect rather than purely as by-product of the
partitioning of antimicrobial into the bilayer. HT61 clearly has some lytic effect on the bacterial membrane, specifically in this case that of _S. aureus_, as it induced lysis of protoplast
membranes and a significant release of ATP. HT61 was also able to induce the release of carboxyfluorescein from liposomes made to represent a simple model of _S. aureus_ lipid membranes.
This suggests that HT61’s action on the membrane may be comparable to a weak detergent and confirms HT61 as a membrane-acting antimicrobial and provide an explanation for HT61’s high
activity against non-multiplying _S. aureus_,27 although these data do not prove that HT61's primary mode of action is on the membrane. A previously study found that there is no
difference in PG, cardiolipin or lysyl-PG content in methicillin-resistant and sensitive strains of _S. aureus_ membranes40 and, as HT61 is actually more active against methicillin-resistant
_S. aureus_ than the methicillin sensitive Oxford strain of _S. aureus_,27 it seems reasonable to assume that HT61 would have the same action on methicillin-resistant _S. aureus_ membranes
as were observed in this study. CHX did not lyse the _S. aureus_ protoplast membranes or result in the release of ATP and did not induce significant release of carboxyfluorescein from
liposomes. This also raises questions about whether the primary mode of action of CHX is indeed activity against the bacterial membrane or whether there is also an internal target. It has
previously been suggested that CHX causes cytoplasmic precipitation and is bactericidal at high concentrations and affects the membrane and is bacteriostatic at low concentrations,31 but
this has recently been superseded by the proposal that the primary mode of action is at the membrane due to the overwhelming evidence of membrane activity.3, 7 However, it seems that osmotic
pressure in assays and systems that measure membrane activity may enhance the activity of membrane-active antimicrobials and could therefore mask the true mode of action of these
antimicrobials and would need to be taken into account when drawing conclusions from these assays. CHX tolerance has been thought to be mediated through a proton motive force-dependent
efflux pump, which would suggest CHX has an internal target.41 A membrane-active antimicrobial that directly and catastrophically affects the bacterial membrane would be able to circumvent a
resistance mechanism such as an efflux pump. Indeed, it has been recently shown that HT61 can act in synergy with CHX against _S. aureus_, increasing the potency of the two
antimicrobials.32 However, this study has shown that resistance, or at least tolerance, to membrane-acting antimicrobials is a real possibility if there are changes in the composition of the
lipid bilayer by reduction of the amount of anionic lipid present.15, 16, 17, 18, 19 This suggests that the primary target for CHX may indeed be within the cell interior as has been
previously proposed (where it causes precipitation of cytoplasmic material), and that the membrane disruption is actually an off-target side-effect of it gaining entry to the cell.42
CONCLUSIONS HT61 and CHX are both membrane-active antimicrobials that non-specifically target anionic lipids and partition into the lipid bilayer, conferring specificity to the cytoplasmic
membrane of bacteria, resulting in increased permeability. However, HT61 also has a demonstrable lytic effect on both bacterial and model membranes, which results in the leakage of the
intracellular components and lysis. This action is absent from bacterial and model membranes following challenge with CHX, raising questions over the suggestion that CHX and HT61 primary
mode of action is via damage to the cytoplasmic membrane. REFERENCES * Epand, R. F. _et al_. Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins.
_Antimicrob. Agents Chemother._ 54, 3708–3713 (2010). Article CAS PubMed PubMed Central Google Scholar * Hurdle, J. G., O'Neill, A. J., Chopra, I. & Lee, R. E. Targeting
bacterial membrane function: an underexploited mechanism for treating persistent infections. _Nat. Rev. Microbiol._ 9, 62–75 (2011). Article CAS PubMed PubMed Central Google Scholar *
Hubbard, A. T. M. _et al_. Mechanism of action of a membrane-active quinoline-based antimicrobial on natural and model bacterial membranes. _Biochemistry_ 56, 1163–1174 (2017). Article CAS
PubMed Google Scholar * Domenech, O. _et al_. Interactions of oritavancin, a new lipoglycopeptide derived from vancomycin, with phospholipid bilayers: Effect on membrane permeability and
nanoscale lipid membrane organization. _Biochim. Biophys. Acta_ 1788, 1832–1840 (2009). Article CAS PubMed Google Scholar * Zhang, L., Rozek, A. & Hancock, R. E. Interaction of
cationic antimicrobial peptides with model membranes. _J. Biol. Chem._ 276, 35714–35722 (2001). Article CAS PubMed Google Scholar * Jung, D., Rozek, A., Okon, M. & Hancock, R. E.
Structural transitions as determinants of the action of the calcium-dependent antibiotic daptomycin. _Chem. Biol._ 11, 949–957 (2004). Article CAS PubMed Google Scholar * Castillo, J. A.
_et al_. Comparative study of the antimicrobial activity of bis(Nalpha-caproyl-L-arginine)-1,3-propanediamine dihydrochloride and chlorhexidine dihydrochloride against _Staphylococcus
aureus_ and _Escherichia coli_. _J. Antimicrob. Chemother_ 57, 691–698 (2006). Article CAS PubMed Google Scholar * Higgins, D. L. _et al_. Telavancin, a multifunctional lipoglycopeptide,
disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant _Staphylococcus aureus_. _Antimicrob. Agents. Chemother._ 49, 1127–1134 (2005). Article CAS PubMed
PubMed Central Google Scholar * Ooi, N. _et al_. XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. _J. Antimicrob. Chemother._ 64, 735–740 (2009).
Article CAS PubMed Google Scholar * Silverman, J. A., Perlmutter, N. G. & Shapiro, H. M. Correlation of daptomycin bactericidal activity and membrane depolarization in
_Staphylococcus aureus_. _Antimicrob. Agents Chemother._ 47, 2538–2544 (2003). Article CAS PubMed PubMed Central Google Scholar * Matsuzaki, K. _et al_. Relationship of membrane
curvature to the formation of pores by magainin 2. _Biochemistry_ 37, 11856–11863 (1998). Article CAS PubMed Google Scholar * Matsumoto, K., Kusaka, J., Nishibori, A. & Hara, H.
Lipid domains in bacterial membranes. _Mol. Microbiol._ 61, 1110–1117 (2006). Article CAS PubMed Google Scholar * Danner, S., Pabst, G., Lohner, K. & Hickel, A. Structure and
thermotropic behavior of the _Staphylococcus aureus_ lipid lysyl-dipalmitoylphosphatidylglycerol. _Biophys. J._ 94, 2150–2159 (2008). Article CAS PubMed Google Scholar * Sievers, S. _et
al_. Changing the phospholipid composition of _Staphylococcus aureus_ causes distinct changes in membrane proteome and membrane-sensory regulators. _Proteomics_ 10, 1685–1693 (2010). Article
CAS PubMed Google Scholar * Friedman, L., Alder, J. D. & Silverman, J. A. Genetic changes that correlate with reduced susceptibility to daptomycin in _Staphylococcus aureus_.
_Antimicrob. Agents Chemother._ 50, 2137–2145 (2006). Article CAS PubMed PubMed Central Google Scholar * Bayer, A. S., Schneider, T. & Sahl, H. G. Mechanisms of daptomycin
resistance in _Staphylococcus aureus_: role of the cell membrane and cell wall. _Ann. NY Acad. Sci._ 1277, 139–158 (2013). Article CAS PubMed Google Scholar * Mishra, N. N. _et al_.
Daptomycin resistance in enterococci is associated with distinct alterations of cell membrane phospholipid content. _PLoS ONE_ 7, e43958 (2012). Article CAS PubMed PubMed Central Google
Scholar * Kilelee, E., Pokorny, A., Yeaman, M. R. & Bayer, A. S. Lysyl-phosphatidylglycerol attenuates membrane perturbation rather than surface association of the cationic
antimicrobial peptide 6W-RP-1 in a model membrane system: implications for daptomycin resistance. _Antimicrob. Agents Chemother._ 54, 4476–4479 (2010). Article CAS PubMed PubMed Central
Google Scholar * Zhang, T. _et al_. Cardiolipin prevents membrane translocation and permeabilization by daptomycin. _J. Biol. Chem._ 289, 11584–11591 (2014). Article CAS PubMed PubMed
Central Google Scholar * Oliva, B. _et al_. Biological properties of novel antistaphylococcal quinoline-indole agents. _Antimicrob. Agents Chemother._ 47, 458–466 (2003). Article CAS
PubMed PubMed Central Google Scholar * Ooi, N. _et al_. XF-70 and XF-73, novel antibacterial agents active against slow-growing and non-dividing cultures of _Staphylococcus aureus_
including biofilms. _J. Antimicrob. Chemother._ 65, 72–78 (2010). Article CAS PubMed Google Scholar * Zhang, T. _et al_. Daptomycin forms cation- and size-selective pores in model
membranes. _Biochim. Biophys. Acta_ 1838, 2425–2430 (2014). Article CAS PubMed Google Scholar * Belley, A. _et al_. Oritavancin kills stationary-phase and biofilm _Staphylococcus aureus_
cells _in vitro_. _Antimicrob. Agents Chemother_ 53, 918–925 (2009). Article CAS PubMed Google Scholar * Dhar, N. & Mckinney, J. D. Microbial phenotypic heterogeneity and antibiotic
tolerance. _Curr. Opin. Microbiol._ 10, 30–38 (2007). Article CAS PubMed Google Scholar * Kim, J. S. _et al_. Bacterial persisters tolerate antibiotics by not producing hydroxyl
radicals. _Biochem. Biophys. Res. Commun._ 413, 105–110 (2011). Article CAS PubMed Google Scholar * Farrell, D. J., Robbins, M., Rhys-Williams, W. & Love, W. G. Investigation of the
potential for mutational resistance to XF-73, retapamulin, mupirocin, fusidic acid, daptomycin, and vancomycin in methicillin-resistant _Staphylococcus aureus_ isolates during a 55-passage
study. _Antimicrob. Agents Chemother._ 55, 1177–1181 (2011). Article CAS PubMed Google Scholar * Hu, Y., Shamaei-Tousi, A., Liu, Y. & Coates, A. A new approach for the discovery of
antibiotics by targeting non-multiplying bacteria: a novel topical antibiotic for staphylococcal infections. _PLoS ONE_ 5, e11818 (2010). Article PubMed PubMed Central Google Scholar *
Julian, K. _et al_. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate _Staphylococcus aureus_ strain in a patient with endocarditis. _Antimicrob. Agents Chemother._ 51,
3445–3448 (2007). Article CAS PubMed PubMed Central Google Scholar * Randall, C. P., Mariner, K. R., Chopra, I. & O'neill, A. J. The target of daptomycin is absent from
_Escherichia coli_ and other gram-negative pathogens. _Antimicrob. Agents Chemother._ 57, 637–639 (2013). Article CAS PubMed PubMed Central Google Scholar * Cheung, H. Y. _et al_.
Differential actions of chlorhexidine on the cell wall of _Bacillus subtilis_ and _Escherichia coli_. _PLoS ONE_ 7, e36659 (2012). Article CAS PubMed PubMed Central Google Scholar *
Jones, C. G. Chlorhexidine: is it still the gold standard? _Periodontology 2000_ 15, 55–62 (1997). Article CAS PubMed Google Scholar * Hu, Y. M. & Coates, A. R. M. Enhancement by
novel anti-methicillin-resistant _Staphylococcus aureus_ compound HT61 of the activity of neomycin, gentamicin, mupirocin and chlorhexidine: _in vitro_ and _in vivo_ studies. _J. Antimicrob.
Chemother._ 68, 374–384 (2013). Article CAS PubMed Google Scholar * Berney, M. _et al_. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in
combination with flow cytometry. _Appl. Environ. Microbiol._ 73, 3283–3290 (2007). Article CAS PubMed PubMed Central Google Scholar * Stocks, S. M. Mechanism and use of the commercially
available viability stain, BacLight. _Cytometry A_ 61, 189–195 (2004). Article CAS PubMed Google Scholar * Kohanski, M. A., Dwyer, D. J. & Collins, J. J. How antibiotics kill
bacteria: from targets to networks. _Nat. Rev. Microbiol_ 8, 423–435 (2010). Article CAS PubMed PubMed Central Google Scholar * Macheboeuf, P. _et al_. Penicillin binding proteins: key
players in bacterial cell cycle and drug resistance processes. _FEMS Microbiol. Rev._ 30, 673–691 (2006). Article CAS PubMed Google Scholar * O'neill, A. J., Miller, K., Oliva, B.
& Chopra, I. Comparison of assays for detection of agents causing membrane damage in _Staphylococcus aureus_. _J. Antimicrob. Chemother._ 54, 1127–1129 (2004). Article CAS PubMed
Google Scholar * Mcdonald, J. H. _Handbook of Biological Statistics_ 2nd edn (Sparky House Publishing, Baltimore, Maryland, (2009). Google Scholar * De Winter, J. C. F. Using the Student’s
t-test with extremely small sample sizes. _Pract. Assess. Res. Eval._ 18, 1–12 (2013). Google Scholar * Shireen, T., Singh, M., Dhawan, B. & Mukhopadhyay, K. Characterization of cell
membrane parameters of clinical isolates of _Staphylococcus aureus_ with varied susceptibility to alpha-melanocyte stimulating hormone. _Peptides_ 37, 334–339 (2012). Article CAS PubMed
Google Scholar * Smith, K., Gemmell, C. G. & Hunter, I. S. The association between biocide tolerance and the presence or absence of qac genes among hospital-acquired and
community-acquired MRSA isolates. _J. Antimicrob. Chemother._ 61, 78–84 (2008). Article CAS PubMed Google Scholar * Russell, A. D. Similarities and differences in the responses of
microorganisms to biocides. _J. Antimicrob. Chemother_ 52, 750–763 (2003). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank Dr Alex O’Neill
of the University of Leeds for his advice throughout this study. This work was supported by a studentship from St George’s, University of London (grant number 12269-10). AUTHOR INFORMATION
Author notes * Alasdair TM Hubbard Present address: 3Current address: Nuffield Department of Clinical Medicine, University of Oxford, Oxford OX3 9DU, UK, * Richard D Harvey Present address:
4Current address: Institute of Pharmacy, Martin-Luther-University Halle-Wittenberg, Halle (Saale), Germany., AUTHORS AND AFFILIATIONS * Division of Clinical Sciences, Infection and Immunity
Research Centre, St George's, University of London, London, UK Alasdair TM Hubbard & Anthony RM Coates * Institute of Pharmaceutical Science, King’s College London, London, UK
Richard D Harvey Authors * Alasdair TM Hubbard View author publications You can also search for this author inPubMed Google Scholar * Anthony RM Coates View author publications You can also
search for this author inPubMed Google Scholar * Richard D Harvey View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to
Richard D Harvey. ETHICS DECLARATIONS COMPETING INTERESTS ARMC is a director and shareholder of Helperby Therapeutics Group plc. The remaining authors declare no conflict of interest. RIGHTS
AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hubbard, A., Coates, A. & Harvey, R. Comparing the action of HT61 and chlorhexidine on natural and model
_Staphylococcus aureus_ membranes. _J Antibiot_ 70, 1020–1025 (2017). https://doi.org/10.1038/ja.2017.90 Download citation * Received: 16 March 2017 * Revised: 14 June 2017 * Accepted: 02
July 2017 * Published: 02 August 2017 * Issue Date: October 2017 * DOI: https://doi.org/10.1038/ja.2017.90 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative






