- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The goal of phytoremediation is to use plants to immobilize, extract or degrade organic and inorganic pollutants. In the case of organic contaminants, plants essentially act
indirectly through the stimulation of rhizosphere microorganisms. A detailed understanding of the effect plants have on the activities of rhizosphere microorganisms could help optimize
phytoremediation systems and enhance their use. In this study, willows were planted in contaminated and non-contaminated soils in a greenhouse, and the active microbial communities and the
expression of functional genes in the rhizosphere and bulk soil were compared. Ion Torrent sequencing of 16S rRNA and Illumina sequencing of mRNA were performed. Genes related to carbon and
amino-acid uptake and utilization were upregulated in the willow rhizosphere, providing indirect evidence of the compositional content of the root exudates. Related to this increased
nutrient input, several microbial taxa showed a significant increase in activity in the rhizosphere. The extent of the rhizosphere stimulation varied markedly with soil contamination levels.
The combined selective pressure of contaminants and rhizosphere resulted in higher expression of genes related to competition (antibiotic resistance and biofilm formation) in the
contaminated rhizosphere. Genes related to hydrocarbon degradation were generally more expressed in contaminated soils, but the exact complement of genes induced was different for bulk and
rhizosphere soils. Together, these results provide an unprecedented view of microbial gene expression in the plant rhizosphere during phytoremediation. SIMILAR CONTENT BEING VIEWED BY OTHERS
SOIL DEPTH AND PHYSICOCHEMICAL PROPERTIES INFLUENCE MICROBIAL DYNAMICS IN THE RHIZOSPHERE OF TWO PERUVIAN SUPERFOOD TREES, CHERIMOYA AND LUCUMA, AS SHOWN BY PACBIO-HIFI SEQUENCING Article
Open access 22 August 2024 SOIL BACTERIAL COMMUNITY STRUCTURES IN RELATION TO DIFFERENT OIL PALM MANAGEMENT PRACTICES Article Open access 30 November 2020 SHIFTS IN RHIZOSPHERE MICROBIAL
COMMUNITIES IN _OPLOPANAX ELATUS_ NAKAI ARE RELATED TO SOIL CHEMICAL PROPERTIES UNDER DIFFERENT GROWTH CONDITIONS Article Open access 07 July 2022 INTRODUCTION The rhizosphere comprises the
surface of the roots and the surrounding soil area where plant root exudates sustain a high microbial activity and high microbial density (Smalla et al., 2001; Kowalchuk et al., 2002).
However, bacterial diversity in the rhizosphere is generally lower than in the bulk soil (Marilley and Aragno, 1999), and microbial community composition is very different (Smalla et al.,
2001; Kowalchuk et al., 2002; Griffiths et al., 2006; Kielak et al., 2008), suggesting a strongly selective environment. This selection pressure results from the exudation of specialized
antimicrobials and signaling molecules (for example, flavonoids, salicylic acid and phytoalexins), carbon (for example, organic acids and aromatic compounds) and nitrogen (for example, amino
acids) compounds. The rhizosphere is thereby selectively enriched in microorganisms that are adapted to highly competitive environments and to the utilization of specific plant compounds
(Berg et al., 2002; Gomes et al., 2003; Berg et al., 2005; Haichar et al., 2008). These compounds are not only exuded for the benefit of microbes, but more often they profit the plant
itself. For instance, organic acids such as malate, citrate and oxalate are often present in the rhizosphere, and in addition to being a carbon source for many microbes, they are involved in
many plant processes such as metal detoxification, nutrient acquisition and alleviation of stress (Jones, 1998). Interactions in the rhizosphere have evolved over millions of years and can
be seen as a way for plants to reach a minimal stress level by, among others, deterring pathogens, increasing their nitrogen and phosphorus uptake and detoxifying the environment. Plants
confronted with stressful environments normally respond by increasing root exudation (Jones et al., 2004; Qin et al., 2007; Naik et al., 2009), which leads to increased microbial biomass in
the rhizosphere (Esperschutz et al., 2009). One biotechnological application of the ‘rhizosphere effect’ is phytoremediation. The goal of phytoremediation is to remove pollutants from the
environment or render them harmless by using plants to stabilize, filter, volatilize, extract or degrade organic and inorganic pollutants (Salt et al., 1998; Pilon-Smits, 2005). For the
degradation of organic contaminants, plants essentially act indirectly through the specific stimulation of rhizosphere and endophytic microorganisms (Barac et al., 2004; Kuiper et al., 2004;
Taghavi et al., 2005). Phytoremediation takes advantage of the long-evolved intimate relationships between plant and microbes, of the stimulating effect of plants on microbes and of the
natural hardiness and competitiveness of rhizosphere microbes to remediate contaminated soils or to mobilize inorganic contaminants and favor their accumulation in plant tissues. It is one
of the least expensive and most environmentally friendly remediation techniques, being on average tenfold less expensive than traditional excavation techniques (‘dig and dump’) (Glass, 1999)
and causing very little disruption to the environment. However, phytoremediation often proceeds slowly, as the stimulation of degrading microbes is mainly restricted to the immediate zone
of influence of the roots (Pilon-Smits, 2005). For these reasons, other approaches are often preferred and phytoremediation is restricted to niche markets where time is not an issue and the
contamination is moderate and superficial. To avoid this limitation, fast-growing trees that rapidly develop deep-root systems and produce large biomass, such as poplars and willows, were
suggested (Schnoor et al., 1995). Willow trees have several key advantages for phytoremediation when compared with other plants: they are genetically very diverse (400 species and over 200
hybrids, Newsholme, 2003), some species can be harvested frequently by coppicing, they are pioneer plants that have invasive growth strategies and very effective nutrient uptake systems,
they grow fast and have high evapotranspiration rates and high productivity (Pulford and Watson, 2003). Although several studies have assessed the microbial communities associated with
willows growing in contaminated soils (Leigh et al., 2006; de Carcer et al., 2007a, 2007b; Kuffner et al., 2008; Hrynkiewicz et al., 2009; Zimmer et al., 2009; Weyens et al., 2013; Bell et
al., 2014a), the details of willow interactions with microbes are still not well understood. Clearly, plant–microbe interactions are at the center of the phytoremediation process of organic
contaminants and have a role in the mobilization of inorganic contaminants, but which rhizosphere genes and organisms are involved and how these complex interactions are affected by
contaminant type and concentration have not been elucidated. This knowledge is crucial to fully optimize the degradation processes that occur in the rhizosphere. In this study, our main
objective was to understand what microorganisms are activated and what microbial genes are upregulated in the rhizosphere of willow growing in contaminated and non-contaminated soils as
compared with bulk soil. We harvested the rhizosphere of willows planted in pots containing contaminated and non-contaminated soil, as well as soil from non-planted pots. Gene expression
profiles were contrasted using metatranscriptomic analyses based on Illumina sequencing, and microbial communities were compared based on Ion Torrent sequencing of 16S rRNA. Our results
indicate major shifts in the potential for carbon and amino-acid uptake, and utilization, nutrient cycling and hydrocarbon degradation in the rhizosphere of willows, with a much stronger
stimulation in contaminated soils. To our knowledge, this is the first functional metatranscriptomic profiling of microbial activities in the rhizosphere, providing an unprecedented level of
detail on plant–microbial interactions. MATERIALS AND METHODS For more details, see the Supplementary Material and Methods. GREENHOUSE EXPERIMENT Contaminated and non-contaminated soils
were collected at a former petrochemical plant site at Varennes, QC, Canada (geographic coordinates: contaminated: 45.699145 and −73.430997, and non-contaminated: 45.700788 and −73.430302).
Various petrochemical activities had been carried out on this site, starting in 1953 to the shutdown of the plant in 2008. The soils have been contaminated for decades by mixed petrochemical
residues (see Supplementary Table S1 for detailed soil analyses). After sampling, soils were mixed thoroughly and distributed in 20 l pots. Willow cuttings (_Salix purpurea_ cultivar Fish
Creek) were first grown for 8 weeks in sterile potting media and then transferred to the pots containing Varennes soil on 4 October 2011. At the time of the transfer, the longest stem that
grew from the cuttings was ∼20 cm long and the potting media was densely colonized by roots. Half of the pots remained unplanted and each treatment was replicated six times. The experimental
design was a split-plot with soil contamination randomized first and planted or unplanted randomized in the subplots. The plants were grown in a greenhouse at the Institut de recherche en
biologie végétale, Montreal, under natural daylight supplemented with high-pressure sodium-vapor lamps, and temperatures of 20 °C in day and 18 °C at night. The moisture in every pot was
maintained near-field capacity by frequent watering, and saucers were used under pots to prevent leaching of contaminants. SOIL SAMPLING For molecular analyses, pots were sampled at the end
of the experiment (26 April 2012, ∼6 months after planting). For rhizosphere soil, plants were completely recovered from the pots and shaken vigorously to remove excess soil. Soil still
adhering to the roots at this stage was considered as rhizosphere soil. Soil from unplanted pots was taken at a depth of ∼5 cm. At least five different soil subsamples were collected from
each pot and homogenized in 50 ml Falcon tubes and immediately flash frozen in liquid nitrogen. In total, the entire process for one pot never took more than a few minutes. Tubes were
transported from the greenhouse to the lab under dry ice and kept frozen at −80 °C until the nucleic acid was extracted. For chemical analyses, pots were sampled at the beginning and at the
end of the experiment. From each pot, soil was collected from at least five different zones and homogenized in an amber glass container. Soil samples were sent to Maxxam Analytics (Montreal,
QC, Canada), where soil was analyzed for C10–C50 hydrocarbons (sum of all aliphatic hydrocarbon compounds with chain lengths from C10–C50) and polycyclic aromatic hydrocarbons (PAHs)
according to standard protocols. The percentage degradation was calculated separately for each of the pots. NUCLEIC ACID EXTRACTION Approximately 2 g of frozen soil was weighed and extracted
using MoBio RNA PowerSoil total RNA isolation kit with the RNA PowerSoil DNA elution accessory kit (MoBio, Carlsbad, CA, USA). RNA extracts were treated with Ambion TURBO DNAse (Life
Technologies, Burlington, ON, Canada) and the absence of DNA was confirmed by 16S rRNA gene universal PCR. ION TORRENT 16S RRNA SEQUENCING Reverse-transcriptase (RT)-PCR of the partial 16S
rRNA was performed using the universal primers F343 and R533 containing the 10-bp multiplex identifiers and adaptor sequences for Ion Torrent sequencing described previously (Yergeau et al.,
2012; Bell et al., 2013b). The sequencing of the pooled library was done using the Ion Torrent Personal Genome Machine system (Life Technologies). Sequences were binned and filtered using a
custom-made Perl script. Taxonomic identities were assigned to sequences using the ‘multiclassifier’ (http://pyro.cme.msu.edu/). Weighted-normalized Unifrac distances between each sample
pair were calculated using the FastUnifrac website (Hamady et al., 2010) based on the GreenGene core data set. ILLUMINA MRNA SEQUENCING rRNA was subtracted following the protocol described
by Stewart et al. (2010). Total rRNA-subtracted RNA was reverse-transcribed using the SuperScript III kit (Invitrogen). Illumina libraries were prepared following the protocol of Meyer and
Kircher (2010), with indices 1–24 pooled together and sent for eight lanes of Illumina HiSeq 2000 paired-end 2 × 101 bp sequencing at McGill University and Génome Québec Innovation Center,
Montréal. Data from the different lanes were pooled together and the resulting 48 files were filtered in pairs using a custom-made Perl script. The resulting high-quality sequences were
submitted to MG-RAST 3.0 (Meyer et al., 2008) for automated annotation. DATA ANALYSIS All statistical analyses were carried out in R (v 2.13.2, The R Foundation for Statistical Computing).
Normal distribution and variance homogeneity of the data were tested using the ‘shapiro.test’ and ‘bartlett.test’ functions, respectively. If the data were not normally distributed or did
not show homogeneous variance, they were log transformed before analysis of variance (ANOVA) analyses. ANOVA were carried out using the ‘aov’ function, whereas _t_-test were performed using
the ‘t.test’ function. Multivariate tests of hypothesis were carried out using Permanova with the ‘adonis’ function of the ‘vegan’ package. The Unifrac matrix was used for principal
coordinate analyses (PCoA) that were carried out using the ‘pcoa’ function of the ‘ape’ package. RESULTS SOIL PHYSICO-CHEMICAL CHARACTERISTICS AND PLANT BIOMASS The non-contaminated soils
contained no PAH and no C10–C50 hydrocarbons at the beginning of the experiment (Table 1; see Supplementary Table S1 for a description of the contaminants). There was only one exception in
the to-be planted pots, where C10–C50 hydrocarbons were detected at a level of 110 mg kg−1 (Supplementary Table S1). At the end of the experiment, all the soils in the non-contaminated pots
had C10–C50 and PAH concentrations below the detection limit. For the contaminated soils, no significant differences at _P_<0.05 (_t_-test) were observed between the planted and unplanted
pots at the beginning of the experiment or at the end for both C10–C50 and PAHs (Table 1, Supplementary Table S1). The concentrations of C10–C50 and PAHs were more variable at the beginning
of the experiment (C10–C50: 480–4200 mg kg−1; PAH: 15.6–186.4 mg kg−1) than at the end of the experiment (C10–C50: 270–660 mg kg−1; PAH: 3.0–19.3 mg kg−1). There was a large decrease in
hydrocarbon concentrations in all contaminated soils during the incubation period, but there was no significant difference between planted and unplanted pots at _P_<0.05 (_t_-test), with
degradation ranging from 0% to 91% for C10–C50 and from 65% to 93% for PAH. At the final time of sampling (April 2012), contaminated and non-contaminated soils from the unplanted pots and
from the rhizosphere of willows were sampled for chemical analyses. Water-extractable PO4 was not detected in any of the samples, whereas the concentration of water-extractable K was
significantly higher in contaminated soil independent of the soil compartment (379 μM kg−1 vs 261 μM kg−1), and the concentration of water-extractable NO3 was significantly lower in the
rhizosphere of willows independent of contamination levels (59.5 μM kg−1 vs 454.9 μM kg−1) (B Cloutier-Hurteau, M-C Turmel and F Courchesne, personal communication). For willow biomass,
there was no significant difference between willows growing in contaminated or non-contaminated soils for the shoots (average: 36.0 g), the roots (average: 6.9 g) and total plant biomass
(average: 50.3 g), but the willows growing in contaminated soils had significantly higher leaf biomass (8.4 g vs 6.3 g) (B Cloutier-Hurteau, M-C Turmel and F Courchesne, personal
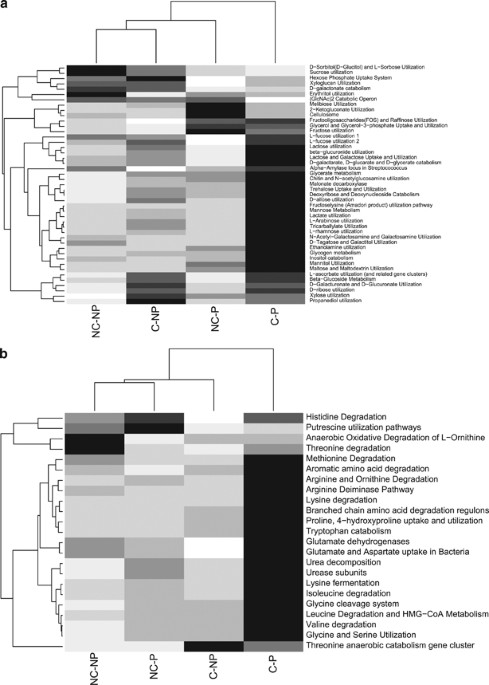
communication). CARBON AND AMINO-ACID UTILIZATION The expression of several gene categories (MG-RAST ‘level 3’) related to carbon uptake and utilization was significantly influenced by the
presence of plants and soil contamination levels (Figure 1a). Most gene categories were more actively expressed in the rhizosphere of contaminated soils, including all gene categories
related to organic acids (Figure 1a). The non-planted soils clustered together and shared similar expression levels of several gene categories, but the rhizosphere soils showed very
dissimilar patterns of expression and did not cluster together (Figure 1a). Permanova tests for the effect of the different treatments on all the gene categories selected yielded significant
results for contamination level (F=4.75, _P_=0.0010) and willow presence (F=7.43, _P_=0.0001), but not for the interaction between contamination and willow. Based on the F-ratios, the
strongest effect was seen when comparing non-planted vs rhizosphere soils. The expression of the phosphoenolpyruvate carboxykinase (_pckA_) gene, which encodes the enzyme that catalyses the
key rate-limiting step in gluconeogenesis, was significantly induced by willow presence (F=7.40, _P_=0.015), but not by contamination level (Figure 2a). Similarly, the expression of
carbohydrate uptake ABC transporter (CUT1 family), which is usually involved in the import of oligosaccharides and their derivatives, was significantly enhanced in the rhizosphere of willows
(F=7.19, _P_=0.015), but not affected by contamination levels (Figure 2a). Most gene categories (MG-RAST ‘level 3’) related to amino-acid utilization and degradation were more expressed in
the rhizosphere of willows planted in contaminated soils (Figure 1b). This was also true for the degradation of aromatic amino acids such as tryptophan and histidine (Figure 1b). In the case
of histidine degradation, this gene category was also highly expressed in the rhizosphere of willows planted in non-contaminated soils (Figure 1b). The non-contaminated soils clustered
together, whereas the contaminated willow rhizosphere soil was a clear outlier (Figure 1b). Permanova tests for the effect of treatments on the overall expression pattern of amino-acid
utilization gene categories resulted in a significant interaction term (F=5.28, _P_=0.002), suggesting a differential effect of willows in contaminated and non-contaminated soils.
Contamination level and willow presence also had a significant influence on gene expression patterns (F=4.69, _P_= 0.0034 and F=6.63, _P_=0.00030, respectively). Based on the F-ratios, the
strongest effect was seen when comparing non-planted vs rhizosphere soils. COMPETITION AND COOPERATION There was a significant effect of contamination level (F=16.15, _P_=0.00067) and willow
presence (F=29.33, _P_=0.000027), but not of the interaction term on the expression of genes related to antibiotic resistance. Antibiotic resistance genes were more expressed in the
rhizosphere of willow across both contamination levels (Figure 2b). Similarly, antibiotic resistance genes were significantly more expressed in contaminated soils, regardless of willow
presence. These two trends resulted in a higher expression of antibiotic resistance genes in the rhizosphere of willows planted in contaminated soils as compared with all other treatments
(Figure 2b). The expression of quorum sensing and biofilm formation genes was significantly affected by contamination level (F=26.14, _P_=0.000053), willow presence (F=23.47, _P_=0.000098)
and the interaction term (F=8.40, _P_=0.0089). ACTIVE MICROBIAL COMMUNITY COMPOSITION Two methods were used to determine active microbial community composition: taxonomic classification of
all sequenced mRNA in MG-RAST and 16S rRNA sequencing. The first method, with the normalization used in this study, gives the activity (total abundance of mRNA) for different taxa, whereas
the second method gives an overview of the active community composition with relative activity values (percentage of all ribosomes) for different taxa. There was a significant effect of
contamination level (F=27.22, _P_= 0.000042) and the interaction term (F=17.14, _P_=0.00051) on the total abundance of mRNA related to Bacteria (bacterial activity), whereas the effect of
willow was marginally significant (F=3.36, _P_=0.082) (Figure 3a). This was confirmed by RT-quantitative PCR based on bacterial 16S rRNA, which identified the contaminated willow rhizosphere
soil as having significantly higher bacterial activity. In contrast, fungi were significantly more active in the rhizosphere of willows (F=43.22, _P_= 0.0000021) as compared with bulk soil,
and the interaction term was significant (F=7.36, _P_=0.013), whereas contamination level had no significant effect (Figure 3a). This different rhizosphere effect on bacterial and fungal
activity was also visible at the phylum/class level, where most fungal phyla (_Basidiomycota_, _Ascomycota_ and _Glomeromycota_) were more active in the rhizosphere of willows planted in
non-contaminated soils (Figure 3b). _Actinobacteria_ and _Firmicutes_ were also more active in the rhizosphere of willows planted in non-contaminated soils. In contrast, several of the most
abundant bacterial phyla/classes (_Alphaproteobacteria_, _Betaproteobacteria_, _Gammaproteobacteria_ and _Acidobacteria_) were more active in the rhizosphere of willows planted in
contaminated soils (Figure 3b). Most phyla/classes showed low activity in the bulk soil, and contaminated and non-contaminated bulk soils clustered together (Figure 3b). _Euryarchaeota_ were
more active in the contaminated bulk soil, whereas _Crenarchaeota_ and _Thaumarchaeota_ were more active in the rhizosphere of willows planted in non-contaminated soil (Figure 3b). When
testing the effects of the treatments on the activities at the phylum/class level using Permanova, the interaction term was significant (F=9.20, _P_=0.0001) as well as both single factors
(contamination level: F=4.61, _P_=0.0044, willow presence: F=13.39, _P_=0.0001), with a dominance of the willow effect (higher F-ratio). The significant differences observed at the
phylum/class level persisted at lower taxonomic levels. At the genus level, the interaction term (F=9.60, _P_=0.0001) and both single factors (contamination: F=4.74, _P_=0.0021; willow
presence: F=6.96, _P_=0.0003) were significant in Permanova tests. The effect of willow presence was then tested on each genus, separately for contaminated and non-contaminated soils
(Supplementary Table S2). The activities of several genera were significantly affected by willows both in contaminated and non-contaminated samples. However, more genera were significantly
affected by willows in the non-contaminated soils (78 less active with willows and 1963 more active with willows, total 2041) than in the contaminated soils (8 less active with willows and
110 more active with willows, total 118). Among these, 42 genera were significantly affected by willows at both contamination levels, and the 24 bacterial and fungal genera among those are
listed in Supplementary Table S2. Among the genera consistently activated by willows at both contamination levels, some were previously reported as having interesting characteristics for
phytoremediation, for instance, _Methylibium petroleiphilum_ is involved in aromatic hydrocarbon and methyl tert-butyl ether degradation (Nakatsu et al., 2006), _Mesorhizobium_ is a
well-known genus involved in nitrogen fixation, some species are resistant to metals (Vidal et al., 2009; Huang et al., 2010) and can degrade acetonitrile (Feng and Lee, 2009), some
_Variovorax_ species display plant growth promoting activities and are able to degrade various contaminants (Han et al., 2011) and _Parvibaculum_ species are able to degrade linear carbon
chains, including alkanes (Schleheck et al., 2004; Rosario-Passapera et al., 2012). Some other genera showed contrasting responses to willows when exposed to different concentrations of
contaminants (Supplementary Table S2). Most of the genera that were positively affected by willows in contaminated soils and negatively affected in non-contaminated soils belonged to the
_Alphaproteobacteria_ or the _Gammaproteobacteria_, whereas the two genera negatively affected by willows in contaminated soils, but positively affected in non-contaminated soil belonged to
the _Blastocladiomycota_ (Supplementary Table S2). The active community composition based on 16S rRNA gene sequencing revealed that contamination level was the main influencing factor
(Figure 4a). However, within this dominant effect of contamination levels, willow presence did have some effect on bacterial community composition. At lower taxonomic levels (Unifrac
distances), willow presence only had a significant effect on microbial communities in the contaminated soils (Permanova: non-contaminated soils: F=1.29, _P_=0.14; and contaminated soils:
F=1.95, _P_=0.016). This effect can also be visualized in the principal coordinate ordinations (Figure 4b). In contrast, active species richness (number of operational taxonomical units at
97% similarity) was not affected by willow presence or contamination levels. NITROGEN CYCLE GENES Nitrogen cycling is one of the key soil functions in the context of soil bioremediation,
where nutrients are often limiting due to an unbalanced C:N ratio. Willow significantly increased the expression of ammonia monooxygenase genes, as willow presence was the only significant
factor in ANOVA tests (F=5.68, _P_=0.027) (Figure 5a). Willows presence appeared to have a stronger effect on ammonia monooxygenase expression in non-contaminated soils (Figure 5a). Willow
had a significant influence on the expression of several other genes, with an increased expression of genes related to nitrogen fixation (nitrogenase) and nitrate reduction in the
rhizosphere, and higher expression of several genes related to denitrification in the bulk soils (Figure 5b). The overall effect of the treatments on the different nitrogen cycle genes was
tested by Permanova, which highlighted significant effects of willow presence (F=2.39, _P_=0.034), contamination levels (F=2.75, _P_=0.016) and the interaction term (F=2.46, _P_=0.027).
HYDROCARBON DEGRADATION GENES The expression of alkane hydroxylase genes was significantly affected by contamination level (F=23.50, _P_=0.000098), willow presence (F=10.52, _P_=0.0041) and
the interaction term (F=5.73, _P_=0.027), with a dominating effect of contamination (higher F-ratio) (Figure 6a). Alkane hydroxylases genes were more expressed in the rhizosphere of willows
planted in contaminated soils than in any other treatment (Figure 6a). For the expression of genes related to the degradation of aromatic compounds, Permanova tests revealed a strong effect
of contamination (F=7.76, _P_=0.0001) and of willow presence (F=3.90, _P_=0.0056), whereas the interaction term was significant but with a weaker effect (F=2.63, _P_=0.030). Within
contaminated soils, willows induced the expression of several aromatic degradation gene categories and pathways, whereas some others were repressed by the presence of willows and others were
highly expressed across contaminated soils (Figure 6b). With a few exceptions, the vast majority of aromatic hydrocarbon degradation gene categories showed relatively low expression levels
in the non-contaminated soils (Figure 6b). DISCUSSION Several studies have addressed the transcriptomics of the rhizosphere (Diehn and Relman, 2001; Mark et al., 2005; Matilla et al., 2007;
Ramachandran et al., 2011), but always using a controlled approach with a single bacterium, one or a few plants in sterile substrates or purified root exudates. In this study, we used a
metatranscriptomic approach and assessed the whole microbial community associated with willow roots growing in non-sterile soil from a contaminated site. This extremely powerful approach has
often been proposed (Kowalchuk et al., 2010; Schenk et al., 2012; Turner and Poole, 2013) and was recently applied to examine taxonomic changes in the rhizosphere (Turner et al., 2013) but,
to our knowledge, this is the first time it has been used to assess the functions, activities and the interactions in the rhizosphere at the community level. The results presented here
give, with an unprecedented level of detail, a portrait of the microbial activities in the rhizosphere of willows as compared with bulk soil, and link these shifts to the potential for
phytoremediation of organic contaminants by willows. METATRANSCRIPTOMICS OF THE RHIZOSPHERE Although the composition of the active microbial community (based on 16S rRNA genes; Figure 4) did
not vary much with the introduction of willows, being more strongly affected by the contamination levels, the community members drastically changed their activities (based on mRNA; Figure
3). Microbes in the rhizosphere of willow had significantly different expression patterns for genes involved in carbon and amino-acid uptake and utilization, strong, indirect evidence for
the use of compounds in root exudates. The overall increase in the expression of phosphoenolpyruvate carboxykinase (involved in the rate-controlling step of glucose synthesis; repressed by
sugars) and CUT1 transport gene (involved in the transport of oligosaccharides) expression in the rhizosphere and the high expression of organic acid uptake and utilization genes in the
contaminated rhizosphere suggest that simple sugars are not the main bacterial carbon source in the rhizosphere of willows. Organic acids were previously reported as the main source of
carbon in the rhizosphere of many plants, including poplar (Jones, 1998; Lugtenberg et al., 2001; Naik et al., 2009; Ramachandran et al., 2011). The effect of willow presence varied
depending on the contamination level of the soil. For instance, bacteria appeared to be generally stimulated in contaminated soils along with many functions, whereas fungi were more clearly
stimulated in non-contaminated soils. Similarly, the rhizosphere effect of birch on soil microbial communities was reported to depend on PAH concentration (Sipilä et al., 2008). One possible
explanation for this result is that plants recruit the rhizosphere microbial communities from the adjacent bulk soil (de Ridder-Duine et al., 2005; Dennis et al., 2010; Lundberg et al.,
2012), and that contaminated and non-contaminated soils harbored different starting microbial communities (Figure 4). Along with specialized carbon compounds, plants also exude several
antimicrobial compounds that create a strongly selective environment in the rhizosphere (Bais et al., 2006). Several studies reported decreased microbial diversity in the rhizosphere
(Marilley and Aragno, 1999; Kowalchuk et al., 2002), supporting the concept of a more selective environment. In our case, the presence of contaminants in the soils is imposing a further
selection pressure on microorganisms. Under this double-selection pressure, only microorganisms that can use specialized carbon sources, can cope with the presence of contaminants and are
highly competitive will be significantly activated by willows. Our results showed that more microbial genera were activated by willows in non-contaminated soils than in contaminated soils.
We also observed significant increases in the expression of antibiotic and toxic compound resistance genes in the rhizosphere, with stronger increases in contaminated soils, alluding to
increased microbial competition or a less hospitable rhizosphere environment. Plants exude antimicrobial compounds (for example, phytoalexins, salicylic acid and flavonoids) that can lead to
increased expression of antibiotic resistance genes. Salicylic acid was reported to induce the expression of antibiotic resistance genes in _Escherichia coli_ (Cohen et al., 1993), whereas
multidrug efflux pump genes of _Rhizobium etli_ were induced by bean root flavonoids and were needed for resistance to phytoalexins, flavonoids and salicylic acid, and effective colonization
of the rhizosphere (Gonzalez-Pasayo and Martinez-Romero, 2000). Mutation in multidrug efflux pumps reduced the fitness of _Pseudomonas putida_ in the rhizosphere of corn (Matilla et al.,
2007). Increased selection pressure in the contaminated rhizosphere soil also potentially led to an increased expression of genes related to cooperation and communication (quorum sensing and
biofilm formation category). Biofilm formation is an essential feature of rhizosphere colonization (Lugtenberg et al., 2001; Ramey et al., 2004) and bacteria living in biofilms have
increased resistance to environmental stresses (Costerton et al., 1999; Hogan and Kolter, 2002). Consequently, genes related to biofilm formation were reported to be over-expressed during
rhizosphere colonization, and their inactivation led to reduced fitness (Matilla et al., 2007; Ramachandran et al., 2011; Fan et al., 2012). Some of the genes included in the quorum sensing
and biofilm formation category (for example, N-acyl homoserine lactone synthetase) are also involved in plant–microbe communication, as N-acyl homoserine lactone will induce plants to
release root exudates (Mathesius et al., 2003). In the present study, we observed an extremely pronounced induction of carbon uptake and utilization genes in the rhizosphere of willows
growing in contaminated soils, indirectly suggesting an increased exudation, which could explain the different rhizosphere effects in contaminated and non-contaminated soils. Poplars root
exudation of C was stimulated up to 100-fold by increasing Al, Cu and Zn concentrations (Qin et al., 2007; Naik et al., 2009). These increases in the exudation are mechanisms by which plants
reduce the toxicity of trace elements in soil, as organic acids can effectively bind many compounds. Stressed beech trees were also shown to have a larger microbial biomass in their
rhizosphere (Esperschutz et al., 2009). Our contaminated soils did contain a range of trace elements, but at relatively low concentrations, in the same range as the non-contaminated soils (B
Cloutier-Hurteau, M-C Turmel and F Courchesne, personal communication), which is unlikely to have caused significantly higher stress to the plants in the contaminated soils. We also did not
observe any clear indication of an increasingly stressful environment for the plants growing in contaminated soils. In fact, plant biomass was not reduced in contaminated soils and leaf
biomass even increased when willows were planted in contaminated soils. As suggested by Henry et al. (2007), one way to improve phytoremediation would be by increasing plant stress levels
through various manipulations (for example, soil nutrient and water content), in order to increase stimulation of the microbes in the rhizosphere. The concentration of nitrate was lower in
the rhizosphere of willows planted in both contaminated and non-contaminated soil. The chemical data matched well with the observed patterns in the expression of genes related to the
nitrogen cycle: high nitrate concentration is conducive to the expression of denitrification genes (Barnard et al., 2005) (several of these genes were more expressed in the bulk soil) and
inhibits nitrogen fixation (Streeter and Wong, 1988) (these genes were highly expressed in the rhizosphere). The lower concentration of nitrate might be because of the increased microbial
activity observed in the rhizosphere, especially in contaminated soils, where bacteria were significantly more active and nitrate at its lowest concentration, being below the detection limit
for two of the three replicates analyzed. Nitrate reduction was shown to be stimulated by root exudates (Henry et al., 2008), and, in this study, several nitrate reductase genes were more
expressed in the rhizosphere of willow, offering another potential explanation for the lower nitrate concentration observed in the rhizosphere. Taken together, these data suggest that the
stimulation of the rhizosphere hydrocarbon-degrading microorganisms by willows is not because of an increased nitrate input from the roots. However, measurements of other available nitrogen
forms would be necessary to convincingly rule out if increased nitrogen input has a role in willow phytoremediation. Indeed, nitrogen transfer from willow to the rhizosphere microorganisms
might take the form of amino acids, and gene expression related to their uptake and utilization were highly elevated in the rhizosphere as compared to the bulk soil. INCREASED EXPRESSION OF
HYDROCARBON DEGRADATION GENES In soils contaminated with organic pollutants, the goal of phytoremediation is to stimulate rhizobacteria to degrade the organic contaminants. Considering this,
our 6-month pot experiment was successful in showing a generally higher expression of many hydrocarbon-degrading genes in the rhizosphere of willow. As expected, the expression of
hydrocarbon degradation genes was mainly governed by soil contamination. However, within this overarching effect of contaminant presence, willows did have significant effects on the
expression of functional genes related to hydrocarbon degradation. Willows induced the expression of several genes that were expressed at very low levels in the contaminated bulk soil. One
of the most interesting features of phytoremediation is that many plant-derived chemicals (root degradation compounds, secondary metabolites, etc.) stimulate microorganisms to degrade
contaminants (Donnelly et al., 1994; Fletcher and Hegde, 1995; Haby and Crowley, 1996; Miya and Firestone, 2001; Isidorov and Jdanova, 2002). Many of these plant-derived compounds are PAH
analogs (Singer et al., 2003), and the rhizosphere of plants growing in uncontaminated soils is already enriched in PAH-degrading microorganisms (Daane et al., 2001). Many rhizosphere
organisms also express aromatic degradation genes even in the absence of aromatic contaminants in the soil. In the absence of contaminant, it has been shown that _Pseudomona_s
aromatic-degrading genes are upregulated in the corn rhizosphere, and that mutation in these genes results in a reduced fitness to rhizosphere conditions (Matilla et al., 2007). In the
rhizosphere of various plants growing in non-contaminated soils, the shikimate and protocatechuate transport systems of _Rhizobium leguminosarum_ were induced, and, when these genes were
mutated, it led to reduced efficiency in rhizosphere colonization (Ramachandran et al., 2011). When exposing _Pseudomonas aeruginosa_ to root exudates, genes related to aromatic compound
catabolism and protocatechuate 3,4-dioxygenase were significantly upregulated (Mark et al., 2005). More specifically, several willow species are recognized for their production of salicylic
acid and related compounds. Salicylate is derived from the amino acid phenylalanine and is degraded through the catechol or gentisate pathways (Ishiyama et al., 2004). Salicylate-degrading
bacteria were previously found to be enriched beneath willows in comparison with other vegetation (Schmidt et al., 2000). Salicylate has been shown to induce transcription of genes involved
in the degradation of PAH (Schell, 1985) and polychlorinated biphenyl (Master and Mohn, 2001), to enhance the microbial degradation of naphthalene (Yen and Gunsalus, 1982; Van Der Meer et
al., 1992) and other PAHs, such as fluoranthene, pyrene, benz[a]anthracene, chrysene and benz[a]pyrene (Chen and Aitken, 1999), and to function as a growth substrate for polychlorinated
biphenyl-degrading bacteria (Singer et al., 2003). Here, within a complex microbial community, many PAH-degrading genes were significantly more expressed in the rhizosphere of willows
planted in contaminated soils. More tests would be necessary to discriminate the role of salicylate among other possible mechanisms explaining this increased gene expression. If salicylate
is confirmed as having a major role in enhancing the survival of PAH-degrading microorganisms and increasing PAH degradation activities, improved phytoremediation could be achieved by
breeding willow cultivars that have increased production of salicylate. Some researchers have investigated the efficacy of amending PAH-contaminated soil with salicylate in an effort to
induce PAH degradation by indigenous soil microorganisms (Ogunseitan et al., 1991; Colbert et al., 1993). The major weakness of phytoremediation is that the effect of plants is largely
limited to the rhizosphere, and, as a consequence, phytoremediation treatments typically proceed at slower rates than other bioremediation techniques. Even with fast-growing deep-rooting
trees such as willows, phytoremediation treatment during this 6-month experiment did not significantly increase global hydrocarbon degradation in the pots, as the final hydrocarbon
concentrations were not significantly different between the planted and unplanted pots. The probable cause is that sufficient rhizosphere soil was not available for chemical analyses, thus
soil outside the direct influence of roots (bulk soil) was included in samples for the determination of hydrocarbon concentrations. However, the fact that the contamination levels did not
differ significantly at the end of our study was an added benefit, as it allowed us to discriminate between the effect of willow and contamination, without having the confounding effect of
different contamination levels in planted and unplanted pots. CONCLUSIONS This study provided the first in-depth metatranscriptomic view of microbial gene expression in the rhizosphere. Our
results indicate a strong rhizosphere effect, with increased expression of many genes related to carbon and amino-acid utilization, nitrogen cycling and hydrocarbon degradation. This
rhizosphere effect was in many cases more pronounced in contaminated soils. The large increase in the expression of hydrocarbon degradation genes in the rhizosphere of willow is promising,
even though after only 6 months, we did not observe a significant effect of willows on hydrocarbon concentration outside the rhizosphere compartment. Several potential mechanisms explaining
the stimulation of rhizosphere microbes were suggested: increased nitrogen inputs, increased plant exudation due to stressful conditions and exudation of salicylate and other PAH analogs.
Future studies will be focused on precisely determining the mechanism behind the observed rhizosphere stimulation of hydrocarbon degraders and devising potential ways to improve
phytoremediation. REFERENCES * Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM . (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. _Annu Rev
Plant Biol_ 57: 233–266. Article CAS PubMed Google Scholar * Barac T, Taghavi S, Borremans B, Provoost A, Oeyen L, Colpaert JV _et al_ (2004). Engineered endophytic bacteria improve
phytoremediation of water-soluble, volatile, organic pollutants. _Nat Biotechnol_ 22: 583–588. Article CAS PubMed Google Scholar * Barnard R, Leadley PW, Hungate BA . (2005). Global
change, nitrification, and denitrification: a review. _Glob Biogeochem Cy_ 19: GB1007. Article CAS Google Scholar * Bell TH, El-Din Hassan S, Lauron-Moreau A, Al-Otaibi F, Hijri M,
Yergeau E _et al_ (2014). Linkage between bacterial and fungal rhizosphere communities in hydrocarbon-contaminated soils is related to plant phylogeny. _ISME J_ 8: 331–343. Article CAS
PubMed Google Scholar * Bell TH, Yergeau E, Maynard C, Juck D, Whyte LG, Greer CW . (2013). Predictable bacterial composition and hydrocarbon degradation in Arctic soils following diesel
and nutrient disturbance. _ISME J_ 7: 1200–1210. Article CAS PubMed PubMed Central Google Scholar * Berg G, Roskot N, Steidle A, Eberl L, Zock A, Smalla K . (2002). Plant-dependent
genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different _Verticillium_ host plants. _Appl Environ Microbiol_ 68: 3328–3338. Article CAS PubMed PubMed
Central Google Scholar * Berg G, Zachow C, Lottmann J, Gotz M, Costa R, Smalla K . (2005). Impact of plant species and site on rhizosphere-associated fungi antagonistic to _Verticillium
dahliae_ Kleb. _Appl Environ Microbiol_ 71: 4203–4213. Article CAS PubMed PubMed Central Google Scholar * Chen S-H, Aitken MD . (1999). Salicylate stimulates the degradation of
high-molecular weight polycyclic aromatic hydrocarbons by Pseudomonas saccharophila P15. _Environ Sci Technol_ 33: 435–439. Article CAS Google Scholar * Cohen SP, Levy SB, Foulds J,
Rosner JL . (1993). Salicylate induction of antibiotic resistance in _Escherichia coli_: activation of the mar operon and a mar-independent pathway. _J Bacteriol_ 175: 7856–7862. Article
CAS PubMed PubMed Central Google Scholar * Colbert SF, Isakeit T, Ferri M, Weinhold AR, Hendson M, Schroth MN . (1993). Use of an exotic carbon source to selectively increase metabolic
activity and growth of _Pseudomonas putida_ in soil. _Appl Environ Microbiol_ 59: 2056–2063. CAS PubMed PubMed Central Google Scholar * Costerton JW, Stewart PS, Greenberg EP . (1999).
Bacterial biofilms: A common cause of persistent infections. _Science_ 284: 1318–1322. Article CAS PubMed Google Scholar * Daane L, Harjono I, Zylstra G, Häggblom M . (2001). Isolation
and characterization of polycyclic aromatic hydrocarbon-degrading bacteria associated with the rhizosphere of salt marsh plants. _Appl Environ Microbiol_ 67: 2683–2691. Article CAS PubMed
PubMed Central Google Scholar * De Carcer DA, Martin M, Karlson U, Rivilla R . (2007a). Changes in bacterial populations and in biphenyl dioxygenase gene diversity in a polychlorinated
biphenyl-polluted soil after introduction of willow trees for rhizoremediation. _Appl Environ Microbiol_ 73: 6224–6232. Article PubMed PubMed Central CAS Google Scholar * De Carcer DA,
Martin M, Mackova M, Macek T, Karlson U, Rivilla R . (2007b). The introduction of genetically modified microorganisms designed for rhizoremediation induces changes on native bacteria in the
rhizosphere but not in the surrounding soil. _ISME J_ 1: 215–223. Article PubMed Google Scholar * De Ridder-Duine AS, Kowalchuk GA, Gunnewiek P, Smant W, van Veen JA, de Boer W . (2005).
Rhizosphere bacterial community composition in natural stands of Carex arenaria (sand sedge) is determined by bulk soil community composition. _Soil Biol Biochem_ 37: 349–357. Article CAS
Google Scholar * Dennis PG, Miller AJ, Hirsch PR . (2010). Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? _FEMS
Microbiol Ecol_ 72: 313–327. Article CAS PubMed Google Scholar * Diehn M, Relman DA . (2001). Comparing functional genomic datasets: lessons from DNA microarray analyses of host-pathogen
interactions. _Curr Opin Microbiol_ 4: 95–101. Article CAS PubMed Google Scholar * Donnelly PK, Hegde RS, Fletcher JS . (1994). Growth of PCB-degrading bacteria on compounds from
photosynthetic plants. _Chemosphere_ 28: 981–988. Article Google Scholar * Esperschutz J, Pritsch K, Gattinger A, Welzl G, Haesler F, Buegger F _et al_ (2009). Influence of chronic ozone
stress on carbon translocation pattern into rhizosphere microbial communities of beech trees (_Fagus sylvatica_ L.) during a growing season. _Plant Soil_ 323: 85–95. Article CAS Google
Scholar * Fan B, Carvalhais L, Becker A, Fedoseyenko D, von Wiren N, Borriss R . (2012). Transcriptomic profiling of Bacillus amyloliquefaciens FZB42 in response to maize root exudates.
_BMC Microbiol_ 12: 116. Article CAS PubMed PubMed Central Google Scholar * Feng Y-S, Lee C-M . (2009). The potential of the acetonitrile biodegradation by _Mesorhizobium_ sp. F28. _J
Haz Mat_ 164: 646–650. Article CAS Google Scholar * Fletcher JS, Hegde RS . (1995). Release of phenols by perennial plant roots and their potential importance in bioremediation.
_Chemosphere_ 31: 3009–3016. Article CAS Google Scholar * Glass DJ . (1999) _U.S. and International Markets for Phytoremediation, 1999–2000. D_. Glass Associates: Needham: MA, USA., pp
270. Google Scholar * Gomes NCM, Fagbola O, Costa R, Rumjanek NG, Buchner A, Mendona-Hagler L _et al_ (2003). Dynamics of fungal communities in bulk and maize rhizosphere soil in the
tropics. _Appl Environ Microbiol_ 69: 3758–3766. Article CAS PubMed PubMed Central Google Scholar * Gonzalez-Pasayo R, Martinez-Romero E . (2000). Multiresistance Genes of _Rhizobium
etli_ CFN42. _Mol Plant-Microbe Interact_ 13: 572–577. Article CAS PubMed Google Scholar * Griffiths RI, Bailey MJ, McNamara NP, Whiteley AS . (2006). The functions and components of the
Sourhope soil microbiota. _Appl Soil Ecol_ 33: 114–126. Article Google Scholar * Haby PA, Crowley DE . (1996). Biodegradation of 3-chlorobenzoate as affected by rhizodeposition and
selected carbon substrates. _J Environ Qual_ 25: 304–310. Article CAS Google Scholar * Haichar F, Marol C, Berge O, Rangel-Castro JI, Prosser JI, Balesdent J _et al_ (2008). Plant host
habitat and root exudates shape soil bacterial community structure. _ISME J_ 2: 1221–1230. Article CAS PubMed Google Scholar * Hamady M, Lozupone C, Knight R . (2010). Fast UniFrac:
facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. _ISME J_ 4: 17–27. Article CAS PubMed Google Scholar
* Han J-I, Choi H-K, Lee S-W, Orwin PM, Kim J, LaRoe SL _et al_ (2011). Complete genome sequence of the metabolically versatile plant growth-promoting endophyte _Variovorax paradoxus_ S110.
_J Bacteriol_ 193: 1183–1190. Article CAS PubMed Google Scholar * Henry A, Doucette W, Norton J, Bugbee B . (2007). Changes in crested wheatgrass root exudation caused by flood, drought,
and nutrient stress. _J Environ Qual_ 36: 904–912. Article CAS PubMed Google Scholar * Henry S, Texier S, Hallet S, Bru D, Dambreville C, Cheneby D _et al_ (2008). Disentangling the
rhizosphere effect on nitrate reducers and denitrifiers: insight into the role of root exudates. _Environ Microbiol_ 10: 3082–3092. Article CAS PubMed Google Scholar * Hogan D, Kolter R
. (2002). Why are bacteria refractory to antimicrobials? _Curr Opin Microbiol_ 5: 472–477. Article CAS PubMed Google Scholar * Hrynkiewicz K, Ciesielska A, Haug I, Baum C . (2009).
Ectomycorrhiza formation and willow growth promotion as affected by associated bacteria: role of microbial metabolites and use of C sources. _Biol Fertil Soils_ 46: 139–150. Article CAS
Google Scholar * Huang A, Teplitski M, Rathinasabapathi B, Ma L . (2010). Characterization of arsenic-resistant bacteria from the rhizosphere of arsenic hyperaccumulator Pteris vittata.
_Can J Microbiol_ 56: 236–246. Article CAS PubMed Google Scholar * Ishiyama D, Vujaklija D, Davies J . (2004). Novel pathway of salicylate degradation by _Streptomyces_ sp. Strain WA46.
_Appl Environ Microbiol_ 70: 1297–1306. Article CAS PubMed PubMed Central Google Scholar * Isidorov V, Jdanova M . (2002). Volatile organic compounds from leaves litter. _Chemosphere_
48: 975–979. Article CAS PubMed Google Scholar * Jones DL . (1998). Organic acids in the rhizosphere - a critical review. _Plant Soil_ 205: 25–44. Article CAS Google Scholar * Jones
DL, Hodge A, Kuzyakov Y . (2004). Plant and mycorrhizal regulation of rhizodeposition. _New Phytol_ 163: 459–480. Article CAS PubMed Google Scholar * Kielak A, Pijl AS, van Veen JA,
Kowalchuk GA . (2008). Differences in vegetation composition and plant species identity lead to only minor changes in soil-borne microbial communities in a former arable field. _FEMS
Microbiol Ecol_ 63: 372–382. Article CAS PubMed Google Scholar * Kowalchuk GA, Buma DS, de Boer W, Klinkhamer PGL, van Veen JA . (2002). Effects of above-ground plant species composition
and diversity on the diversity of soil-borne microorganisms. _Antonie Van Leeuwenhoek_ 81: 509–520. Article PubMed Google Scholar * Kowalchuk GA, Yergeau E, Leveau JHJ, Sessitch A,
Bailey M . (2010). Plant-associated microbial communities. In: Liu W-T, Jansson JK (eds) _Environmental Molecular Microbiology_. Caister Academic Press: Norwich, UK, pp 133–147. Google
Scholar * Kuffner M, Puschenreiter M, Wieshammer G, Gorfer M, Sessitsch A . (2008). Rhizosphere bacteria affect growth and metal uptake of heavy metal accumulating willows. _Plant Soil_
304: 35–44. Article CAS Google Scholar * Kuiper I, Lagendijk EL, Bloemberg GV, Lugtenberg BJJ . (2004). Rhizoremediation: A Beneficial Plant-Microbe Interaction. _Mol Plant-Microbe
Interact_ 17: 6–15. Article CAS PubMed Google Scholar * Leigh MB, Prouzova P, Mackova M, Macek T, Nagle DP, Fletcher JS . (2006). Polychlorinated biphenyl (PCB)-degrading bacteria
associated with trees in a PCB-contaminated site. _Appl Environ Microbiol_ 72: 2331–2342. Article CAS PubMed PubMed Central Google Scholar * Lugtenberg BJJ, Dekkers L, Bloemberg GV .
(2001). Molecular determinants of rhizosphere colonization by Pseudomonas. _Annu Rev Phytopathol_ 39: 461–490. Article CAS PubMed Google Scholar * Lundberg DS, Lebeis SL, Paredes SH,
Yourstone S, Gehring J, Malfatti S _et al_ (2012). Defining the core _Arabidopsis thaliana_ root microbiome. _Nature_ 488: 86–90. Article CAS PubMed PubMed Central Google Scholar *
Marilley L, Aragno M . (1999). Phylogenetic diversity of bacterial communities differing in degree of proximity of _Lolium perenne_ and _Trifolium repens_ roots. _Appl Soil Ecol_ 13:
127–136. Article Google Scholar * Mark GL, Dow JM, Kiely PD, Higgins H, Haynes J, Baysse C _et al_ (2005). Transcriptome profiling of bacterial responses to root exudates identifies genes
involved in microbe-plant interactions. _Proc Natl Acad Sci USA_ 102: 17454–17459. Article CAS PubMed PubMed Central Google Scholar * Master ER, Mohn WW . (2001). Induction of bphA,
encoding biphenyl dioxygenase, in two polychlorinated biphenyl-degrading bacteria, psychrotolerant Pseudomonas strain Cam-1 and MesophilicBurkholderia strain LB400. _Appl Environ Microbiol_
67: 2669–2676. Article CAS PubMed PubMed Central Google Scholar * Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anollés G, Rolfe BG _et al_ (2003). Extensive and specific
responses of a eukaryote to bacterial quorum-sensing signals. _Proc Natl Acad Sci USA_ 100: 1444–1449. Article CAS PubMed PubMed Central Google Scholar * Matilla M, Espinosa-Urgel M,
Rodriguez-Herva J, Ramos J, Ramos-Gonzalez M . (2007). Genomic analysis reveals the major driving forces of bacterial life in the rhizosphere. _Genome Biol_ 8: R179. Article PubMed PubMed
Central CAS Google Scholar * Meyer F, Paarmann D, D'Souza M, Olson R, Glass EM, Kubal M _et al_ (2008). The metagenomics RAST server - a public resource for the automatic
phylogenetic and functional analysis of metagenomes. _BMC Bioinformatics_ 9: 386. Article CAS PubMed PubMed Central Google Scholar * Meyer M, Kircher M . (2010). Illumina sequencing
library preparation for highly multiplexed target capture and sequencing. _Cold Spring Harb Protoc_ doi:10.1101/pdb.prot5448. * Miya RK, Firestone MK . (2001). Enhanced phenanthrene
biodegradation in soil by slender oat root exudates and root debris. _J Environ Qual_ 30: 1911–1918. Article CAS PubMed Google Scholar * Naik D, Smith E, Cumming JR . (2009). Rhizosphere
carbon deposition, oxidative stress and nutritional changes in two poplar species exposed to aluminum. _Tree Physiol_ 29: 423–436. Article CAS PubMed Google Scholar * Nakatsu CH,
Hristova K, Hanada S, Meng X-Y, Hanson JR, Scow KM _et al_ (2006). Methylibium petroleiphilum gen. nov., sp. nov., a novel methyl tert-butyl ether-degrading methylotroph of the
Betaproteobacteria. _Int J Syst Evol Microbiol_ 56: 983–989. Article CAS PubMed Google Scholar * Newsholme C . (2003) _Willows: The Genus Salix_. Timber Press, Incorporated: Portland,
OR, USA, pp 256. Google Scholar * Ogunseitan OA, Delgado IL, Tsai YL, Olson BH . (1991). Effect of 2-hydroxybenzoate on the maintenance of naphthalene-degrading pseudomonads in seeded and
unseeded soil. _Appl Environ Microbiol_ 57: 2873–2879. CAS PubMed PubMed Central Google Scholar * Pilon-Smits E . (2005). Phytoremediation. _Ann Rev Plant Biol_ 56: 15–39. Article CAS
Google Scholar * Pulford I, Watson C . (2003). Phytoremediation of heavy metal-contaminated land by trees—a review. _Environ Int_ 29: 529–540. Article CAS PubMed Google Scholar * Qin R,
Hirano Y, Brunner I . (2007). Exudation of organic acid anions from poplar roots after exposure to Al, Cu and Zn. _Tree Physiol_ 27: 313–320. Article CAS PubMed Google Scholar *
Ramachandran V, East A, Karunakaran R, Downie JA, Poole P . (2011). Adaptation of _Rhizobium leguminosarum_ to pea, alfalfa and sugar beet rhizospheres investigated by comparative
transcriptomics. _Genome Biol_ 12: R106. Article CAS PubMed PubMed Central Google Scholar * Ramey BE, Koutsoudis M, SBv Bodman, Fuqua C . (2004). Biofilm formation in plant-microbe
associations. _Curr Opin Microbiol_ 7: 602–609. Article CAS PubMed Google Scholar * Rosario-Passapera R, Keddis R, Wong R, Lutz RA, Starovoytov V, Vetriani C . (2012). _Parvibaculum
hydrocarboniclasticum_ sp. nov., a mesophilic, alkane-oxidizing alphaproteobacterium isolated from a deep-sea hydrothermal vent on the East Pacific Rise. _Int J Syst Evol Microbiol_ 62:
2921–2926. Article CAS PubMed Google Scholar * Salt DE, Smith RD, Raskin I . (1998). Phytoremediation. _Annu Rev Plant Physiol Plant Mol Biol_ 49: 643–668. Article CAS PubMed Google
Scholar * Schell MA . (1985). Transcriptional control of the nah and sal hydrocarbon-degradation operons by the nahR gene product. _Gene_ 36: 301–309. Article CAS PubMed Google Scholar
* Schenk PM, Carvalhais LC, Kazan K . (2012). Unraveling plant-microbe interactions: can multi-species transcriptomics help? _Trends Biotechnol_ 30: 177–184. Article CAS PubMed Google
Scholar * Schleheck D, Tindall BJ, Rossello-Mora R, Cook AM . (2004). _Parvibaculum lavamentivorans_ gen. nov., sp. nov., a novel heterotroph that initiates catabolism of linear
alkylbenzenesulfonate. _Int J Syst Evol Microbiol_ 54: 1489–1497. Article CAS PubMed Google Scholar * Schmidt SK, Lipson DA, Raab TK . (2000). Effects of willows (_Salix brachycarpa_) on
populations of salicylate-mineralizing microorganisms in alpine soils. _J Chem Ecol_ 26: 2049–2057. Article CAS Google Scholar * Schnoor JL, Licht LA, McCutcheon SC, Lee Wolfe N,
Carreira LH . (1995). Phytoremediation of organic and nutrient contaminants. _Environ Sci Technol_ 29: 318A–323A. Article CAS PubMed Google Scholar * Singer AC, Crowley DE, Thompson IP .
(2003). Secondary plant metabolites in phytoremediation and biotransformation. _Trends Biotechnol_ 21: 123–130. Article CAS PubMed Google Scholar * Sipilä TP, Keskinen AK, Akerman ML,
Fortelius C, Haahtela K, Yrjälä K . (2008). High aromatic ring-cleavage diversity in birch rhizosphere: PAH treatment-specific changes of IE3 group extradiol dioxygenases and 16S rRNA
bacterial communities in soil. _ISME J_ 2: 968–981. Article CAS PubMed Google Scholar * Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S _et al_ (2001). Bulk and rhizosphere
soil bacterial communities studied by denaturing gradient gel electrophoresis: Plant-dependent enrichment and seasonal shifts revealed. _Appl Environ Microbiol_ 67: 4742–4751. Article CAS
PubMed PubMed Central Google Scholar * Stewart FJ, Ottesen EA, DeLong EF . (2010). Development and quantitative analyses of a universal rRNA-subtraction protocol for microbial
metatranscriptomics. _ISME J_ 4: 896–907. Article CAS PubMed Google Scholar * Streeter J, Wong PP . (1988). Inhibition of legume nodule formation and N2 fixation by nitrate. _Crit Rev
Plant Sci_ 7: 1–23. Article CAS Google Scholar * Taghavi S, Barac T, Greenberg B, Borremans B, Vangronsveld J, van der Lelie D . (2005). Horizontal gene transfer to endogenous endophytic
bacteria from poplar improves phytoremediation of toluene. _Appl Environ Microbiol_ 71: 8500–8505. Article CAS PubMed PubMed Central Google Scholar * Turner T, Poole P . (2013).
Transcriptomics and metatranscriptomic analysis of the response of rhizosphere bacteria to environmental change. In de Bruijn FJ (ed). Molecular Microbial Ecology of the Rhizosphere. John
Wiley & Sons, Inc.: Hoboken, NJ, USA, pp 1123–1128. * Turner TR, Ramakrishnan K, Walshaw J, Heavens D, Alston M, Swarbreck D _et al_ (2013). Comparative metatranscriptomics reveals
kingdom level changes in the rhizosphere microbiome of plants. _The ISME J_ 7: 2248–2258. Article CAS PubMed Google Scholar * Van Der Meer JR, De Vos WM, Harayama S, Zehnder A . (1992).
Molecular mechanisms of genetic adaptation to xenobiotic compounds. _Microbiol Rev_ 56: 677–694. CAS PubMed PubMed Central Google Scholar * Vidal C, Chantreuil C, Berge O, Maure L,
Escarre J, Bena G _et al_ (2009). _Mesorhizobium metallidurans_ sp. nov., a metal-resistant symbiont of _Anthyllis vulneraria_ growing on metallicolous soil in Languedoc, France. _Int J Syst
Evol Microbiol_ 59: 850–855. Article CAS PubMed Google Scholar * Weyens N, Schellingen K, Beckers B, Janssen J, Ceulemans R, van der Lelie D _et al_ (2013). Potential of willow and its
genetically engineered associated bacteria to remediate mixed Cd and toluene contamination. _J Soils Sediments_ 13: 176–188. Article CAS Google Scholar * Yen K, Gunsalus I . (1982).
Plasmid gene organization: naphthalene/salicylate oxidation. _Proc Natl Acad Sci USA_ 79: 874–878. Article CAS PubMed PubMed Central Google Scholar * Yergeau E, Lawrence JR, Sanschagrin
S, Waiser MJ, Korber DR, Greer CW . (2012). Next-generation sequencing of microbial communities in the Athabasca River and its tributaries in relation to oil sands mining activities. _Appl
Environ Microbiol_ 78: 7626–7637. Article CAS PubMed PubMed Central Google Scholar * Zimmer D, Baum C, Leinweber P, Hrynkiewicz K, Meissner R . (2009). Associated bacteria increase the
phytoextraction of cadmium and zinc from a metal-contaminated soil by mycorrhizal willows. _Int J Phytorem_ 11: 200–213. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS
Werther Guidi, Michel Labrecque, Benoît Cloutier-Hurteau, Marie-Claude Turmel and François Courchesne provided soil analysis data, Yves Terrat and Sébastien Halary helped with soil sampling
and Danielle Ouellette performed the RT-quantitative PCR analyses. We are grateful to ConocoPhillips for providing us with access to the Varennes field site. This project was supported by
the Genome Canada and Genome Québec funded GenoRem Project. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * National Research Council Canada, Energy, Mining and Environment, Montreal, Quebec,
Canada Etienne Yergeau, Sylvie Sanschagrin, Christine Maynard & Charles W Greer * Biodiversity Center, Institut de recherche en biologie végétale, Université de Montréal and Jardin
botanique de Montréal, Montreal, Quebec, Canada Marc St-Arnaud Authors * Etienne Yergeau View author publications You can also search for this author inPubMed Google Scholar * Sylvie
Sanschagrin View author publications You can also search for this author inPubMed Google Scholar * Christine Maynard View author publications You can also search for this author inPubMed
Google Scholar * Marc St-Arnaud View author publications You can also search for this author inPubMed Google Scholar * Charles W Greer View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Etienne Yergeau. ETHICS DECLARATIONS COMPETING INTERESTS The GenoRem project contains several industrial partners,
including ConocoPhillips, the company that provided us with access to their site for this study. Our manuscript has in no way been modified by ConocoPhillips, nor has any industrial partner
commented on, or had any influence in, the analysis of our results. The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies this paper on
The ISME Journal website SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE S1 (PDF 292 KB) SUPPLEMENTARY TABLE S2 (DOC 65 KB) SUPPLEMENTARY INFORMATION (DOC 56 KB) RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yergeau, E., Sanschagrin, S., Maynard, C. _et al._ Microbial expression profiles in the rhizosphere of willows depend on soil
contamination. _ISME J_ 8, 344–358 (2014). https://doi.org/10.1038/ismej.2013.163 Download citation * Received: 13 June 2013 * Revised: 08 August 2013 * Accepted: 09 August 2013 * Published:
26 September 2013 * Issue Date: February 2014 * DOI: https://doi.org/10.1038/ismej.2013.163 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS *
_Salix purpurea_ * metatranscriptomic * rhizosphere * contaminated soils * phytoremediation