- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Kendomycin, an ansamycin-type natural product first reported in 1996, possesses a series of attractive bioactivities and a unique all-carbon macrocyclic skeleton. To the date, seven
total syntheses, two formal total syntheses and a number of synthetic studies on this hot molecule have been reported. In this short review article, we mainly survey and comment on these
efforts regarding the difficult macrocyclization strategies. SIMILAR CONTENT BEING VIEWED BY OTHERS CHIMERIC NATURAL PRODUCTS DERIVED FROM MEDERMYCIN AND THE NATURE-INSPIRED CONSTRUCTION OF
THEIR POLYCYCLIC SKELETONS Article Open access 02 September 2022 TOTAL SYNTHESIS OF STRUCTURALLY DIVERSE PLEUROMUTILIN ANTIBIOTICS Article 26 September 2022 SYNTHESIS OF (+)-RIBOSTAMYCIN BY
CATALYTIC, ENANTIOSELECTIVE HYDROAMINATION OF BENZENE Article 26 May 2022 INTRODUCTION Kendomycin [(−)-TAN2162, 1] is a secondary metabolite isolated from _Streptomyces_ species. It was
first reported in 1996 by Takeda Pharmaceutical Co. Ltd, Osaka, Japan (identification of the planar structure) as an antagonist for an endothelin receptor from _Streptomyces_ AL-71389,1, 2
and later in 1998 by Su _et al._3 (ascertainment of the relative stereochemistry) as an antiosteoporotic agent from _Streptomyces_ NRRL-21370. In 2000, Bode and Zeeck4 re-isolated this
compound from _Streptomyces violaceoruber_ 3844-33C and identified its absolute configuration by the advanced Mosher’s ester method. The authors reported its cytotoxic effects on three human
tumor cell lines in addition to its remarkable antibacterial activity against both Gram-positive and Gram-negative bacteria, notably the STA MU50 strain, which belongs to not only
methicillin-resistant _Staphylococcus aureus_ but also vancomycin-intermediate _Staphylococcus aureus_ strains. Further biological studies showed that its cytotoxicity may be derived from
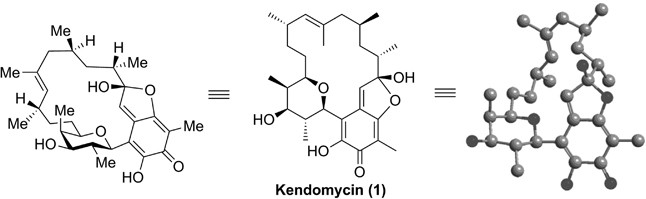
proteasome inhibition5, 6 and Bcl-xl inhibition.7 Structurally, kendomycin (1, Figure 1) belongs to the ansamycin family and contains a cyclophane framework. It comprises a quinone methide
chromophore set in the macrocycle, which was heavily assembled with stereogenic centers particularly on the tetrahydropyran segment. Notably, distinguished from the macrolactam connection of
usual ansamycins, the aliphatic ansa-chain of 1 connects to the chromophore via carbon–carbon bonds. The biosynthetic pathway of 1 was disclosed first by Bode and Zeeck,4, 8 and further by
Müller and co-workers9 (Scheme 1). It was proposed that the ansa-chain is added onto the benzoic acid 2 or its benzoquinone derivative 3 with a type-I polyketide synthase assembly line. The
generated 4 undergoes a pyran-cyclization to form 5, which further forms the macrocarbocyclic intermediate 6 through aldol condensation between the malonate terminus and the quinone
carbonyl. Finally, dehydration and hemiacetal formation furnish 1. The biogenetic studies provided informative hints for the design of chemical syntheses. The combination of attractive
bioactivities and the unique chemical structure of kendomycin (1) has been of great interests to the synthetic and medicinal communities. To date, seven total syntheses, two formal total
syntheses, and a number of synthetic efforts have been published. The key points that should be overcome in the total synthesis of 1 are the following: (I) efficient macrocyclization, (II)
selective installation of the multiple stereogenic centers and (III) construction of the sensitive quinone-methide-lactol moiety. For point (III), all of the reported synthetic strategies
left this difficult task for the last steps of the total synthesis. Though the syntheses reported by most research groups have suffered from this painful transformation, we think that this
obstacle has basically been surmounted through the efforts in many laboratories. For point (II), the proposed biosynthetic pathways and a varieties of synthetic examples from other
macrocyclic natural products suggest that it is much easier to install the multiple stereogenic centers before the macrocyclization step. Fortunately, owing to the rapid development of the
asymmetric synthesis field, more reliable protocols (especially reagent-controlled protocols) reduce the difficulties associated with point (II) in total synthesis research, though trials
are still needed to find the most efficient way. Therefore, points (II) and (III) have not been the major problem for total synthesis of 1. Efficient cyclization of the 18-membered
carbocycle, point (I), is still the primary challenge for further synthetic studies on this hot molecule and its structure–activity relationship research. Several review articles have been
published that summarize the synthetic efforts toward 1, including two early-period comprehensive reviews,10, 11 two personal accounts,12, 13 and one specific review on the olefin metathesis
strategy.14 In this short review, we will provide a survey and discussion on the macrocyclization strategies (both successful and failed) reported so far in the synthetic studies of 1.
CHRONOLOGICAL EFFORTS OF THE MACROCYCLIZATION STRATEGIES FOR KENDOMYCIN The pioneer trial for the construction of the macrocycle of kendomycin (1) was first reported by Mulzer _et al._15
They attempted to connect the C13–C14 double bond by ring-closing olefin metathesis (RCM) based on a series of benzofuran substrates 7–14 (Scheme 2).12, 16, 17, 18, 19 However, none of the
substrates produced macrocyclic products under various conditions with Grubbs 2nd-generation catalyst or Schrock catalyst. The byproducts were thought to mainly be dimers and oligomers. They
rationalized that the planar benzofuran system might cause the two olefin terminuses to not be spatially near enough for the intramolecular reaction. Another strategy that Mulzer, Martin
and co-workers tried for the C13–C14 connection was through the Horner–Wadsworth–Emmons reaction (Scheme 3).15 Two substrates 15 and 16 were prepared. While compound 15 failed to provide the
desired cyclization product 17 under various conditions, compound 16 did macrocyclize to afford 18. The subsequent task was the removal of the C15 carbonyl. However, it resisted direct
reduction, and a two-step procedure using Luche reduction and Barton–McCombie deoxygenation led to a double-bond migrated undesired product 19, possibly via [3,3]-sigmatropic
rearrangement12, 15 or allyl radical rearrangement.11 Therefore, though the Horner–Wadsworth–Emmons strategy succeeded in the macrocyclization step, it unfortunately could not lead to the
final target 1. In 2004, Lee and co-workers achieved the first total synthesis of kendomycin (1) by talentedly using a _C_–glycosidation for the macrocyclization (Scheme 4).20 While their
initial cyclization attempt failed on _tert_-butyldimethylsilyl (TBS)-protected phenol 20 under various conditions (no cyclization product, mainly hydrolysis of the anomeric acetate), the
macrocyclization proceeded smoothly using the TBS-deprotected free phenol 21 with Lewis acid SnCl4. At –5 °C the _O_–glycosidation product 22 was possibly afforded and then rearranged to the
_C_–glycosidation product 23 at room temperature. Notably, the cyclization process was highly selective, providing only the single desired diastereomer, though with modest unstable yield
(40–70%). Subsequently, three steps completed the asymmetric total synthesis of kendomycin (1). The second total synthesis of 1 was reported by Smith _et al._ in 2005.21, 22 While the Mulzer
group failed with the benzofuran-type substrates (_vide supra_),15 the Smith group continued challenging the RCM strategy at the C13–C14 double bond with furan-ring-opened substrates
(Scheme 5). In a series of seven prepared substrates, compounds 24, 25 and 26 provided the macrocyclic RCM products, while 27 (C19 epimer of 26), 28 (C19 ketone analog of 26), 29 (C4–OH of
26) and 30 (C4–OH of 27) failed. The RCM reactions of 24–26 were highly selective, affording only one isomer, though with the undesired _Z_-geometry (for 25, the product geometry could not
be determined). The best yield was obtained from 26 to macrocycle 33 (57% from 2:1 epimer mixture of 26 and 27 or 86% calculated for 26 only). Though not completely understanding the RCM
reactivity difference for the substrates with only subtle changes, the Smith group proposed that in compound 26, the opened furan ring, the hydrogen bonding between C19–OH and C1–OMe, and
the bulky TBSO at C4 (fixing the C4a–C5 rotamer) should together contribute greatly to the conformation of the cyclization transition state. Several extra steps were necessary to isomerize
the double bond geometry from 33 to 34, which was subsequently transformed to kendomycin (1). Arimoto and co-workers also reported their attempts for the RCM macrocyclization between C13 and
C14 (Scheme 6).23, 24 With compound 35 having a more rigid right chain compared to Smith’s intermediate 26, the macrocyclization gave a single isomer, however, also with the undesired
_Z_-geometry. With 5 mol% Grubbs 2nd-generation catalyst, the yield was unstable ranging from trace to 45%. A higher amount of catalyst (60 mol%) was necessary to obtain reproducible results
(53%). Due to the low efficiency of this key reaction, they later changed to another strategy to complete the total synthesis (_vide infra_). In 2008, Panek and co-workers achieved the
third total synthesis of 1.25, 26 Their key macrocyclization (Scheme 7) was an intramolecular Barbier-type organometallic addition (Williams and Shamim27 seemingly also intended to apply a
Barbier-type reaction for macrocyclization though at C4a–C5 bond of kendomycin. However no further report was published) with substrate 37 using freshly prepared SmI2 in tetrahydrofuran. The
C19–C20 single bond connected highly selectively, providing a single diastereomer 38 in modest 60% yield. Subsequently, three steps furnished the final target 1. Later in 2008, Rychnovsky
and co-workers reported their formal total synthesis of 1.28 Actually, in 2006, they published an early formal total synthesis of 1 with Prins tetrahydropyran cyclization to a Smith’s
intermediate that was ahead of the key macrocyclization step.29 In the 2008 paper, the Rychnovsky group applied Prins reaction to the macrocyclization step (Scheme 8). Under acetic acid and
BF3·OEt2 conditions, the C9–OH and C5–aldehyde of compound 39 first intramolecularly linked to form an oxocarbenium ion 40. Then, Prins reaction between C5 and C6 followed by an acetate trap
afforded the desired product 41 (33%) together with the fluoride-trapped byproduct 42 (48%). Though minimization of the amount of 42 failed under other conditions, this reaction is still a
very efficient transformation—highly selectively constructing C5, C6 and C7 (three) stereogenic centers, one tetrahydropyran ring and one macrocyclic ring in one step. Subsequent removal of
the acetate and sulfonyl protecting groups provided the Lee group’s intermediate 43, thereby achieving a formal total synthesis of 1. Despite the failure of macrocyclization at the C13–C14
double bond by the RCM and Horner–Wadsworth–Emmons strategies (_vide supra_), Mulzer and co-workers persisted in their pursuit of total synthesis of 1 at different cyclization sites. In
2009, they disclosed two distinct total synthesis routes.30, 31 One was based on their long-time RCM assumption, but at other disconnection sites than C13–C14 (Scheme 9). They first prepared
substrate 44, its C5-epimer 45, and its C5-ketone analog 46 to test RCM between C9 and C10. While 45 and 46 yielded no cyclization products, 44 did macrocyclize and afford 47 in 46% yield.
However, 47 could not undergo the subsequent tetrahydropyran formation under various conditions such as iodination, oxymercuration or selenocyclization. Although there would have been
additional options (for example, Mitsunobu inversion at C5–OH), they abandoned the C9–C10 disconnection approach at this point12 and turned to pursue the possibility of RCM between C19 and
C20. However, after the preparation of segments 49 and 50, it was found to be impossible to connect them together to afford the RCM precursor 51. Thus, their choice was changed once again to
another disconnection site between C11 and C12. In this case, with Grubbs 2nd-generation catalyst, the prepared substrate 53 (7:2 epimer mixture at C5) efficiently provided the macrocyclic
product 54 in 62% yield as the single _E_-isomer (only the major epimer cyclized). Subsequent hydrogenation of the newly formed double bond and tetrahydropyran cyclization under acidic
condition afforded the Lee group’s intermediate 43. Alternatively, 43 could also be synthesized from 53 through a more efficient sequence (tetrahydropyran cyclization, RCM and then
hydrogenation). The Mulzer group further developed a shorter route to achieve the total synthesis of 1 from 43. In the same paper, the Mulzer group reported another novel strategy from seco
acid 57 via Keck macrolaztonization/photo-Fries rearrangement sequence affording cleanly compound 59, which was further transformed to the same key intermediate 43 after two steps (Scheme
10). In 2010, another novel total synthesis of 1 was reported by Nakata, Saikawa, and co-workers using a key intramolecular Dötz benzannulation and Claisen rearrangement to construct the
macrocycle (Scheme 11).32, 33 Actually, among kendomycin synthetic studies, White _et al._34 first introduced an intermolecular Dötz benzannulation to construct the aromatic ring, though
they encountered great problems in the later macrocyclization step.35 Instead, the Nakata and Saikawa group elegantly designed a different type of Fisher carbene substrate 62 (easily
prepared from tetrahydropyran alkyne 60) and applied it to an intramolecular Dötz benzannulation, which could simultaneously construct the macrocycle. The desired ansa-product 64 formed
smoothly and was obtained as the sole isolated product in modest yield. After TBS protection, using Ac2O to _in situ_ trap the newly generated phenol in the _N_, _N_-dimethylaniline solvent,
the thermal Claisen rearrangement constructed the macrocarbocycle 66 highly efficiently with 85% yield from the oxa-metacyclophane 65. With the TBS analog of 60, the Nakata and Saikawa
group also obtained the related derivative of 66 with similar efficiency. Several additional steps completed the total synthesis of 1. Interestingly, the Nakata and Saikawa group reported
the Dötz benzannulation of substrate 67 without the tetrahydropyran ring, providing the desired 68 in only 14% yield along with considerable amount of byproduct 71 (38%), which was formed by
a metalla-Claisen-type rearrangement of the carbene complex to generate 70, followed by reductive elimination. In 2014, Arimoto and co-workers achieved their total synthesis of 1.36
Unsatisfied with their previous RCM strategy at C13–C14 (_vide supra_), this time they changed the key reaction to a Pd-catalyzed Tsuji–Trost macro-etherification on hydroquinone 72 (Scheme
12). The yield of the desired C1–_O_-tethered product 73 and its regioselectivity over the C4–_O_-tethered byproduct 74 were found to be highly sensitive to the reaction conditions.
Extensive studies finally provided 73 in 71% isolated yield (10:1 ratio to 74). Subsequent TBS protection gave 75, which afforded the Claisen rearrangement product 76 in almost quantitative
yield under their previous developed condition.24 Several more steps from 76 accomplished the total synthesis of 1. In 2014, Fürstner and co-workers reported a formal total synthesis of 1
(Scheme 13).37 Their key macrocyclizaton step was a ring-closing alkyne metathesis to form a C19–C20 triple bond, which readily favored the benzofuran construction. The ring-closing alkyne
metathesis trails on substrates 77–80 were as troublesome as they expected. Only 80 gave the desired product, yet with high load of the expensive catalyst 81 and modest erratic yield. The
steric bulkiness of both the catalyst and C20 position of 77–80 was inferred to be the major cause. Thus, the relatively loose C4a-unsubstituted diyne 83 was applied, and the ring-closing
alkyne metathesis proceeded exceptionally easily, furnishing the desired product 84 at ambient temperature in essentially quantitative and well-reproducible yield with 5 mol% catalyst 81.
Subsequent deprotection and Au-catalyzed hydroalkoxylation established the benzofuran 58, which was the same as the intermediate of the Mulzer group’s photo-Fries strategy. CONCLUDING
REMARKS For the syntheses of macrocycle natural products, the choice of tactics for the cyclization step, which often defines the overall efficiency of the total synthesis, is the key in
synthetic strategies.38 For the synthesis of macrocycles larger than 12-membered rings, there are two basic strategies: direct cyclization and the cyclization-ring contraction sequence.
Construction of a larger macro-heterocycle followed by a ring contraction to the desired macrocarbocycle is traditionally the popular approach via a circuitous route because a carbon–carbon
bond formation is generally more difficult than a carbon–heteroatom bond formation. However, with the rapid development of synthetic methodology for carbon–carbon bond connections, direct
macrocyclization to construct carbon–carbon bonds is the current trend of the field. From information gleaned in the previous sections, readers can obtain insights for alternative
macrocyclization strategies for kendomycin. To establish efficient total syntheses, all of the research groups initially attempted the macrocyclization by carbon–carbon bond formation. The
early five total/formal syntheses from the Lee, Smith, Panek, Rychovsky, and Mulzer groups all adopted direct cyclization with carbon–carbon bond connection (notwithstanding a definite ring
contraction through _O_-glycosidation of an intermediate, the Lee group’s cyclization was efficiently achieved in one pot). However, those strategies suffered from unstable/unsatisfactory
yields or needed further steps to modify the connected bond (_Z_/_E_ isomerization in the Smith synthesis; olefin saturation in the Mulzer synthesis). Interestingly, after the Mulzer’s
synthesis using Fries-rearrangement strategy, all later successful syntheses included a ring contraction step in their macrocycle syntheses. Although the combined yields were still not
satisfactory for some of the attempts, the most recent strategy from the Fürstner group was truly efficient with 95% yield of ring-closing alkyne metathesis heterocycle formation and 85%
yield of Fries-rearrangement contraction. Actually, several groups including ours (unpublished) used simplified model compounds to verify their strategies for macrocyclization, however, the
conditions that worked for the model compounds frequently had serious difficulties when applied to the actual intermediate and needed further optimization. Nevertheless, the strategic
changes in kendomycin total syntheses from direct cyclization in the earlier studies to traditional ring contraction illustrate that the modern synthetic methodology is still far from
perfection. Another factor in strategy design is the selectivity, especially diastereoselectivity, for complex natural products. While the construction of a series of stereogenic centers in
linear precursor molecules is not a significant problem for modern synthetic chemistry, achieving diastereo-control during macrocyclization is not trivial. To avoid this challenge, the
retrosynthetic analyses of macrocycles usually disconnect a bond not neighboring to chiral carbons, and set forth all chirality well in the macrocyclization substrates. Moreover, in the
kendomycin structure, the northern part is structurally simpler and sterically less hindered compared to the heavily functionalized southern part. Therefore, it is not surprising that the
initial cyclizations were tried between the C13–C14 bond by many research groups. However, the unsatisfactory experimental results at this position made the retrosynthetic design switch to
the disconnection in the southern part. The syntheses of the Lee and Rychnovsky groups were efficient in terms of stereoselectivity in the macrocyclization, though with unstable or low
yields. Particularly, the latter successfully established three stereogenic centers simultaneously. Cyclization used by other groups did not have selectivity problems, except for
regiocontrol in Arimoto’s Tsuji–Trost macroetherifcation strategy. Notably, the Mulzer group successfully developed a highly diastereoselective construction of C5 after the macrocyclization.
Figure 2 summarizes the yet unexplored (or failed) potential macrocyclization sites in kendomycin (1). For C11–C12, C12–C13, C15–C16, C16–C17, C17–C18, and C18–C19 bonds, connection of
these bonds needs to control a heteroatom–free chiral carbon diastereoselectivity, and thus, macrocyclization at those positions seems really difficult. However, a challenge in highly
efficient cyclization might be the future direction of kendomycin synthesis. The C14–C15 bond, at which cyclization might be achieved by intramolecular cross-coupling, has also not been
examined. Another attractive possible cyclization is at the C20–C20a bond via a biomimetic aldol reaction on quinone substrates. Extensive structure–activity relationship studies for 1 have
not been reported to date.4, 7, 8, 33 However, the results of the Nakata and Saikawa group suggested that the ansa-macrocyclic skeleton is essential for its antimicrobial activity.33
Therefore, the macrocyclization step is unavoidable for the future structure–activity relationship studies and should be thought and designed for more convenient preparation of
structure–activity relationship libraries. Thus far, the reported total and formal total syntheses of 1 are all from long-standing groups in the total synthesis field. It is predictable that
with the large growth of kendomycin research, more groups in synthetic methodology and medicinal chemistry will join the field and develop more efficient synthetic pathways to 1. REFERENCES
* Funahashi, Y., Kawamura, N. & Ishimaru, T. Endothelin receptor antagonists manufacture. JP08231551. _Chem. Abstr._ 126, 6553 (1996). Google Scholar * Funahashi, Y., Ishimaru, T.
& Kawamura, N. Endothelin receptor antagonists manufacture. JP08231552. _Chem. Abstr._ 125, 326518 (1996). Google Scholar * Su, M. H., Hosken, M. I., Hotovec, B. J. & Johnston, T.
L. Antiosteoporotic compound. US5728727. _Chem. Abstr._ 128, 239489 (1998). Google Scholar * Bode, H. B. & Zeeck, A. Structure and biosynthesis of kendomycin, a carbocyclic
ansa-compound from _Streptomyces_. _J. Chem. Soc., Perkin Trans._ 1, 323–328 (2000). Article Google Scholar * Elnakady, Y. A. _et al_. Evidence for the mode of action of the highly
cytotoxic _Streptomyces_ polyketide kendomycin. _Chembiochem._ 8, 1261–1272 (2007). Article CAS Google Scholar * Beck, P. _et al_. Interactions of the natural product kendomycin and the
20S proteasome. _J. Mol. Biol._ 426, 3108–3117 (2014). Article CAS Google Scholar * Janssen, C. O. _et al_. Interaction of kendomycin and semi-synthetic analogues with the anti-apoptotic
protein Bcl-xl. _Bioorg. Med. Chem. Lett._ 18, 5771–5773 (2008). Article CAS Google Scholar * Bode, H. B. & Zeeck, A. Biosynthesis of kendomycin: origin of the oxygen atoms and
further investigations. _J. Chem. Soc., Perkin Trans._ 1, 2665–2670 (2000). Article Google Scholar * Wenzel, S. C., Bode, H. B., Kochems, I. & Müller, R. A type I/type III polyketide
synthase hybrid biosynthetic pathway for the structurally unique ansa compound kendomycin. _Chembiochem._ 9, 2711–2721 (2008). Article CAS Google Scholar * Shan, M., Wang, L., Zhang, Q.
& O’Dooherty, G. A. Tactics in total synthesis of (-)-kendomycin. _Chemtracts_ 22, 1–17 (2009). CAS Google Scholar * Martin, H. J., Magauer, T. & Mulzer, J. In pursuit of a
competitive target: total synthesis of the antibiotic kendomycin. _Angew. Chem. Int. Ed._ 49, 5614–5626 (2010). Article CAS Google Scholar * Martin, H. J., Magauer, T. & Mulzer, J. In
pursuit of an elusive target: our kendomycin story. _Strategies Tactics Org. Synth._ 8, 261–289 (2012). Article CAS Google Scholar * Saikawa, Y., Tanaka, K. & Nakata, M. Construction
of ansa-skeleton via intramolecular Dötz benzannulation: total synthesis of kendomycin. _Yuki Gosei Kagaku Kyokaishi_ 72, 1143–1153 (2014). Article CAS Google Scholar * Bicchielli, D.
_et al_. Olefin metathesis as key step in the synthesis of bioactive compounds: challenges in the total synthesis of (–)-kendomycin. _Curr. Org. Synth._ 9, 397–405 (2012). Article CAS
Google Scholar * Mulzer, J., Pichlmair, S., Green, M. P., Marques, M. M. B. & Martin, H. J. Toward the synthesis of the carbacylic _ansa_ antibiotic kendomycin. _Proc. Natl Acad. Sci.
USA_ 101, 11980–11985 (2004). Article CAS Google Scholar * Martin, H. J., Drescher, M., Kählig, H., Schneider, S. & Mulzer, J. Synthesis of the C1–C13 fragment of kendomycin:
atropisomerism around a _C_-aryl glycosidic bond. _Angew. Chem. Int. Ed._ 40, 3186–3188 (2001). Article CAS Google Scholar * Marques, M. M. B., Pichlmair, S., Martin, H. J. & Mulzer,
J. Stereocontrolled synthesis of _C_-arylglycosides applied to the south west fragment of the antibiotic kendomycin. _Synthesis_ 34, 2766–2770 (2002). Google Scholar * Pichlmair, S.,
Marques, M. M. B., Green, M. P., Martin, H. J. & Mulzer, J. A novel approach toward the synthesis of kendomycin: selective synthesis of a _C_–aryl glycoside as a single atropisomer.
_Org. Lett._ 5, 4657–4659 (2003). Article CAS Google Scholar * Green, M. P. _et al_. Synthesis of analogue structures of the _p_-quinone methide moiety of kendomycin. _Org. Lett._ 6,
3131–3134 (2004). Article CAS Google Scholar * Yuan, Y., Men, H. & Lee, C. Total synthesis of kendomycin: a macro-_C_–glycosidation approach. _J. Am. Chem. Soc._ 126, 14720–14721
(2004). Article CAS Google Scholar * Smith, A. B. III, Mesaros, E. F. & Meyer, E. A. Total synthesis of (-)-kendomycin exploiting a Petasis-Ferrier rearrangement/ring-closing olefin
metathesis synthetic strategy. _J. Am. Chem. Soc._ 127, 6948–6949 (2005). Article CAS Google Scholar * Smith, A. B. III, Mesaros, E. F. & Meyer, E. A. Evolution of a total synthesis
of (-)-kendomycin exploiting a Petasis-Ferrier rearrangement/ring-closing olefin metathesis strategy. _J. Am. Chem. Soc._ 128, 5292–5299 (2006). Article CAS Google Scholar * Sengoku, T.,
Uemura, D. & Arimoto, H. Ring-closing metathesis approach to a 16-membered macrocycle of kendomycin. _Chem. Lett._ 36, 726–727 (2007). Article CAS Google Scholar * Sengoku, T.,
Arimoto, H. & Uemura, D. Synthetic approach to kendomycin: preparation of the C-glycosidic core. _Chem. Commun._ 40, 1220–1221 (2004). Article Google Scholar * Lowe, J. T. & Panek,
J. S. Total synthesis of (-)-kendomycin. _Org. Lett._ 10, 3813–3816 (2008). Article CAS Google Scholar * Lowe, J. T. & Panek, J. S. Stereocontrolled [4+2]-annulation accessing
dihydropyrans: synthesis of the C1a-C10 fragment of kendomycin. _Org. Lett._ 7, 1529–1532 (2005). Article CAS Google Scholar * Williams, D. R. & Shamim, K. Efforts toward the total
synthesis of (-)-kendomycin. _Org. Lett._ 7, 4161–4164 (2005). Article CAS Google Scholar * Bahnck, K. B. & Rychnovsky, S. D. Formal synthesis of (-)-kendomycin featuring a
Prins-cyclization to construct the macrocycle. _J. Am. Chem. Soc._ 130, 13177–13181 (2008). Article CAS Google Scholar * Bahnck, K. B. & Rychnovsky, S. D. Rapid stereocontrolled
assembly of the fully substituted _C_-aryl glycoside of kendomycin with a Prins cyclization: a formal synthesis. _Chem. Commun._ 42, 2388–2390 (2006). Article Google Scholar * Magauer, T.,
Martin, H. J. & Mulzer, J. Total synthesis of the antibiotic kendomycin by macrocyclization using photo-Fries rearrangement and ring-closing metathesis. _Angew. Chem. Int. Ed._ 48,
6032–6036 (2009). Article CAS Google Scholar * Magauer, T., Martin, H. J. & Mulzer, J. Ring-closing metathesis and photo-Fries reaction for the construction of the ansamycin
antibiotic kendomycin: development of a protecting group free oxidative endgame. _Chem. Eur. J._ 16, 507–519 (2010). Article CAS Google Scholar * Tanaka, K. _et al_. Total synthesis of
kendomycin featuring intramolecular Dötz benzannulation. _Org. Lett._ 12, 1700–1703 (2010). Article CAS Google Scholar * Tanaka, K. _et al_. Synthesis and biological evaluation of
kendomycin and its analogues. _J. Org. Chem._ 79, 9922–9947 (2014). Article CAS Google Scholar * White, J. D. & Smits, H. Application of the Dötz reaction to Construction of a major
portion of the ansa macrocycle (-)-kendomycin. _Org. Lett._ 7, 235–238 (2005). Article CAS Google Scholar * Smits, H . _Studies towards the total synthesis of (-)-kendomycin_ (PhD thesis,
Oregon State University). _Chem. Abstr._ 148, 144520 (1998). Google Scholar * Sengoku, T. _et al_. Total synthesis of the antibiotic kendomycin: a macrocyclization using the Tsuji–Trost
etherification. _Angew. Chem. Int. Ed._ 53, 4213–4216 (2014). Article CAS Google Scholar * Hoffmeister, L., Persich, P. & Fürstner, A. Formal total synthesis of kendomycin by way of
alkyne metathesis/gold catalysis. _Chem. Eur. J._ 20, 4396–4402 (2014). Article CAS Google Scholar * Gulder, T. & Baran, P. S. Strained cyclophane natural products: macrocyclization
at its limits. _Nat. Prod. Rep._ 29, 899–934 (2012). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We are grateful for the financial support of Grants-in-Aid for
Scientific Research (nos.18032010 and 21310136) from the JSPS. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * State Key Laboratory of Bioactive Substance and Function of Natural Medicines,
Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China Shu Xu * Department of Medicinal Chemistry, Beijing Key Laboratory of Active
Substances Discovery and Drugability Evaluation, Institute of Materia Medica, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China Shu Xu * Graduate School
of Life Sciences, Tohoku University, Sendai, Japan Hirokazu Arimoto Authors * Shu Xu View author publications You can also search for this author inPubMed Google Scholar * Hirokazu Arimoto
View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Hirokazu Arimoto. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Xu, S., Arimoto, H. Strategies for construction of the
all-carbon macrocyclic skeleton of the ansamycin antibiotic—kendomycin. _J Antibiot_ 69, 203–212 (2016). https://doi.org/10.1038/ja.2016.5 Download citation * Received: 27 November 2015 *
Revised: 09 January 2016 * Accepted: 15 January 2016 * Published: 10 February 2016 * Issue Date: April 2016 * DOI: https://doi.org/10.1038/ja.2016.5 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative