- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The structures of xanthoradones A and B, new potentiators of imipenem activity against methicillin-resistant _Staphylococcus aureus_ produced by _Penicillium radicum_ FKI-3765-2,
were elucidated by spectroscopic studies, including various NMR experiments. These compounds have an asymmetric biaryl skeleton, which contains dihydronaphthopyranone and naphthoquinone
moieties. SIMILAR CONTENT BEING VIEWED BY OTHERS WYCHIMICINS, A NEW CLASS OF SPIROTETRONATE POLYKETIDES FROM _ACTINOCRISPUM WYCHMICINI_ MI503-A4 Article 07 September 2022 DESIGN AND
SYNTHESIS OF 4-[4-FORMYL-3-(2-NAPHTHYL)PYRAZOL-1-YL]BENZOIC ACID DERIVATIVES AS POTENT GROWTH INHIBITORS OF DRUG-RESISTANT _STAPHYLOCOCCUS AUREUS_ Article 29 June 2020 NEW POTENTIATORS OF
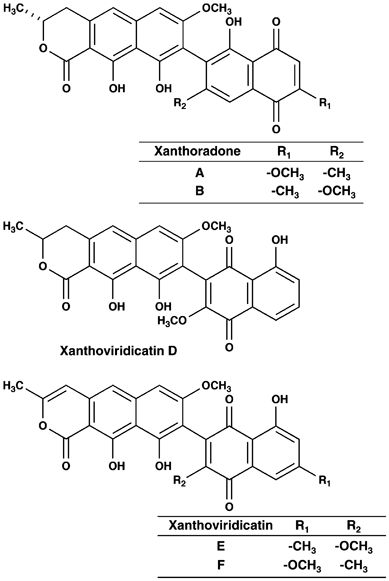
AMPHOTERICIN B ACTIVITY, SHODOAMIDES A TO C PRODUCED BY _PSEUDOPHIALOPHORA_ SP. BF-0158 Article 21 July 2023 INTRODUCTION Two new compounds, designated xanthoradones A and B (Figure 1), were
isolated as potentiators of imipenem activity against methicillin-resistant _Staphylococcus aureus_ from the culture broth of _Penicillium radicum_ strain FKI-3765-2.1 The taxonomy of the
producing strain, fermentation, isolation and biological properties of the xanthoradones were described in a previous paper.1 In this study, the physico-chemical properties and structure
elucidation of xanthoradones are described. RESULTS PHYSICO-CHEMICAL PROPERTIES The physico-chemical properties of xanthoradones A and B are summarized in Table 1. They had similar UV
spectra with absorption maxima at 218 nm, 263–267 nm and 373–375 nm. The IR absorption at 1606–1778 cm−1 and 3403–3407 cm−1 suggested the presence of carbonyl and hydroxyl groups in their
structures. These data indicated that they share the same skeleton. STRUCTURE ELUCIDATION OF XANTHORADONE A The molecular formula of xanthoradone A was determined to be C27H22O9 on the basis
of high-resolution electrospray ionization time-of-flight mass spectrum (HRESI-TOF-MS) measurement. The 13C NMR spectrum (in CDCl3) showed 27 resolved signals, which were classified into
two methyl carbons, one methylene carbon, four sp2 methine carbons, two oxygenated methyl carbons, one oxygenated methine carbon, nine sp2 quaternary carbons, five oxygenated sp2 quaternary
carbons and three carbonyl carbons by analyzing the DEPT and heteronuclear single quantum coherence (HSQC) spectra. The 1H NMR spectrum (in CDCl3) displayed 22 proton signals, nine of which
were suggested to be three hydroxy protons (δ 9.73, 12.5 and 13.9) and two oxygenated methyl protons (δ 3.84 and 3.92), as reported for xanthoviridicatin F.2 These results supported the
molecular formula. The connectivity of proton and carbon atoms was established by the 13C–1H HSQC spectrum (Table 2). Analyses of 1H–1H COSY revealed the presence of the partial structure I,
as shown in Figure 2a. Furthermore, 13C–1H long-range couplings of 2_J_ and 3_J_ observed in the 13C–1H heteronuclear multiple bond coherence (HMBC) spectrum gave the following linkages
(Figure 2b): (1) Cross peaks from H2-3 (δ 3.00, 3.06) to C-4 (δ 133.5), C-5 (δ 116.1) and C-13 (δ 99.7); from H-5 (δ 6.99) to C-3 (δ 34.7), C-6 (δ 140.0), C-7 (δ 98.1), C-11 (δ 108.4) and
C-13; from H-7 (δ 6.71) to C-5, C-8 (δ 160.3), C-9 (δ 109.0) and C-11; from OH-10 (δ 9.73) to C-9, C-10 (δ 154.7) and C-11; from OH-12 (δ 13.9) to C-11, C-12 (δ 162.7) and C-13; and from
H3-15 (δ 3.84) to C-8 indicated that a naphthalene skeleton connects the partial structure I at C-4. Furthermore, the findings that the chemical shift of C-2 (δ 76.8) corresponds to that of
an oxygenated carbon and that the OH-12 proton (δ 13.9) shifted to a lower field because of hydrogen bonding indicated that C-2 and C-13 are connected through an ester bond. This connection
was supported by the chemical shift of C-1 (δ 171.5) corresponding to an ester bond and the long-range coupling of 4_J_ observed from H-5 to C-1 in 13C–1H HMBC (Figure 2). Thus, these data
indicated that xanthoradone A was found to have a dihydronaphthopyranone skeleton. (2) Cross peaks from H-2′ (δ 6.08) to C-1′ (δ 190.6), C-3′ (δ 160.9), C-4′ (δ 179.8) and C-10′ (δ 112.0);
from H-6′ (δ 7.67) to C-4′, C-5′ (δ 129.8), C-7′ (δ 147.2), C-8′ (δ 130.6), C-10′ and C-12′ (δ 20.5); from OH-9′ (δ 12.5) to C-8′, C-9′ (δ 159.7) and C-10′; from H3-11′ (δ 3.92) to C-3′; and
from H3-12′ (δ 2.21) to C-6′ (δ 121.3), C-7′ and C-8′ indicated that xanthoradone A has another naphthoquinone skeleton. This was supported by the chemical shifts of two carbonyl carbons
(C-1′ (δ 190.6) and C-4′ (δ 179.8)) derived from a quinone skeleton and the UV absorption (263 nm). Finally, the correlations observed between H3-15 and OH-9′, and between OH-10 and H3-12′
in rotating frame nuclear overhauser effect spectroscopy (ROESY) experiments (Figure 3), and the long-range coupling of 4_J_ observed from H-7 to C-8′ in 13C–1H HMBC experiments (Figure 2b)
indicated that the two substructures of dihydronaphthopyranone and naphthoquinone are connected at C-9–C-8′. Taken together, the structure of xanthoradone A was elucidated as shown in Figure
1. The structure satisfied the degree of unsaturation and the molecular formula. STRUCTURE ELUCIDATION OF XANTHORADONE B The molecular formula C27H22O9 of xanthoradone B was the same as
that of xanthoradone A. The 1H NMR spectrum of xanthoradone B was almost identical to that of xanthoradone A except for a weak coupling constant (_J_=2.0 Hz) between H-2′ and H-11′ in
xanthoradone B, which was not observed in xanthoradone A. In fact, cross peaks observed from H-2′ (δ 6.73) to C-4′ (δ 185.0), C-10′ (δ 116.1) and C-11′ (δ 16.4); from H-6′ (δ 7.33) to C-4′,
C-5′ (δ 133.0), C-7′ (δ 163.6), C-8′ (δ 110.3) and C-10′; from OH-9′ (δ 12.3) to C-8′, C-9′ (δ 161.1) and C-10′; from H3-11′ (δ 2.16) to C-2′ (δ 135.8), C-3′ (δ 148.3) and C-4′; and from
H3-12′ (δ 3.88) to C-7′ in the 13C–1H HMBC experiments indicated the presence of a naphthoquinone skeleton (Figure 4). This was also supported by the chemical shifts of two carbonyl carbons
(C-1′ (δ 189.0) and C-4′ (δ 185.0)) derived from a quinone skeleton and the UV absorption (267 nm). Taken together, the structure of xanthoradone B was elucidated as shown in Figure 1; the
oxygenated methyl group at R1 and the methyl group at R2 in xanthoradone A are reversed in xanthoradone B (Figure 1). The structure satisfied the degree of unsaturation and the molecular
formula. DISCUSSION In this study, xanthoradones A and B were isolated from _Penicillium radicum_ strain FKI-3765-2, and their planar structures were elucidated by NMR studies (Figure 1).
They were found to have a common asymmetric biaryl skeleton containing a dihydronaphthopyranone and a naphthoquinone. Regarding the stereochemistry of xanthoradones, both xanthoradone A and
B have one chiral carbon at C-2 in the structure. The orientation of the methyl group at C-2 of the δ-lactone in xanthoradone A was investigated by 1H–1H spin decoupling experiments. On
irradiation at H3-14 (δ 1.57), a complex multiplet at H-2 (δ 4.79) became a simple doublet of doublets with _J_2,3=10.0 and 4.0 Hz. The large coupling constant (_J_2,3=10.0 Hz) indicated
that H-2 and H_ax_-3 (δ 3.00) are oriented in an axial–axial direction. This was supported by the coupling constants of H_ax_-3 (_J_=17.0, 10.0 Hz). Thus, the methyl group at C-2 of the
δ-lactone in xanthoradone A was determined to be equatorial in orientation as shown in Figure 1. Similarly, the orientation of the methyl group at C-2 of the δ-lactone in xanthoradone B was
also deduced, as shown in Figure 1. Some structurally related natural products, such as xanthoviridicatin D produced by _Penicillium viridicatum_3 and xanthoviridicatins E and F, as HIV-1
integrase inhibitors, produced by _Penicillium chrysogenum_,2 have been reported previously (Figure 1). However, the binding pattern of the two substructures differs between xanthoradones
and xanthoviridicatins; C-9 of a dihydronaphthopyranone moiety connects with C-8′ of the aromatic site of the naphthoquinone moiety in xanthoradones, whereas the former connects with C-2′ of
the quinone site of the naphthoquinone moiety in known xanthoviridicatins. By comparison with fungal biaryl compounds containing a dihydronaphthopyranone moiety, such as vioxanthin,4
rubrosulphin,5 viomellein5 and luteosporin,6 the absolute configuration of xanthoradones at C-2 can be deduced to be in 2_R_ configuration. Furthermore, xanthoradones A and B have a chiral
axis in the structures. The configuration of the axis might be _M_ by comparing the CD spectra of (_P_) and (_M_)-vioxanthins;4 xanthoradones show a negative cotton effect at 281–289 nm and
a positive cotton effect at 266–269 nm similar to (_M_)-vioxanthin. METHODS GENERAL EXPERIMENTAL PROCEDURES The UV spectra were recorded on a spectrophotometer (8453 UV-Visible
spectrophotometer; Agilent Technologies, Santa Clara, CA, USA). IR spectra were recorded on a Fourier transform infrared spectrometer (FT-710; Horiba, Kyoto, Japan). Optical rotations were
measured using a digital polarimeter (DIP-1000; JASCO, Tokyo, Japan). ESI-TOF-MS and HRESI-TOF-MS spectra were recorded on a mass spectrometer (JMS-T100LP; JEOL, Tokyo, Japan). Various NMR
spectra were measured using a spectrometer (XL-400; Varian, Palo Alto, CA, USA). REFERENCES * Yamazaki, H., Nonaka, K., Masuma, R., Ōmura, S. & Tomoda, H. Xanthoradones, new potentiators
of imipenem activity against methicillin-resistant _Staphylococcus aureus_, produced by _Penicillium radicum_ FKI-3765-2 I. Taxonomy, fermentation, isolation and biological properties. _J.
Antibiot._ 62, 431–434 (2009). Article CAS Google Scholar * Singh, S. B. _et al_. Isolation, structure, and HIV-1 integrase inhibitory activity of xanthoviridicatin E and F, two novel
fungal metabolites produced by _Penicillium chrysogenum_. _Helv. Chim. Acta._ 86, 3380–3385 (2003). Article CAS Google Scholar * Stack, M. E., Mazzola, E. P. & Eppley, R. M. Structure
of xanthoviridicatins D and xanthoviridicatin G, metabolites of _Penicillium viridicatum_: application of proton and carbon-13 NMR spectroscopy. _Tetrahedron Lett._ 52, 4989–4992 (1979).
Article Google Scholar * Bode, S. E., Drochner, D. & Muller, M. Synthesis, biosynthesis, and absolute configuration of vioxanthin. _Angew. Chem. Int. Ed. Engl._ 46, 5916–5920 (2007).
Article CAS PubMed Google Scholar * Durley, R. C., MacMillan, J., Simpson, T. J., Glen, A. T. & Turner, W. B. Fungal products. Part XIII. Xanthomegnin, viomellin, rubrosulphin, and
viopurpurin, pigments from _Aspergillus sulphureus_ and _Aspergillus melleus_. _J. Chem. Soc. Perkin. Trans. 1_ 2, 163–169 (1975). Article Google Scholar * Mori, H., Kawai, K., Ohbayashi,
F., Kitamura, J. & Nozawa, Y. Genotoxicity of quinone pigments from pathogenic fungi. _Mutat. Res._ 122, 29–34 (1983). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by a grant-in-aid for Scientific Research (B) 21310146 (to HT) from the Ministry of Education, Culture, Sports, Science and Technology, Japan and the
Sasakawa Scientific Research Grant (to HY) from The Japan Science Society. We express our thanks to Ms N Sato for performing NMR experiments, and Dr K Nagai and Ms A Nakagawa for measuring
mass spectra. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Graduate School of Pharmaceutical Sciences, Kitasato University, Tokyo, Japan Hiroyuki Yamazaki & Hiroshi Tomoda * Kitasato
Institute for Life Sciences, Kitasato University, Tokyo, Japan Satoshi Ōmura Authors * Hiroyuki Yamazaki View author publications You can also search for this author inPubMed Google Scholar
* Satoshi Ōmura View author publications You can also search for this author inPubMed Google Scholar * Hiroshi Tomoda View author publications You can also search for this author inPubMed
Google Scholar CORRESPONDING AUTHOR Correspondence to Hiroshi Tomoda. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yamazaki, H., Ōmura, S. &
Tomoda, H. Xanthoradones, new potentiators of imipenem activity against methicillin-resistant _Staphylococcus aureus_, produced by _Penicillium radicum_ FKI-3765-2 II. Structure elucidation.
_J Antibiot_ 62, 435–437 (2009). https://doi.org/10.1038/ja.2009.61 Download citation * Received: 16 April 2009 * Revised: 29 May 2009 * Accepted: 25 June 2009 * Published: 17 July 2009 *
Issue Date: August 2009 * DOI: https://doi.org/10.1038/ja.2009.61 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * fungal metabolites * imipenem
potentiator * methicillin-resistant _Staphylococcus aureus_ (MRSA) * structure elucidation * xanthoradone * xanthoviridicatin