- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The extent of carbon (C) and nitrogen (N) export to the deep ocean depends upon the efficacy of the biological pump that transports primary production to depth, thereby preventing
its recycling in the upper photic zone. The dinitrogen-fixing (diazotrophic) _Trichodesmium_ spp. contributes significantly to oceanic C and N cycling by forming extensive blooms in
nutrient-poor tropical and subtropical regions. These massive blooms generally collapse several days after forming, but the cellular mechanism responsible, along with the magnitude of
associated C and N export processes, are as yet unknown. Here, we used a custom-made, 2-m high water column to simulate a natural bloom and to specifically test and quantify whether the
programmed cell death (PCD) of _Trichodesmium_ mechanistically regulates increased vertical flux of C and N. Our findings demonstrate that extremely rapid development and abrupt, PCD-induced
demise (within 2–3 days) of _Trichodesmium_ blooms lead to greatly elevated excretions of transparent exopolymers and a massive downward pulse of particulate organic matter. Our results
mechanistically link autocatalytic PCD and bloom collapse to quantitative C and N export fluxes, suggesting that PCD may have an impact on the biological pump efficiency in the oceans.
SIMILAR CONTENT BEING VIEWED BY OTHERS THE RATE AND FATE OF N2 AND C FIXATION BY MARINE DIATOM-DIAZOTROPH SYMBIOSES Article Open access 24 August 2021 DYNAMIC DIEL PROTEOME AND DAYTIME
NITROGENASE ACTIVITY SUPPORTS BUOYANCY IN THE CYANOBACTERIUM _TRICHODESMIUM_ Article 10 January 2022 AEROBIC BACTERIA PRODUCE NITRIC OXIDE VIA DENITRIFICATION AND PROMOTE ALGAL POPULATION
COLLAPSE Article Open access 12 May 2023 INTRODUCTION _Trichodesmium_ surface blooms (hereafter, referred to as ‘blooms’) were reported and described near the Torres Straits as ‘sea-sawdust’
by Captain James Cook and Sir Joseph Banks on the Endeavor’s first voyage (Banks, 1770), and near the Abrolhos Islands (east of Bahia, Brazil) in 1832 by Charles Darwin (Darwin, 1909). More
recently, remote sensing has revealed the scope of these massive blooms in the subtropical and tropical oceans, extending over 5.4 × 106 km2 (Subramaniam et al., 1999; Westberry and Siegel,
2006) and consisting of up to 1.2 × 107 cells l−1 (Capone et al. 1998; Lugomela et al., 2002; Rodier and Le Borgne, 2008, 2010). These blooms develop swiftly and are characterized by high
rates of CO2 and N2 fixation, up to 640 pg C per cell per day and 29 pg N per cell per day, respectively, as well as rapid doubling times (∼2.6 day) (Capone et al., 1998; Rodier and Le
Borgne, 2008, 2010; Luo et al., 2012). The elicitors and signals causing these rapid surface-bloom formations of _Trichodesmium_ are still vague (Berman-Frank et al., 2007; Rodier and Le
Borgne, 2010; Bergman et al., 2012). Notably, these massive _Trichodesmium_ blooms often collapse abruptly (within 3–5 days), with mortality rates paralleling bloom development rates (Rodier
and Le Borgne, 2008, 2010; Bergman et al., 2012) and with different processes (not necessarily mutually exclusive in natural populations) being implicated in their termination. Bloom
crashes may result from viral lysis (Hewson et al., 2004) or autocatalytic programmed cell death (PCD), which is induced by nutrient (iron (Fe) starvation) or high light (oxidative) stress
in both laboratory and natural populations (Berman-Frank et al., 2004, 2007). PCD has indeed been well documented in a variety of diverse phytoplankton lineages including bloom formers like
_Trichodesmium_ (Berman-Frank et al., 2004; Bidle and Falkowski, 2004), implicating it as an important feature of algal ecophysiology. However, the nature and mechanistic controls of PCD in
phytoplankton are not well understood and, moreover, the consequences of this cellular process on ecosystem and biogeochemical dynamics are virtually unexplored. Indeed, the fate and
quantitative contribution of _Trichodesmium_ blooms to the vertical export or recycling of newly fixed nitrogen (N) and carbon (C) in the ocean following bloom collapse is poorly studied
(Mulholland, 2007; Bergman et al., 2012), despite its global-scale importance (62–137 Tg N per year and 2.1–18 Tg C per year) (Luo et al., 2012). _Trichodesmium_ may release up to 50–80% of
recently fixed N2 in natural communities and cultures as dissolved organic N and ammonium (NH4+) (Mulholland, 2007). Low δ15N of exported material collected from the HOTS and BATS time
series stations indicates that recently fixed N is transferred out of the euphotic zone (Karl et al., 2002), although _Trichodesmium_ is rarely recovered in sediment traps and is only grazed
by three representatives of the Miraciidae family of pelagic harpacticoid copepods, mostly by _Macrosetella gracilis_ (O’Neil, 1998). Our previous findings indicate that PCD in
_Trichodesmium_ may trigger rapid sinking due to concomitant internal cellular degradation, vacuole loss and increased production of extracellular polysaccharide aggregates, operationally
defined as transparent exopolymeric particles (TEPs) (Berman-Frank et al., 2004, 2007). In the present study, we used a customized, experimental water column to specifically test and
quantify for the first time whether PCD-induced-bloom demise of _Trichodesmium_ mechanistically regulates increased vertical fluxes of C and N, thereby facilitating its rapid export from
surface waters to depth. MATERIALS AND METHODS EXPERIMENTAL SETUP Triplicate experiments were conducted with Fe-free medium to determine the relationship between PCD-induced bloom
termination, and C and N export fluxes. In all experiments, measurements were performed to assess culture state (cell abundance and Chl _a_), PCD induction (caspase-specific activity and
metacaspase gene expression) and organic export (total organic C (TOC), particulate organic C (POC), dissolved organic C (DOC), TEP and particulate organic N (PON) levels). Prior to
surface-bloom induction, _Trichodesmium erythraeum_ IMS101 was cultivated as _‘pre-bloom’_ exponential-phase batch cultures (0.5 × 106 cells ml−1) in a 10-l air-bubbled YBC II medium (Chen
et al., 1996) at 25 °C with a 12:12 light/dark cycle at ∼80 mMol quanta m−2s−1. Although _Trichodesmium_ IMS101 stock cultures were not axenic, cohabitating bacteria accounted for <1% of
the total biomass during exponential growth (as determined by epifluorescence microscopy counts). All culture transfers were performed ascetically and media in water columns was kept sterile
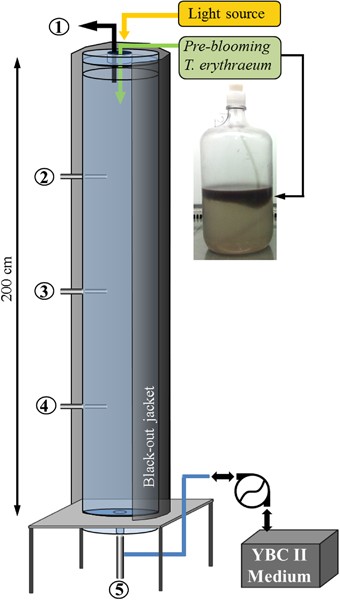
throughout the experiments. Experiments were all conducted in a custom-made water column (200 cm high, 13.8 cm (∅) cylinder, five sampling ports) that was fabricated to resemble and model a
water column (Figure 1, Supplementary Figure S1). The cylinder was kept in dark (using a black-out jacket) except for surface illumination in a 12:12 light:dark cycle at ∼600 mMol quanta
m−2s−1. The custom-made external halogen light source was positioned 0.5 m from the surface water column, illuminating fixture by a fiber optic cord to avoid any thermal stress. Experiments
were set by pumping 30 l of sterile YBC II (Fe-free) medium from the bottom of the cylinder using a peristaltic pump (Masterflex 6600, Cole-Parmer Instruments, Vernon Hills, IL, USA). After
the water column was filled with YBC II, ∼600 million _Trichodesmium_ cells (from the ‘pre-blooming’ batch culture) were poured from the top. Each experiment was conducted over 5 days and
five depths were routinely sampled: surface layer (0.5 cm), 50, 100, 150 and 200 cm deep. All experiments were initiated (_T_0) after a bloom was formed (3 h since _Trichodesmium_ addition),
defined as >95% of the cells in the water column above 0.5 cm. To minimize bacterial contamination, the water column system was cleaned and disinfected with 10% HCl for 3–5 h prior to
each run. RNA EXTRACTION Surface filaments (10 ml) were collected onto 25-mm diameter, 5-μm pore-size polycarbonate filters placed in a 2-ml eppendorf tube and snap-frozen in liquid N2.
Total RNA was extracted from _Trichodesmium_ cells using TRIzol Reagent (Invitrogen, Life Technologies, Santa Cruz, CA, USA) followed by RNeasy mini Kit (Qiagen, Valencia, CA, USA). DNase
(Turbo DNase, Ambion, Life Technologies) was used to any gDNA contamination from the RNA sample and RNeasy MinElute Cleanup Kit (Qiagen) to concentrate and clean the samples. Total RNA
concentration was determined using a NanoDrop ND-1000 Spectrophotometer (peqLab Biotechnologie, Erlangen, Germany). First-strand complementary DNA was generated from total RNA by reverse
transcription using SuperScript III First-Strand Synthesis System (Invitrogen Life Technologies). Reverse transcription reactions used equal total amounts (500 ng) of RNA and were primed
with random hexamers, allowing the investigation of multiple candidate genes from the same source first-strand complementary DNA. QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION (QRT-PCR)
Specific oligonucleotide DNA primer sets were designed as metacaspase 2 and 9 genes (_MC2_ (NCBI Accession no. YP_722351) and _MC9_ (NCBI Accession no. YP_724070; Table 1) (Berman-Frank et
al., 2004). Expression levels of target genes (TG; metacaspase and iron stress-inducible genes) were normalized to 16S rRNA housekeeping gene (HK) and calibrated to an initial time point
(Table 1). Triplicate first-strand complementary DNA aliquots from each sample served as templates in real-time PCR. Reactions containing either ‘no template’ (NTC) or DNase-treated RNA (to
exclude genomic DNA contamination) as template served as negative controls. All NTC and RNA-only samples were always below the detection limit. Brilliant II QPCR Reagents (Stratagene, La
Jolla, CA, USA) for _in situ_ monitoring of PCR progression. Reaction mixture (total of 10 μl) contained 4 μl diluted complementary DNA (∼20 ng RNA), 5 μl SYBRgreen (Brilliant II QPCR
Reagents, Stratagene), 0.5 μl per primer (250 nM) and 0.5 μl DEPC water. Transcript levels were determined by Corbett 6000 Rotor-Gene Q real-time PCR cycler using Brilliant SYBR Green
(Brilliant II QPCR Reagents, Stratagene). Q-RT-PCR-running conditions were: 7 min in 95 °C followed by 45 cycles of 10 s in 95 °C, 30 s in 60 °C and 20 s in 72 °C ending in melting, 72 °C–95
°C (1 °C steps). Samples were then analyzed by Rotor-Gene Q software subjected to the CT method Statistics (Stratagene). Standard curves were generated using serially diluted linear plasmid
DNA that contained each gene amplicon (Bidle and Bender, 2008) with measured amplification efficiencies being between 88% and 93%. Melt curves were performed with one distinct peak for both
metacaspase and 16S primers, whereas a small second peak was also apparent for the IsiBTery primer set (Supplementary Figure S2). The copy numbers of TG relative to housekeeping genes (HK)
were estimated as 2[−1(_C_tHK—_C_tTG)] (shorthand as 2(−ΔΔ_C_t)) according to Pfaffl (2001). MEASUREMENTS OF CASPASE ACTIVITY Surface filaments (15 ml) were collected onto 25-mM diameter,
5-μM pore-size polycarbonate filters, placed in a 2-ml eppendorf tube and snap-frozen in liquid N. Cells were resuspended in Lauber buffer (50 mM HEPES (pH 7.3), 100 mM NaCl, 10% sucrose,
0.1% 3-[(3- cholamidopropyl)-dimethylammonio]-1 propanesulfonate and 10 mM dithiothreitol) and sonicated on ice (four times, each for a 30 S cycle) using an ultracell disruptor (Sonic
Dismembrator, Fisher Scientific, Waltham, MA, USA). Cell extracts were centrifuged then (10 000 _g_, 2 min), and supernatant was collected for caspase activity and protein assays.
Caspase-specific activity was determined using the fluorogenic, peptide substrate IETD-AFC (Calbiochem, Rockland, MA, USA) at a 50 mM final concentration. Kinetic analysis was performed on
substrate cleavage over a 4-h period at 26 °C with measurements taken at 15 min intervals using a Fluoroskan Ascent Fl (Labsystems, Waltham, MA, USA) plate reader (Ex 405 nM, Em 485 nm).
Cleavage rates were normalized to total protein, as determined by BCA Protein Assay (Thermo Scientific, Rockford, IL, USA). CELL MEASUREMENTS Cells were counted using a Sedgwick-Rafter Cell
(Pyser-SGI, Kent, UK) and a light microscope (Nikon, Melville, NY, USA; Eclipse 80i). Filament number and size were counted using Nikon plan fluor × 4/0.75 lens and cell length was measured
by Nikon plan fluor X40/0.75 lens. Image analyses were done using Image J software (http://rsbweb.nih.gov). _Trichodesmium_ cells are usually cylindrical, therefore, cell volume of the
cylinder (length × π × _r_2) was calculated accordingly: _Trichodesmium_ cell length × π × (1/2 × the cell width)2. Cellular C content was determined according to the equation
_C_=0.433·V0.863 (Verity et al., 1992) as, where C represents cell C content (_C_cell, pg) and V represents cell volume (μM3). CHLOROPHYLL _A_ CONCENTRATIONS Filaments were collected on GF/F
filters (Whatman, Kent, UK) and Chl _a_ was extracted by boiling for 6 min in 90% methanol. Pigment concentration was analyzed spectrophotometrically at 664 and 750 nM (CARY100, Varian,
Santa Clara, CA, USA) according to Tandeau de Marsac and Houmard (1988). PARTICULATE ORGANIC C Samples (50 ml) were filtered through pre-combusted (4 h, 450 °C) GF/F filters, dried overnight
at 60 °C and stored in a desiccator until further analysis. POC was determined using a CHN analyzer Perkin Elmer (Waltham, MA, USA) CHNS/O elemental analyzer, PE 2400, after carbonate
removal from the filters using overnight fuming with concentrated HCl vapor. DISSOLVED ORGANIC C Duplicate water samples (40 ml) were first pre-filtered through combusted GF/F filters to
remove particulates and collected in acid-washed glass tubes. HCl (37%) was added (40 μl) to each sample and stored at 4 °C until analysis by TOC analyzer (Shimadzu ASI-L Autosampler,
Columbia, MD, USA). TOC was determined as POC+DOC. TEP DETERMINATION Water samples (20 ml) were filtered gently (∼100–150 mbar) onto 0.4-μm polycarbonate filters and TEP concentrations (μg
gum xanthan equivalents l−1) were measured according to Passow and Alldredge (1995). A conversion factor of 0.51 was employed to convert from milligrams of gum xanthan equivalents to
milligrams of C (Engel and Passow, 2001). RESULTS AND DISCUSSION FORMATION OF _TRICHODESMIUM_ SURFACE BLOOM We experimentally induced _T. erythraeum_ IMS101 blooms (Figure 1, Supplementary
Figure S1) by introducing a dense culture (3 × 106cells l−1) into an Fe-free water column. Immediately after addition, the trichomes were uniformly distributed (1 × 106–2 × 107 l−1)
throughout the upper 100 cm (Figure 2a). The customized water column was operated without turbulence, at an uniform temperature (26 °C), darkened from all sides and illuminated from the
surface by ∼600 μMol quanta m−2 s−1 (12:12 h light/dark). Bloom initiation always followed cell aggregation from single trichome morphology into puff and tuft colony formation (Figure 2b).
An extremely dense (1.3 × 109±3.3 × 107 l−1) ‘slick’-like bloom developed at the surface (upper 0.5 cm) from these ascending and aggregated colonies at a rate of 4 × 108cells h−1 (Figure
2c). Integrated cell abundance remained constant (3 × 108±9 × 107 m−3) in the water column throughout bloom development simulations, whereas surface cell numbers increased rapidly,
indicating that active ascent was due to positively buoyant cells. After ∼3 h, 96–98% of the cells had concentrated at the surface, incorporating significant concentrations of TOC and total
organic N (0.72±0.09 mg C m−2 and 0.13±0.03 mg N m−2, respectively), whereas <2% of the cells were evenly distributed below the upper 0.5 cm throughout the rest of the water column
(Figure 2a). Although artificially expedited using dense batch cultures (Figure 1), bloom formation in our water column possessed notable similarities to natural blooms observed in tropical
oceans, where surface slicks of dense _Trichodesmium_ aggregations develop under calm conditions with sea surface temperatures of ±26 °C, high solar irradiance and high concentrations of Fe
and P (Karl et al., 2002; Rodier and Le Borgne, 2008, 2010; Bergman et al., 2012). In these oceanic blooms, the vertical ascent of _Trichodesmium_ cells due to positive buoyancy (Villareal
and Carpenter, 2003; Rodier and Le Borgne, 2008, 2010) occurs simultaneously with rapid growth rates, both processes accounting for the hastened, ultra-dense accumulation of cells at the
surface (Rodier and Le Borgne, 2008, 2010; Bergman et al., 2012). ABRUPT AND SYNCHRONIZED BLOOM DEMISE Once surface blooms were established, we utilized the expression levels of _isiB,_ an
archetypical prokaryotic iron stress-response protein, as a diagnostic indicator of Fe stress (Chappell and Webb, 2010), along with the levels of metacaspase gene expression (Berman-Frank et
al., 2004; Bidle and Falkowski, 2004) and caspase-like catalytic activity (Berman-Frank et al., 2004, 2007) as diagnostic subcellular markers of PCD (see Materials and methods). Whereas
cell numbers remained stable in our experimental water column during the first 24 h (_T_1d) of Fe limitation, slight increases in transcript expression levels of _isiB_, _MC2_ and _MC9_
genes (by 0.5–0.9 logΔΔ_C_t) were already detectable (Table 1, Figures 3a and b). After 2 days (_T_2d) of Fe limitation, the expression levels of _isiB, MC2_ and _MC9_ genes increased
significantly with respect to _T_1 (three-, six- and five-fold, respectively) (Figure 3b). The changes in _MC_ gene expression were paralleled by 26-fold higher caspase activity rates
(718±268 RFU mg per protein h−1, Figure 3b). In the aquatic environment, natural populations of _Trichodesmium_ encounter similar Fe limitation where they can mobilize and reincorporate Fe
from intracellular storages, such as ‘DNA-binding proteins from starved cells’, to meet cellular demands for growth (Castruita et al., 2007). The observed lag time in our system (with no
changes in cell abundance; Figure 3a) between bloom initiation (_T_0) and the induction of PCD (_T_2d) may have resulted from the consistent slow release of intracellular Fe (Castruita et
al., 2007) prior to the PCD-induced collapse. Following bloom collapse (_T_3d), _MC_ gene expression and caspase activity rates were significantly reduced from their maximal levels (_T_2d)
by 76–100% and 40–80%, respectively (Figure 3b). The low metacaspase expression and caspase-specific activity levels measured in the remaining surface trichomes were consistent with the low
constitutive levels of these genes and protein activities, respectively. These observations demonstrate that an Fe-stress induced, PCD cellular response mechanistically triggered an abrupt,
synchronized _Trichodesmium_ bloom collapse. The drastic reduction (by 71%) in cell abundance (Figure 3a) was characterized by a notable loss of recognizable trichome cellular structure and
the transition to green amorphous aggregates composed of dying and decomposing biomass (Figure 3a, Supplementary movie S1). We suggest that the remaining 29% (5.1 × 108cells l−1) of the
biomass helped to ensure the survival of the _Trichodesmium_ population. In different species, cysts, spores and hormogonia cells are known to be resistant to environmental stress and may
also resist PCD, thus forming an inoculum for subsequent blooms (Vardi et al., 1999). Careful examination of microscopic cell images (∼100 of × 40 magnification) showed sporadic short
filaments during and after the crash, although these could not be definitively characterized as hormogonia. Thus, the surviving trichomes probably avoided PCD through an as yet unknown
mechanism(s). The subcellular triggers, signal transduction and regulatory networks of PCD in _Trichodesmium_ are currently unknown. One intriguing aspect of this PCD response is the
potential role that quorum sensing (QS) may have in triggering its onset. QS is known to regulate the cell–cell communication and population response through the activation of different
metabolic pathways including cell death (Dandekar et al., 2012), and QS has been recently documented to control phosphorus acquisition among _Trichodesmium_-epibiont consortia (Hewson et
al., 2009b; Hmelo et al., 2012). Further investigation is needed to identify whether QS has any role in the PCD of _Trichodesmium._ LINKING PCD-INDUCED BLOOM DEMISE TO PARTICULATE C AND N
SEQUESTRATION Our experimental water column enabled us to establish a novel mechanistic link between the PCD-induced bloom collapse and quantitative C:N export fluxes. Given this laboratory
model system lacks some of the complex abiotic (physical and chemical) and biotic (microbial remineralization) interactions within a natural water column, it provides a _‘best case
scenario’_ for flux measurements upon _Trichodesmium_ bloom collapse and, therefore, provides an useful constraint for future modeling efforts. During bloom initiation, TOC was predominantly
incorporated within the _Trichodesmium_ surface bloom (TOC/_C_cell=1, 0.74±0.09 mg Nm−2) and was comprised mostly of POC (Figures 4a and b, Table 2). PON concentrations were 0.13±0.04 mg N
m−2 and POC/PON was 6.4±1. TEP comprised a very small component (only 10%) of the TOC pool (Table 2) and DOC was below the analytical detection limits. TEPs are present in large numbers in
all aquatic environments and regarded as part of the POC pool (Passow, 2002). They are intensely sticky organic microgels that promote aggregation and may act as a bridge between dissolved
and particulate matter (Passow, 2002). Abrupt bloom collapse (_T_2d) from Fe-stress-induced PCD was characterized by a drastic reduction in _C_cell (87%) and removal rates of 11–17 μg C m−2
h−1, as well as a dramatic increase (2.2-fold) in TEP production, which resulted in two—three-fold increases in TEP/TOC and TOC/_C_cell, respectively (Table 3, Figures 4a–c). Similar to the
oceanic milieu (Passow, 2002; Bar-Zeev et al., 2011) and our earlier study (Berman-Frank et al., 2007), these increased ratios reflect the cessation of active incorporation of organic C and
N into _Trichodesmium_ cells and its preferential excretion into the media as polysaccharides and detrital matter. Moreover, it led to greatly enhanced sinking rates of captured aggregates,
likely resulting from TEP scaffolding (De La Rocha and Passow, 2007) of _Trichodesmium_ detrital matter, documented as large (∼1–5 mm) greenish aggregates (Figure 4f). Twenty-four hours
after the bloom collapse (_T_3d), we quantified the sinking velocities of these aggregates at ∼200 m per day (Supplementary movie S1). Sinking rates of the corresponding TOC (composed mainly
from POC) also increased significantly (5.4±1.1 μg C m h−1, Table 3, Supplementary movie S1) at this time (_T_3d) and rapidly accumulated at the bottom of the column (0.32±0.21 mg C m−2).
While organic C and N rapidly sank and accumulated at the chamber bottom at _T_3d, maximal TEP sinking rates (5.2±3.9 μg C m h−1) were measured 24 h later (_T_4d). We suggest that the
temporal uncoupling between the maximal TOC and TEP sinking rates (_T_3d vs _T_4d, respectively) may originate from their differential densities and viscosities (De La Rocha and Passow,
2007). Nevertheless, 5 days (_T_5) after bloom initiation, cell abundance, organic C and N concentrations at the surface layer of the column were drastically reduced, having been efficiently
exported to the bottom of the 2-m water column (Figure 4, Table 3). Notably, TOC measured at the bottom (0.9±0.1 mg C m−2) was predominantly composed of POC (68%), with DOC accounting for
only 12% (Figure 4, Table 2). Likewise, TEP accounted for as much as 90% of the TOC (0.84±0.1 mg C m−2). PON-measured concentrations were 0.1±0.04 mg N m−2 and C:N ratio increased to 7.6
(Figure 4, Table 2). The mechanistic link between PCD cellular processes and associated pulses of C:N export underscores its fundamental role in regulating cell fate, particle flux, and
upper ocean biogeochemistry of bloom-forming diazotrophs. Although export fluxes from natural _Trichodesmium_ or other diazotrophic blooms are poorly defined (Mulholland, 2007; Bergman et
al., 2012), newly fixed particulate organic matter (POM) in the pelagic surface oceans is the primary source for exported C and N to the deep ocean (Buesseler et al., 2007; Arístegui et al.,
2009). Globally, the export efficiency of POM from surface waters via the biological pump varies considerably (from 2 to 50%), with up to a quarter of this material sinking below 1000 m
(Boyd and Trull, 2007), depending on the magnitude of net primary production, sinking velocities and remineralization rates (Boyd and Trull, 2007; De La Rocha and Passow, 2007).
Heterotrophic bacteria have a significant role in decomposing and remineralizing POC, PON and TEP as part of the microbial loop (Passow, 2002; Azam and Malfatti, 2007; Arístegui et al.,
2009; Bar-Zeev et al., 2011; Yamada et al., 2012), resulting in particle solubilization, stoichiometric transformations and changes in export fluxes (Cho and Azam, 1988; Smith et al., 1992;
Schneider et al., 2003; Boyd and Trull, 2007). Heterotrophic bacteria were recently shown to have an active role in gene transcription as epibionts within the _Trichodesmium_ consortia and
are suggested to alter the _Trichodesmium_ metabolism (Hewson et al., 2009b). Our experimental observations represent a contrasting ‘_best case scenario_’, whereby algal bloom, crash and
subsequent vertical flux were largely isolated from these aforementioned microbial transformation processes and, consequently, provide an upper limit of potential vertical C and N fluxes
following _Trichodesmium_ bloom crashes. Sinking velocities are governed by particle concentration (McDonnell and Buesseler, 2010), size (following Stokes law) and density (Arístegui et al.,
2009). Our experimental water column simulations highlight that rapid development and abrupt, PCD-induced collapse of _Trichodesmium_ blooms will serve to mechanistically enhance these
first-order controls on particle flux. Bloom collapse via PCD commences with the internal degradation of cellular components (such as gas vesicles, Berman-Frank et al., 2004) and is
coincident with the massive TEP production. The resulting high concentration of dense, detrital particulate matter coupled with the sticky nature of TEP serves to promote the formation of
large aggregates (De La Rocha and Passow, 2007; Burd and Jackson, 2009; McDonnell and Buesseler, 2010) and preserves C and N in particulate forms through a prompt downward pulse of POM
(Supplementary movie S1). Moreover, we suggest that the toxicity of _Trichodesmium_ and low palatability to grazers (Rodier and Le Borgne, 2008; Kerbrat et al., 2011) combined with the sheer
magnitude of POM export flux will critically limit the amount of recycled matter within the upper mixed layer. Although the sinking rates and degree of export preservation in our model
system cannot _a priori_ be extrapolated to the ocean, our findings provide novel mechanistic context for recent observations of massive, localized _Trichodesmium_ bloom collapses (6 μg C
l−1 per day and 42 ng N l−1 per day, Rodier and Le Borgne, 2008, 2010) and reported vertical fluxes of newly fixed POC and PON from diazotrophs (17 μg C m−2 per day and 1.1 μg N m−2 per day,
Karl et al., 2012). Indeed, the tight link between PCD and C:N export pulses (demonstrated here) highlights the fundamental influence that PCD may exert on the oceanic biogeochemical
cycling associated with bloom-forming natural populations. Parameterizations for the effects of PCD-mediated bloom collapse should be included into future diagnostic models to constrain the
relative POC and PON fluxes from diazotrophs in the modern ocean. REFERENCES * Azam F, Malfatti F . (2007). Microbial structuring of marine ecosystems. _Nat Rev Microbiol_ 5: 782–791.
Article CAS Google Scholar * Arístegui J, Gasol JM, Duarte CM, Herndl GJ . (2009). Microbial oceanography of the dark ocean’s pelagic realm. _Limnol Oceanogr_ 54: 1501–1529. Article
Google Scholar * Banks J . (1770). The Endeavour Journal of Sir Joseph Banks. _Captain James Cook daily Journal,_ Vol. 1 http://southseas.nla.gov.au/journals/cook/17700828. * Bar-Zeev E,
Berman T, Rahav E, Dishon G, Herut B, Kress N _et al_ (2011). Transparent exopolymer particle (TEP) dynamics in the eastern Mediterranean Sea. _Mar Ecol Prog Ser_ 431: 107–118. Article CAS
Google Scholar * Bergman B, Sandh G, Lin S, Larsson J, Carpenter EJ . (2012). _Trichodesmium_-a widespread marine cyanobacterium with unusual nitrogen fixation properties. _FEMS Microbiol
Rev_ 37: 286–302. Article Google Scholar * Berman-Frank I, Bidle KD, Haramaty L, Falkowski PG . (2004). The demise of the marine cyanobacterium, _Trichodesmium_ spp., via an autocatalyzed
cell death pathway. _Limnol Oceanogr_ 49: 997–1005. Article Google Scholar * Berman-Frank I, Rosenberg G, Levitan O, Haramaty L, Mari X . (2007). Coupling between autocatalytic cell death
and transparent exopolymeric particle production in the marine cyanobacterium _Trichodesmium_. _Environ Microbiol_ 9: 1415–1422. Article CAS Google Scholar * Bidle KD, Falkowski PG .
(2004). Cell death in planktonic photosynthetic microorganisms. _Nat Rev Microbiol_ 2: 643–655. Article CAS Google Scholar * Bidle KD, Bender SJ . (2008). Iron starvation and culture age
activate metacaspases and programmed cell death in the marine diatom, _Thalassiosira pseudonana_. _Eukaryot Cell_ 7: 223–236. Article CAS Google Scholar * Boyd PW, Trull TW . (2007).
Understanding the export of biogenic particles in oceanic waters: is there consensus? _Prog Oceanogr_ 72: 276–312. Article Google Scholar * Buesseler KO, Lamborg CH, Boyd PW, Lam PJ, Trull
TW, Bidigare RR _et al_ (2007). the Ocean’s twilight zone. _Science_ 316: 567–570. Article CAS Google Scholar * Burd BA, Jackson AG . (2009). Particle aggregation. _Annu Rev Mar Sci_ 1:
65–90. Article Google Scholar * Capone DG, Subramaniam A, Montoya JP, Voss M, Humborg C, Johansen AM _et al_ (1998). An extensive bloom of the N2-fixing cyanobacterium _Trichodesmium
erythraeum_ in the central Arabian Sea. _Mar Ecol Prog Ser_ 172: 281–292. Article Google Scholar * Castruita M, Elmegreen LA, Shaked Y, Stiefel EI, Morel FMM . (2007). Comparison of the
kinetics of iron release from a marine (_Trichodesmium erythraeum_) Dps protein and mammalian ferritin in the presence and absence of ligands. _J Inorg Biochem_ 101: 1686–1691. Article CAS
Google Scholar * Chappell PD, Webb E . (2010). A molecular assessment of the iron stress response in the two phylogenetic clades of _Trichodesmium_. _Environ Microbiol_ 12: 13–27. Article
CAS Google Scholar * Chen YB, Zehr JP, Mellon M . (1996). Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium _Trichodesmium_ sp IMS 101 in
defined media: evidence for a circadian rhythm. _J Phycol_ 32: 916–923. Article Google Scholar * Cho BC, Azam F . (1988). Major role of bacteria in biogeochemical fluxes in the
ocean's interior. _Nature_ 332: 441–443. Article CAS Google Scholar * Dandekar AA, Chugani S, Greenberg EP . (2012). Bacterial quorum sensing and metabolic incentives to cooperate.
_Science_ 338: 264–266. Article CAS Google Scholar * Darwin CR . (1909) _The Voyage of the Beagle_ CHAPTER I. Collier PF & Son: New York, NY, USA, p 24. Google Scholar * De La Rocha
CL, Passow U . (2007). Factors influencing the sinking of POC and the efficiency of the biological carbon pump. _Deep Sea Res_ 54: 639–658. Article Google Scholar * Engel A, Passow U .
(2001). Carbon and nitrogen content of transparent exopolymer particles (TEP) in relation to their Alcian Blue adsorption. _Mar Ecol Prog Ser_ 219: 1–10. Article CAS Google Scholar *
Hmelo LR, Van Mooy BAS, Mincer TJ . (2012). Characterization of bacterial epibionts on the cyanobacterium _Trichodesmium_. _Aquatic Microbial Ecol_ 67: 1–14. Article Google Scholar *
Hewson I, Govil S, Capone DG, Carpenter EJ, Fuhrman J . (2004). Evidence of _Trichodesmium_ viral lysis and potential significance for biogeochemical cycling in the oligotrophic ocean.
_Aquatic Microbial Ecol_ 36: 1–8. Article Google Scholar * Hewson I, Poretsky RS, Dyhrman ST, Zielinski B, White AE, Tripp HJ _et al_ (2009b). Microbial community gene expression within
colonies of the diazotroph, _Trichodesmium_, from the Southwest Pacific Ocean. _ISME J_ 3: 1286–1300. Article CAS Google Scholar * Karl DM, Michaels A, Bergman B, Capone DG, Carpenter E,
Letelier R _et al_ (2002). Dinitrogen fixation in the world ’s oceans. _Biogeochemistry_ 57: 47–98. Article Google Scholar * Karl DM, Church MJ, Dore JE, Letelier RM, Mahaffeyd C . (2012).
Predictable and efficient carbon sequestration in the North Pacific Ocean supported by symbiotic nitrogen fixation. _Proc Natl Acad Sci USA_ 109: 1842–1849. Article CAS Google Scholar *
Kerbrat S, Amzil Z, Pawlowiez R, Golubic S, Sibat M, Darius HT _et al_ (2011). First evidence of Palytoxin and 42-Hydroxy-palytoxin in the marine cyanobacterium _Trichodesmium_. _Mar Drugs_
9: 543–560. Article CAS Google Scholar * Lugomela C, Lyimo TJ, Bryceson I, Semesi AK, Bergman B . (2002). _Trichodesmium_ in coastal waters of Tanzania: diversity, seasonality, nitrogen
and carbon fixation. _Hydrobiologia_ 477: 1–13. Article Google Scholar * Luo Y-W, Doney SC, Anderson LA, Benavides M, Berman-Frank I, Bode A _et al_ (2012). Database of diazotrophs in
global ocean: abundance, biomass and nitrogen fixation rates. _Earth Syst Sci Data_ 4: 47–73. Article Google Scholar * McDonnell AMP, Buesseler OK . (2010). Variability in the average
sinking velocity of marine particles. _Limnol Oceanogr_ 55: 2085–2096. Article Google Scholar * Mulholland MR . (2007). The fate of nitrogen fixed by diazotrophs in the ocean.
_Biogeosciences_ 4: 37–51. Article CAS Google Scholar * O’Neil JM . (1998). The colonial cyanobacterium _Trichodesmium_ as a physical and nutritional substrate for the harpacticoid
copepod _Macrosetella gracili_s. _J Plankton Res_ 20: 43–59. Article Google Scholar * Passow U, Alldredge A . (1995). A dye-binding assay for the spectrophotometric measurement of
transparent exopolymer particles (TEP). _Limnol Oceanogr_ 40: 1326–1335. Article CAS Google Scholar * Passow U . (2002). Transparent exopolymer particles (TEP) in aquatic environments.
_Prog Oceanogr_ 55: 287–333. Article Google Scholar * Pfaffl MW . (2001). A new mathematical model for relativequantification in real-time RT-PCR. _Nucleic Acids Res_ 29: E45. Article CAS
Google Scholar * Rodier M, Le Borgne R . (2010). Population and trophic dynamics of _Trichodesmium thiebautii_ in the SE lagoon of New Caledonia. Comparison with _T. erythraeum_ in the SW
lagoon. _Mar Pollut Bull_ 61: 349–359. Article CAS Google Scholar * Rodier M, Le Borgne R . (2008). Population dynamics and environmental conditions affecting _Trichodesmium_ spp.
(filamentous cyanobacteria) blooms in the south–west lagoon of New Caledonia. _J Exp Mar Biol Ecol_ 358: 20–32. Article Google Scholar * Schneider B, Schlitzer R, Fischer G, Nöthig EM .
(2003). Depth-dependent elemental compositions of particulate organic matter (POM) in the ocean. _Global Biogeochem Cycles_ 17: 32. Article Google Scholar * Smith DC, Simon M, Alldrege AL,
Azam F . (1992). Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution. _Nature_ 359: 139–142. Article CAS Google Scholar * Subramaniam
A, Carpenter EJ, Falkowski PG . (1999). Bio-optical properties of the marine diazotrophic cyanobacteria _Trichodesmium_ spp a reflectance model for remote-sensing. _Limnol Oceanogr_ 44:
618–627. Article CAS Google Scholar * Tandeau de Marsac N, Houmard J . (1988). Complementary chromatic adaptation: physiological conditions and action spectra. _Methods Enzymol_ 167:
318–328. Article CAS Google Scholar * Vardi A, Berman-Frank I, Rozenberg T, Hadas O, Kaplan A, Levine A . (1999). Programmed cell death of the dinoflagellate _Peridinium gatunense_ is
mediated by CO2 limitation and oxidative stress. _Curr Biol_ 9: 1061–1064. Article CAS Google Scholar * Verdugo P, Santschi HP . (2010). Polymer dynamics of DOC networks and gel formation
in seawater. _Deep Sea Res_ 57: 1486–1493. Article CAS Google Scholar * Verity PG, Robertson CY, Tronzo CR, Andrews MG, Nelson JR, Sieracki ME . (1992). Relationships between cell volume
and the carbon and nitrogen content of marine photosynthetic nanoplankton. _Limnol Oceanogr_ 37: 1434–1446. Article CAS Google Scholar * Villareal TA, Carpenter EJ . (2003). Buoyancy
regulation and the potential for vertical migration in the oceanic cyanobacterium _Trichodesmium_. _Microbial Ecol_ 45: 1–10. Article CAS Google Scholar * Westberry TK, Siegel DA .
(2006). Spatial and temporal distribution of _Trichodesmium_ blooms in the world’s oceans. _Global Biogeochem Cycles_ 20: GB4016. Article Google Scholar * Yamada N, Fukuda H, Ogawa H,
Saito H, Suzumura M . (2012). Heterotrophic bacterial production and extracellular enzymatic activity in sinking particulate matter in the western North Pacific Ocean. _Front Microbiol_ 3:
379. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank Mr Shimon Pilo for technical assistance and the Bar-Ilan University Mechanical Workshop for
constructing the experimental water column. We would like to thank Mr Gad Rosenberg and Dr Sarit Lampert for designing the _16S rRNA_ HK primers. This research was supported by grants from
the United States-Israel Binational Science Foundation (BSF; Grant no. 2008048) to IB-F and KDB and from the United States National Science Foundation (OCE-1061883) to KDB, as well as an MSc
scholarship to IA by the Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University. DISCLAIMER The data in this manuscript are all original and have not been submitted
elsewhere for consideration. AUTHOR INFORMATION Author notes * Edo Bar-Zeev Present address: 3Current address: Department of Chemical and Environmental Engineering, Yale University, New
Haven, CT 06520, USA., AUTHORS AND AFFILIATIONS * Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat Gan, Israel Edo Bar-Zeev, Itamar Avishay & Ilana
Berman-Frank * Environmental Biophysics and Molecular Ecology Group, Institute of Marine and Coastal Sciences, Rutgers University, New Brunswick, NJ, USA Kay D Bidle Authors * Edo Bar-Zeev
View author publications You can also search for this author inPubMed Google Scholar * Itamar Avishay View author publications You can also search for this author inPubMed Google Scholar *
Kay D Bidle View author publications You can also search for this author inPubMed Google Scholar * Ilana Berman-Frank View author publications You can also search for this author inPubMed
Google Scholar CORRESPONDING AUTHOR Correspondence to Edo Bar-Zeev. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary
Information accompanies this paper on The ISME Journal website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 (JPG 233 KB) SUPPLEMENTARY FIGURE 2 (JPG 287 KB) SUPPLEMENTARY MOVIE 1 (MP4
2327 KB) SUPPLEMENTARY INFORMATION (DOC 28 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bar-Zeev, E., Avishay, I., Bidle, K. _et al._ Programmed
cell death in the marine cyanobacterium _Trichodesmium_ mediates carbon and nitrogen export. _ISME J_ 7, 2340–2348 (2013). https://doi.org/10.1038/ismej.2013.121 Download citation *
Received: 07 April 2013 * Revised: 10 June 2013 * Accepted: 15 June 2013 * Published: 25 July 2013 * Issue Date: December 2013 * DOI: https://doi.org/10.1038/ismej.2013.121 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * programmed cell death * bloom * _Trichodesmium_ * carbon and nitrogen export flux







