- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Bacteria and archaea in the dark ocean (>200 m) comprise 0.3–1.3 billion tons of actively cycled marine carbon. Many of these microorganisms have the genetic potential to fix
inorganic carbon (autotrophs) or assimilate single-carbon compounds (methylotrophs). We identified the functions of autotrophic and methylotrophic microorganisms in a vent plume at Axial
Seamount, where hydrothermal activity provides a biogeochemical hot spot for carbon fixation in the dark ocean. Free-living members of the SUP05/Arctic96BD-19 clade of marine
gamma-proteobacterial sulfur oxidizers (GSOs) are distributed throughout the northeastern Pacific Ocean and dominated hydrothermal plume waters at Axial Seamount. Marine GSOs expressed
proteins for sulfur oxidation (adenosine phosphosulfate reductase, sox (sulfur oxidizing system), dissimilatory sulfite reductase and ATP sulfurylase), carbon fixation
(ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO)), aerobic respiration (cytochrome _c_ oxidase) and nitrogen regulation (PII). Methylotrophs and iron oxidizers were also active in
plume waters and expressed key proteins for methane oxidation and inorganic carbon fixation (particulate methane monooxygenase/methanol dehydrogenase and RuBisCO, respectively). Proteomic
data suggest that free-living sulfur oxidizers and methylotrophs are among the dominant primary producers in vent plume waters in the northeastern Pacific Ocean. SIMILAR CONTENT BEING VIEWED
BY OTHERS PURPLE SULFUR BACTERIA FIX N2 VIA MOLYBDENUM-NITROGENASE IN A LOW MOLYBDENUM PROTEROZOIC OCEAN ANALOGUE Article Open access 06 August 2021 A HYDROGENOTROPHIC _SULFURIMONAS_ IS
GLOBALLY ABUNDANT IN DEEP-SEA OXYGEN-SATURATED HYDROTHERMAL PLUMES Article Open access 09 March 2023 MARINE AMMONIA-OXIDISING ARCHAEA AND BACTERIA OCCUPY DISTINCT IRON AND COPPER NICHES
Article Open access 24 March 2021 INTRODUCTION Recent genomic studies demonstrate that many of the 6.5 × 1028 bacteria that inhabit the dark ocean (>200 m) have the genetic potential to
fix carbon (Reinthaler et al., 2010; Swan et al., 2011). These include autotrophic and methylotrophic bacteria that have genes to fix inorganic carbon and assimilate single-carbon compounds,
respectively. Hydrothermal vents are characterized by steep physical and chemical gradients that are established as hot fluids from the deep subsurface mix with cold oxidized seawater. The
stable neutrally buoyant plumes that form above hydrothermal vents contain approximately 0.01% vent fluid (Lupton et al., 1985) and are enriched in sulfides, iron, manganese, methane (CH4),
hydrogen (H2) and other reduced compounds relative to background seawater (Lilley et al., 1995). This steady supply of chemical energy has the potential to fuel autotrophic and
methylotrophic activities in the dark ocean. Vent-associated microbial communities are commonly dominated by bacteria (especially, alpha-, gamma- and epsilon-proteobacteria) that likely
participate in the oxidation of reduced sulfur compounds, ammonia, iron, manganese, CH4 and H2 (Huber et al., 2003, 2006, 2007; Opatkiewicz et al., 2009; Dick and Tebo, 2010; Sylvan et al.,
2012). Members of the SUP05/Arctic96BD-19 clade of marine gamma-proteobacterial sulfur oxidizers (GSOs) are among the dominant groups identified at venting locations (Sunamura et al., 2004;
Anantharaman et al., 2013; Anderson et al., 2013). Marine GSOs were first identified in the tissues of clams and mussels living at hydrothermal vents and cold seeps (Cavanaugh et al., 1987;
Distel et al., 1988). Members of this group are closely related to thiotrophic bacteria that obtain energy from the oxidation of reduced sulfur compounds (Distel et al., 1988). Similar to
other symbiotic relationships in sulfide-rich marine ecosystems, the host provides access to reduced sulfur sources for autotrophic bacteria and the bacterial symbionts provide the host with
a source of organic carbon (Childress et al., 1991; Robinson and Cavanaugh, 1995). Abundant and free-living marine GSOs have recently been identified in diverse seawater samples (Lavik et
al., 2009; Walsh et al., 2009; Swan et al., 2011). Marine GSOs accounted for 45% of the 16_S_ ribosomal RNA (rRNA) gene clones recovered from an oxygen-minimum zone in the South Atlantic and
37% of the bacterial 16_S_ rRNA genes recovered from an anoxic fjord in British Columbia (Lavik et al., 2009; Walsh et al., 2009). Marine GSOs dominated bacterial communities (88–90%) in
the plume layer in the Suiyo Seamount caldera (Sunamura et al., 2004) and were among the key groups identified in diffuse-flow hydrothermal vent fluids in 1998–2000 following an eruption at
Axial volcano in 1998 (Huber et al., 2006). Recent studies have indicated that marine GSOs are abundant and active in the Guaymas Basin in the Gulf of California (Lesniewski et al., 2012;
Anantharaman et al., 2013). We used a tandem mass spectrometry (MS/MS)-based proteomics approach to identify expressed proteins in hydrothermal plume waters at Axial Seamount, an active
volcano along the Juan de Fuca Ridge spreading center (Caress et al., 2012; Chadwick et al., 2012; Dziak et al., 2012). We also compared microbial communities in plumes with microbial
communities in other areas of the northeastern Pacific Ocean not influenced by hydrothermal systems. Our results suggest that chemolithoautotrophic marine GSOs were the dominant primary
producers in hydrothermal plume fluids, with methanotrophic and iron-oxidizing bacteria contributing to a lesser extent. These data support previous findings, which combined suggest that
this ubiquitous group of free-living marine GSOs are active in diverse deep ocean environments (Swan et al., 2011; Anantharaman et al., 2013; Lesniewski et al., 2012). MATERIALS AND METHODS
SAMPLE COLLECTION All samples were collected onboard the _R/V Thomas G Thompson_ during a University of Washington-led research cruise in support of the National Science Foundation’s Ocean
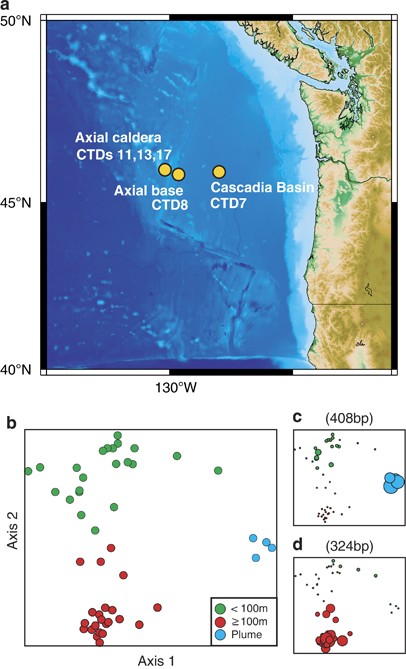
Observatory Initiative—Regional Scale Nodes cruise (19 August to 1 September 2011) from Seattle, WA, to Hydrate Ridge and Axial Seamount (Figure 1). Water samples for microbial and chemical
analyses were collected using a conductivity, temperature and depth (CTD) rosette profiler equipped with 12 l Niskin bottles. Samples were collected from five different stations (CTD7
Cascadia Basin (lat: 45.8602/lon: −127.9353), CTD8 base of Axial Seamount (lat: 45.8202/lon: −129.7567), CTD11 ASHES vent field (lat: 45.9339/lon: −130.0136), CTD13 Coquille vent (lat:
45.9264/lon: −129.9803) and CTD17 ASHES vent field (lat: 45.9340/lon: −130.0138)). Sample numbers, depths and types of analyses are shown in Supplementary Table 1. CONTEXTUAL DATA AND SITE
DESCRIPTION Partial and full water column profile measurements were conducted using a rosette-mounted instrument package that included a Seabird 9plus CTD with dual temperature and
conductivity sensors, a Seabird 43 oxygen sensor (calibrated to onboard Winkler titrations) and a WET Labs C-Star transmissometer. Using the downcast profile measurements, the position and
extent of the hydrothermal plume was identified by large negative deflections in beam transmission (corresponding to the high particulate load generated from precipitating metal sulfides in
the hydrothermal fluid) and subtle positive anomalies in temperature (corresponding to the heat input from high-temperature fluids), following well-established plume identification practices
(Baker et al., 1993). At the summit of Axial Seamount, hydrothermal plumes were 30–100 m thick, with the neutrally buoyant plume having a typical rise height of 75–200 m above the seafloor.
During the profile upcast, 12 l Niskin water sampling bottles (General Oceanics, Miami, FL, USA) were triggered at depths determined to be within the hydrothermal plume and at regular
intervals in the water column above the plume. Water samples were analyzed for CH4 and H2 concentrations using shipboard gas chromatography. Samples were headspace extracted and inlet into a
gas chromatograph (SRI Instruments, Torrance, CA, USA) configured with a helium carrier, a 30 m 5 Å mol sieve column, a 50 °C isothermal temperature program, and flame ionization and pulse
discharge detectors. This method provided the sensitivity to measure the low gas concentrations of background seawater (CH4: 0.5–1 nM, H2: 0.1–0.5 nM). Hydrothermal fluids are enriched in
both CH4 and H2 (Lilley et al., 1982; Proskurowski et al., 2008). NUTRIENTS, DISSOLVED ORGANIC CARBON AND TOTAL DISSOLVED NITROGEN Samples for nutrient, dissolved organic carbon and total
dissolved nitrogen analyses were stored frozen in 60 ml high-density polyethylene bottles until analysis within 2 months of the completion of the cruise. Nutrient concentrations were
assessed by following the protocols of the WOCE Hydrographic Program using a Technicon AAII system at the University of Washington. Dissolved organic carbon and total dissolved nitrogen
concentrations were assessed by high-temperature combustion using a Shimadzu TOC-csh with autoinjection (Dickson et al., 2007). Four-point standard curves using potassium hydrogen phthalate
and potassium nitrate were run daily to calibrate the response of the high-temperature combustion system. Measurements were quality controlled using Consensus Reference Materials distributed
to the international community by the Hansell Laboratory (Hansell, 2005). The Consensus Reference Materials were analyzed at regular intervals during each analytical day. Low-carbon
reference water was employed to determine system blanks. Dissolved organic nitrogen was determined as the difference in concentrations of total dissolved nitrogen and dissolved inorganic
nitrogen (Supplementary Figure 1). MICROBIAL CELL COUNTS Microbial cells were collected from multiple depths, fixed in filtered formalin at a final concentration of 1%, stored at 4 °C
overnight and subsequently filtered (5–10 ml) onto 0.2 μm Osmonics polycarbonate filters. Total cell counts were determined by staining cells from the whole-water and concentrated seawater
sample with the nucleic acid stain 4',6-diamidino-2-phenylindole (DAPI). The suite of oligonucleotide probes used to enumerate bacteria was: EUB-27R (CTGAGCCAKGATCRAACTCT), EUB-338Rpl
(GCWGCCWCCCGTAGGWGT), EUB-700R (CTAHGCATTTCACYGCTACAC), EUB-700Ral (CTACGAATTTCACCTCTACAC) and EUB-1522R (AAGGAGGTGATCCANCCVCA). The negative control oligonucleotide was 338F
(TGAGGATGCCCTCCGTCG) (Morris et al., 2002). Briefly, reactions were performed on membrane sections at 37 °C for 16 h in hybridization buffer (900 mM NaCl, 20 mM Tris (pH 7.4), 0.01% (w/v)
sodium dodecyl sulfate and 15% or 35% formamide) and lineage-specific Cy3-labeled oligonucleotide probe suites. A control hybridization reaction was performed with a low-stringency buffer
containing 15% formamide and a Cy3-labeled complementary probe (338F). Each probe had a final concentration of 2 ng μl−1. Optimal hybridization stringency was achieved by hybridization
washes (20 mM Tris (pH 7.4), 6 mM EDTA, 0.01% sodium dodecyl sulfate and 70 or 150 mM NaCl) for two 10-min intervals. DAPI and fluorescence _in situ_ hybridization cell counts were
determined from 15 fields of view using a Nikon 80i epifluorescence microscope equipped with a Photometrics CoolSNAPHQ2 digital camera, filter sets appropriate for Cy3 and DAPI and the
NIS-Elements Basic Research Acquisition and Analyses package (Nikon Instruments Inc., Melville, NY, USA). For fluorescence _in situ_ hybridization, DAPI images were segmented and overlain
onto corresponding Cy3 image segmentations to identify positive probe signals with corresponding DAPI signals. Negative control counts were determined using the same technique and subtracted
from positive probe counts to correct for autofluorescence and nonspecific binding. COMMUNITY STRUCTURAL ANALYSIS Microbial cells were collected for DNA extraction by filtering 1 liter of
seawater onto sterile Supor-200 0.2 μm polyethersulfone filters. Cell lysis was performed as described previously (Morris et al., 2012) with the following modifications. Onboard, filters
were placed in 2 ml cryovials, flash frozen in liquid nitrogen and stored at −80 °C. To initiate cell lysis, filters were thawed on ice and sliced into sections with autoclaved scissors, and
then 1 ml of sucrose lysis buffer (20 mM EDTA, 400 mM NaCl, 0.75 M sucrose and 50 mM Tris–HCl, pH 8.4) and 20 μl of lysozyme (1 mg ml−1) were added. The filters were incubated for 60 min at
37 °C with shaking prior to adding 20 μl proteinase K (100 μg ml−1) and 200 μl sodium dodecyl sulfate (10%) and subsequently incubated for 2 h at 55 °C with shaking and then stored at −80
°C until DNA extraction was performed. DNA was extracted from 600 μl of cell lysate using a DNeasy Blood and Tissue kit (QIAGEN, Germantown, MD, USA) and used for Terminal Restriction
Fragment Length Polymorphism (TRFLP) analysis as described previously (Morris et al., 2012). DNA concentrations were estimated with the Qubit fluorometer and the Qubit dsDNA HS assay kit.
PCRs for TRFLP were conducted with 1–2 ng DNA per reaction by amplifying the 16_S_ rRNA gene with 5′ end-labeled phosphoramidite fluorochrome 5-carboxy-fluorescein 27F_B
(5′-AGRGTTYGATYMTGGCTCAG-3′), unlabeled 519R (5′-GWATTACCGCGGCKGCTG-3′) primers and _Taq_ polymerase as described previously (Morris et al., 2012). PCR products were digested overnight at 37
°C with _Hae_III. Reaction products were cleaned using columns containing hydrated superfine Sephadex G-50. The resulting terminal restriction fragments (TRFs) were resolved along with
internal size standards (50–500 bp) on an ABI 3730 DNA Analyzer (Applied Biosystems Life Technologies, Carlsbad, CA, USA) located at the Fred Hutchison Cancer Research Center in Seattle, WA,
USA. TRF sizes were estimated with Peak Scanner 1.0 (Applied Biosystems). Fragment sizes were rounded to the nearest whole number, and systematic rounding errors were manually corrected.
The resulting data matrix contained 48 samples (rows) and 382 unique 16_S_ rRNA gene TRFs (columns). TRFs were relativized for nonmetric multidimensional scaling (NMS) analysis by dividing
the area of each fragment by the total area using the software package PC-ORD (McCune and Grace, 2002). Sorenson distances were used for NMS analysis with a random starting configuration,
and the resulting solution was constrained to two dimensions. The final stress for the two-dimensional solution was 14.665 with instability of 0.00454 when using 40 real-data runs. The axes
were rotated to maximize orthogonality (100%). Axis 1 explained 25.7% and axis 2 explained 58.1% of the variance (both axes explained 83.8% of the variance). The null hypothesis that there
was no difference between sample groupings was rejected using the multi-response permutation procedure, which evaluates groupings with real and randomized data. A chance-corrected
within-group agreement statistic of 0.24 indicated within-group homogeneity, and a _P_-value of 0 indicated that observed group differences were statistically significant. 16_S_ RRNA GENE
DIVERSITY Bacterial 16_S_ rRNA gene clone libraries were constructed by amplifying community 16_S_ rRNA genes with bacterial primers 27F_B and 1492R (5′-GGYTACCTTGTTACGACTT-3′) as described
previously (Morris et al., 2012). Briefly, amplifications were performed using community genomic DNA extracted from CTD17 (1450 m) and CTD7 (2850 m), using Apex _Taq_ polymerase (Genesee
Scientific, San Diego, CA, USA), and under the following conditions: 35 cycles, annealing at 55 °C for 1 min, elongation at 72 °C for 2 min and denaturation at 94 °C for 30 s. Amplicons were
purified and cloned using the pGEM-T-Easy vector (Promega, Madison, WI, USA) following the manufacturer’s instructions. The resulting transformations were sent to High Throughput Sequencing
(HTSeq.org, Seattle, WA, USA), where clones were isolated and sequenced by Sanger sequencing using the M13F (5′-TGTAAAACGACGGCCAGT-3′) and M13R (5′-CAGGAAACAGCTATGAC-3′) primers
(Supplementary Table 2). Nearly full-length 16_S_ rRNA gene sequences were obtained for frequently occurring lineages by selecting and purifying a subset of clones using the Qiagen plasmid
mini-prep kit (QIAGEN). Sequencing was performed using the following primer pairs by GeneWiz Inc. (La Jolla, CA, USA): M13R, 519F (5′-CAGC(A/G)GCCGCGGTAATAC-3′), 338F
(5′-ACTCCTACGGGAGGCAGC-3′) and 926R (5′-CCGTCAATTC(A/C)TTT (A/G)AGTTT-3′). Sequences were assembled with the CAP3 Sequence Assembly Program (Huang and Madan, 1999) and annotated by using the
Bayesian method of Wang et al. (2007), which contained a custom training set augmented with marine environmental clades (Iverson et al., 2012), and the SILVA least common ancestor
classification tool (Pruesse et al., 2007). _In silico_ restriction analysis and other DNA sequence manipulation operations were performed with the Sequence Manipulation Suite (Stothard,
2000). Restriction fragments were verified for a subset of clones to confirm predicted fragment sizes. Bacterial 16_S_ rRNA sequences generated during this study were submitted to GenBank
(accession nos. KC522839–KC522949). PHYLOGENETIC RELATIONSHIPS OF MARINE GSOS Phylogenetic relationships were determined using a subset of published 16_S_ rRNA gene sequences. A marine GSO
reference tree was obtained by selecting nearly complete (>1300 bp), high-quality, nonredundant 16_S_ rRNA gene sequences from public data sets (_n_=65). Sequences were first aligned and
trimmed using MUSCLE and then manually pruned to maintain tree topology. A maximum likelihood tree that contained only the highest quality set of unique sequences (_n_=50) was constructed
using RAxML. The GTRGAMMA model was used to evaluate 100 tree topologies with 1000 bootstrap analyses. Trees were visualized using FigTree and rooted with _Methylococcus capsulatus_ (GenBank
accession no. AJ563935). PLUME PROTEOMICS Seawater (∼180 l) was collected from the stable hydrothermal vent plume issuing from the black smoker chimney Inferno (CTD17, 1450 m). Whole water
was transferred to clean 50 l polystyrene reservoirs and concentrated to ∼230 ml with a Pellicon 2 tangential flow filtration system equipped with a 30-kDa Biomax Polyethersulfone cassette
(Millipore Co., Billerica, MA, USA) as described previously (Morris et al., 2010). Cells were collected and concentrated in approximately 2 h. Concentrated cells were flash frozen in liquid
nitrogen and stored at −80 °C until further processing at the University of Washington. Cell counts before and after filtration (6.9 × 1010 and 2.9 × 1010, respectively) indicate that we
recovered 42% of the cells present in 180 l of hydrothermal vent plume water. Cells in the concentrated sample were divided into replicate samples (Av1 and Av2, ∼115 ml each) and harvested
by centrifuging at 4 °C for 60 min (17 000 _g_). The supernatant was discarded, and cell pellets were rinsed with 100 μl of 20 mM Tris buffer (pH 7.4) and stored at −80 °C. Cells were lysed
using a titanium sonicating microprobe (20 s, 10 repetitions) in a 6 M urea and 50 μM ammonium bicarbonate solution. Disulfide bonds were reduced with dithiothreitol and alkylated with
iodoacetic acid. After additions of ammonium bicarbonate and methanol, 2 μg of sequence grade trypsin (Promega) was added to each sample. Enzymatic digestions were incubated for 12 h at 37
°C. Resulting peptides were desalted using a macro-spin C18 column (NestGroup, Southborough, MA, USA) following the manufacturer’s guidelines prior to analysis by mass spectrometry (MS).
Peptide concentrations of Axial volcano hydrothermal vent plume proteome replicates Av1 and Av2 were measured using the Thermo Scientific Nanodrop 2000/2000c, which measures the peptide bond
absorbance at wavelength of 205 nm. Approximately 1 μg of peptide digest was used for each injection into the mass spectrometer. Each sample consisted of a complex mixture of peptides that
was introduced into the mass spectrometer by reverse-phase chromatography using a brand new 15 cm long, 75 μm internal diameter fused silica capillary column packed with C18 particles (Magic
C18AQ, 100 Å, 5 μm; Burker-Michrom Inc., Auburn, CA, USA) fitted with a 2 cm long, 100 μm internal diameter precolumn (Magic C18AQ, 200 Å, 5 μm; Michrom Bioresources Inc.). Peptides were
first trapped on the precolumn (5% acetonitrile, 4 ml min−1, 7 min). Chromatographic separations were performed using an acidified (formic acid, 0.1% v/v) water–acetonitrile gradient (5–35%
acetonitrile in 60 min) with a total run time of 95 min. Mass spectrometry was performed on replicates Av1 and Av2 independently using the Thermo Fisher linear ion trap–Orbitrap (LTQ-OT)
hybrid tandem mass spectrometer (Thermo Fisher, San Jose, CA, USA). Peptides were analyzed using a data-independent method termed Precursor Acquisition Independent from Ion Count (PAcIFIC)
(Panchaud et al., 2009). Rather than requiring the mass spectrometer to select ions for fragmentation based on MS1 data, the PAcIFIC method systematically fragments ions at all m/z channels
(Panchaud et al., 2011). Each method file includes the full 95 min linear high-performance liquid chromatography gradient of 5–35% acetonitrile over 60 min (see above) and covers a 21.5 m
z−1 range using 14 contiguous, unique channels that span 2.5 m z−1 in the mass spectrometer. This results in a total of 45 method files per PAcIFIC analytical cycle to cover a full m/z range
of 400–1400. PROTEIN IDENTIFICATIONS Tandem mass spectra were interrogated against a composite database containing deduced protein sequences from lineages identified in the CTD17 clone
library and lineages that are dominant in the deep ocean (background seawater). The database contained marine GSOs ‘_Candidatus_ Vesicomyosocius okutanii HA’, ‘_Candidatus_ Ruthia magnifica
Cm’, the SUP05 metagenome (Walsh et al., 2009) and SCGC AAA001-B15 (Arctic96BD-19 draft genome); the methylotrophs _Methylobacter tundripaludum_ SV96 and _Methylomicrobium alcaliphilum_;
iron-oxidizing bacteria _Gallionella capsiferriformans_ ES-2 and _Sideroxydans lithotrophicus_ ES-1; abundant lineages in seawater ‘_Candidatus_ Pelagibacter ubique HTCC1062’ and
‘_Candidatus_ Pelagibacter ubique HTCC1002’; ammonia-oxidizing archaea _Nitrosopumilus maritimus_ SCM1, an uncultured marine group II (Iverson et al., 2012); an incomplete hydrothermal vent
metagenome (Xie et al., 2011); and common contaminants. SEQUEST (version UW2011.01.1) was used to correlate observed tandem mass spectra to peptide sequence via theoretical tandem mass
spectra from the composite database described above (Eng et al., 1994, 2008). For a detailed discussion of database considerations in community proteomics, see the publication by Morris et
al. (2010). SEQUEST parameters included a 3.75-Da peptide mass tolerance on MS1 spectra, specifying trypsin as the enzyme; variable oxidation modification on methionine (15.9949 Da) and
static modification on cysteine residues (57.021464 Da) resulting from alkylation. RESULTS DISTRIBUTION OF MARINE GSOS IN THE NORTHEASTERN PACIFIC OCEAN TRFLP profiles from CTD 7, 8, 11 and
13 were conducted to place hydrothermal vent plume communities in a larger regional context (Figure 1a). Community structural analyses identified three distinct groupings (Figure 1b).
Microbial communities in the surface layer (<100 m) were distinct from communities at depths ⩾100 m, and microbial communities directly over hydrothermal vents were distinct from those in
background seawater. TRFLP profiles just above hydrothermal vents at Axial and Coquille were dominated by a 408-bp TRF, which had the strongest positive correlation with ordination axis 1
(0.761; Table 1). The 408 bp TRF was identified as a marine GSO by constructing a 16_S_ rRNA gene clone library (57 clones) from vent plume waters (CTD17, 1450 m) (Figure 1c). A different
marine GSO TRF (324 bp) was observed in relatively low abundance in vent plume samples (0.1–3.2%) and at relatively high abundance in background seawater ⩾100 m, where it reached 10% (Figure
1d). This fragment identity was confirmed by constructing another 16_S_ rRNA gene clone library (53 clones) from 2850 m at the CTD7 station. GSO abundance was not correlated with any single
environmental variable measured in this study. DIVERSITY OF MARINE GSOS IN THE NORTHEASTERN PACIFIC OCEAN Maximum likelihood phylogenetic analyses of nearly full-length 16_S_ rRNA gene
sequences indicate that marine GSOs identified in this study are closely related to marine GSOs that inhabit symbiont, open ocean, coastal and hydrothermal vent environments (Figure 2).
These include sequences recovered from Saanich Inlet, the Yellow Sea, Monterey Bay, the Arabian Sea, the Benguela upwelling system, Suiyo Seamount, the Arctic and Antarctic, and the deep
ocean (Bano and Hollibaugh, 2002; Fuchs et al., 2005; Murray and Grzymski, 2007; Kato et al., 2009; Lavik et al., 2009; Walsh et al., 2009; Zaikova et al., 2010). The Arctic96BD-19 subclade
is comprised of two monophyletic lineages, including one that includes the original Arctic96BD-19 clone and the only known cultured marine GSO (Marshall and Morris, 2013). The SUP05 subclade
is comprised of two or more lineages, including one that includes symbionts of deep-sea clams and one that includes symbionts of deep-sea mussels and free-living representatives that
inhabit both open ocean and coastal environments. DOMINANCE OF MARINE GSOS IN PLUME WATERS Hydrothermal vent plume waters were sampled over the Inferno vent (Figure 3a). Cell concentrations
increased by 4.5-fold from 9.58 × 104 cells per ml at 750 m to an average of 4.27 × 105 cells per ml (±3.69 × 104 cells per ml) below 1445 m over the ASHES vent field (Figure 3b). A 3–4%
drop in beam transmission and a less obvious positive deflection in temperature at 1340–1470 m water depth define the stable and neutrally buoyant hydrothermal plume directly over the
Inferno hydrothermal vent at ASHES. Accompanying water sample measurements of CH4 concentrations and DAPI counts clearly show the enrichment of gases and cells in the plume relative to
background levels. Dissolved oxygen above the Inferno plume shows an oxygen-minimum zone at 800–1100 m water depth and oxygen concentrations in the plume environment of ∼25 μmol kg−1.
Microbial biomass was subsequently concentrated from 180 l of water collected at the same location (CTD17, 1450 m) for 16_S_ rRNA gene and MS/MS proteomic analysis of the Inferno vent plume
microbial community (Supplementary Figure 2). Similar shifts in microbial cell counts, CH4 and beam transmission were not observed at non-venting background locations (Supplementary Figure
2). Fluorescence _in situ_ hybridization, TRFLP and clone library analyses suggest that a single bacterial lineage dominated the Inferno hydrothermal vent plume (Table 1 and Supplementary
Figure 3). Bacteria accounted for 94% of microbial cell counts, suggesting that archaea were a minor component of the plume community. TRFLP and clone library analyses were therefore
conducted using bacteria-specific 16_S_ rRNA gene primers. TRFLP analyses revealed the dominance of a 408-bp TRF (48%). A 16_S_ rRNA gene clone library confirmed the identity of the
bacterial lineage that produced this and other abundant fragments (Table 1). The majority of clones recovered from plume waters (62%) were marine GSOs with 193, 324, 406 and 408 bp TRFs.
Most of them had a 408-bp TRF (28 clones). Five clones were closely related to methylotrophs, and two clones were closely related to known iron oxidizers (Table 1 and Supplementary Table 2).
DOMINANT ACTIVITIES IDENTIFIED IN PLUME WATERS We identified key proteins expressed by plume communities using an MS/MS-based proteomic approach. The presence of 16_S_ rRNA gene sequences
associated with marine GSOs, methylotrophs and iron oxidizers in the CTD17 clone library informed the construction of a protein sequence database for identifying MS/MS spectra, as described
in the Materials and methods section. We confidently identified 239 proteins with ⩾2 peptides in replicate Av1 (Supplementary Table 3) and 131 proteins with ⩾2 peptides in replicate Av2
(Supplementary Table 4). Although fewer proteins were identified in Av2, nearly all (94%) of the proteins identified in Av2 were also identified in Av1 (Supplementary Table 5). Differences
in the total number of proteins identified in replicate samples may result from differences in the amount of biomass obtained during sample processing. Marine GSOs, methylotrophs and iron
oxidizers were active in the Inferno plume (Figure 4). Of the MS/MS spectra assigned to these lineages, 93% were from proteins expressed by marine GSOs and 5% were from proteins expressed by
methanotrophs and iron oxidizers (Figure 4a). Half of the remaining 2% of MS/MS spectra were assigned to proteins expressed by members of the SAR11 clade, and half were assigned to proteins
expressed by other proteobacteria (Supplementary Tables 3 and 4). A significant fraction of the marine GSO proteins (75%) were identified using a SUP05 genome sequence obtained from a
Saanich Inlet metagenome. The remaining marine GSO proteins were identified using genome sequences obtained from marine GSO symbionts (Figure 4b). SUP05 proteins identified using symbiont
genomes, such as cytochrome _c_ oxidase (COX), are likely to be present in vent plume GSOs and symbionts but not present or not identified in GSOs from Saanich Inlet (Walsh et al., 2009).
COX genes were also present and expressed by SUP05 cells in hydrothermal vent plumes from the Guaymas Basin (Anantharaman et al., 2013). Metabolism, genetic information processing and
environmental information processing were the dominant functional classifications identified using the Kyoto Encyclopedia of Genes and Genomes. Of the MS/MS spectra assigned to these
categories, 43.1% were associated with metabolism, 26% were associated with genetic information and processing and 8% were associated with environmental information processing (i.e.,
nitrogen regulatory protein PII) (Figure 4c). A total of 96 metabolic proteins were identified; the majority of these proteins (56.4%) were associated with energy metabolism (Figure 4d).
Proteomic evidence of energy, carbohydrate, amino acid, nucleotide, vitamin and cofactor, and lipid metabolism supports the conclusion that the bacteria identified in Inferno plume waters
were active. CHEMOLITHOAUTOTROPHY AND METHANOTROPHY IN PLUME WATERS Proteomic analysis suggests that hydrothermal vent plume marine GSOs oxidized sulfur, fixed inorganic carbon, respired
oxygen and sensed nitrogen availability in plume waters. Marine GSOs and methylotrophs expressed key proteins associated with carbon fixation (Figure 5). Marine GSOs expressed proteins from
several sulfur oxidation pathways, including key proteins for thiosulfate oxidation (sox), dissimilatory sulfite reductase, adenosine phosphosulfate reductase and ATP sulfurylase (SAT)
(Figure 5a). Several key metabolic proteins were identified in addition to those involved in sulfur metabolism, including components of the Calvin–Benson–Basham cycle
(ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO) and a putative RuBisCO regulator), subunits I and II of COX and a nitrogen regulatory protein (PII). The majority of methylotrophic
proteins identified were associated with CH4 and methanol oxidation (58.4% of spectra) (Figure 5b). These proteins included the β-subunit (PmoB) of particulate methane monooxygenase (pMMO),
which is a characteristic of aerobic methanotrophs, and methanol dehydrogenase (XoxF). We also identified a putative methylotrophic transketolase, which could participate in formaldehyde
fixation via the ribulose monophosphate pathway for carbon fixation used by some methylotrophs (Trotsenko et al., 1986). The formaldehyde-activating enzyme (Fae) was also identified, but
with lower confidence (one peptide with a protein probability of 1), which suggests that the Inferno plume harbored type I methanotrophs that assimilated carbon via the ribulose
monophosphate pathway. DISCUSSION These analyses suggest that free-living marine GSOs were the dominant primary producers in Inferno plume waters at Axial Seamount in 2011. Microbial
community analysis suggests that members of the SUP05 subclade dominated hydrothermal plume waters at Axial volcano (ASHES and Coquille), whereas those from the Arctic96BD-19 subclade were
more abundant in background seawater (⩾100 m). This suggests that vent plume communities harbor diverse marine GSOs dominated by members of the SUP05 subclade and that members of the
Arctic96BD-19 subclade are more ubiquitous in the northeastern Pacific Ocean. We also identified proteins linking energy metabolism with carbon fixation, aerobic respiration and nitrogen
regulation in both marine GSOs and type I methanotrophs (Figure 5). Our findings are consistent with recent observations that marine GSOs are among the active and dominant lineages at
hydrothermal vents. Marine GSOs were among the dominant lineages in plume and diffuse-flow fluids at the Main Endeavour Field and at Axial Seamount on the Juan de Fuca Ridge (Anderson et
al., 2013). A meta-transcriptomic study from Guaymas Basin hydrothermal plumes in the Gulf of California revealed that mRNA transcripts from ammonia oxidizers (_amoAC_), methanotrophs
(_pmoABC_) and marine GSOs (s_oxAY_) were greater in abundance than background seawater abundances (Lesniewski et al., 2012). Methanotrophs dominated plume activities in the Guaymas Basin,
whereas marine GSOs dominated plume activities at Axial volcano and ammonia oxidizers were not a significant component of the Inferno plume community. Both methodological approaches revealed
the dominant lineages and key functions expressed in plumes. Differences are likely due to location and vent chemistry, as the sediment-rich Guaymas Basin provides an abundance of reduced
carbon and nitrogen compounds (Lesniewski et al., 2012). Known bacterial sulfur oxidation systems fall into one of three basic groups. The first group consists of bacteria capable of
complete oxidation of reduced sulfur compounds to sulfate. These organisms have a full set of sulfur oxidation genes (_sox_) including a core set (_sox_ABXYZ) and supplementary genes
(_sox_CDEFGHTRSVW) (Friedrich et al., 2005). A second group uses the branched thiosulfate oxidation pathway via an incomplete sox system (_sox_ABXYZ, but not _sox_C or _sox_D) and the _dsr_
system (Friedrich et al., 2001, 2005; Ghosh and Dam, 2009). Once the more reduced sulfur compounds have been exhausted, this group stores elemental sulfur in globules for later oxidation to
sulfite by _dsr_ proteins (e.g., sulfite reductase) and to sulfate by APS reductase (i.e., AprAB) and ATP sulfurylase (i.e., SAT) (Hensen et al., 2006; Ghosh and Dam, 2009). Lastly, other
bacteria oxidize reduced sulfur compounds via formation of a tetrathionate intermediate. These organisms have no _sox_ genes or an incomplete set of _sox_ genes (Ghosh and Dam, 2009).
Environmental genomic data suggest that marine GSOs harbor incomplete sets of _sox_ and _dsr_ genes, oxidizing sulfur via the formation of a tetrathionate intermediate or via the branched
thiosulfate oxidation pathway. This also suggests that they produce and store elemental sulfur in globules (Walsh et al., 2009). The marine GSO isolate, _Thioglobus singularis_ strain
GSO-PS1, formed external sulfur globules when grown on seawater media (Marshall and Morris, 2013). This suggests that members of the Arctic96BD-19 subclade oxidize sulfur via the formation
of a tetrathionate intermediate or via the branched thiosulfate oxidation pathway. Proteomic data provide further evidence suggesting that marine GSOs utilized the branched thiosulfate
oxidation pathway (Figure 5). In a previous study, critical genes for carbon fixation via the Calvin–Benson–Basham cycle (RuBisCO) were noted in the partial SUP05 metagenome, whereas genes
for a complete tricarboxylic acid cycle, required for heterotrophic growth, were absent, suggesting that SUP05 GSOs are obligate chemolithoautotrophs (Walsh et al., 2009). RuBisCO genes were
also noted in partial Arctic96BD-19 genomes (Swan et al., 2011). We observed marine GSO RuBisCO proteins in the Inferno vent plume, providing functional evidence that free-living marine
GSOs fixed inorganic carbon. Iron oxidizers also contributed to primary production in the plume, as evidenced by the expression of an iron oxidizer RuBisCo (Supplementary Table 3).
Free-living members of the SUP05 subclade are known to thrive in low-oxygen waters (Walsh et al., 2009; Zaikova et al., 2010) and hydrothermal vent plumes (Sunamura et al., 2004), whereas
members of the Arctic96BD-19 GSO clade have been identified in oxygenated inshore and offshore waters, at hydrothermal vents and cold seeps and in the surface layer and deep ocean (Bano and
Hollibaugh, 2002; Fuchs et al., 2005; Murray and Grzymski, 2007; Kato et al., 2009; Lavik et al., 2009; Swan et al., 2011). The Inferno hydrothermal vent plume was sufficiently oxygenated
(dissolved oxygen ranged from 27.6 to 28.6 μmol kg−1) for GSOs to respire aerobically, thus the discovery of COX subunits I and II provides direct evidence that aerobic respiration was
employed (Figure 5). Much less is known about GSO sulfur oxidation in oxygenated waters throughout the deep ocean, where hydrogen sulfide is not present at high concentrations but less
reduced forms of sulfur are available for further oxidation. The presence of CH4 oxidation proteins (pMMO) in the Inferno plume is consistent with the presence of both CH4 and oxygen (Figure
3). The PmoA and PmoC polypeptides of pMMO were not observed. PmoA and PmoC are fully membrane bound, whereas PmoB has soluble domains (Lieberman and Rosenzweig, 2005). Membrane-bound
peptides are difficult to extract and ionize due to their hydrophobic nature, reducing the chance of identification using a whole-cell MS/MS proteomic approach. However, we also identified a
methanol dehydrogenase formaldehyde-activating enzyme (Fae) and putative methylotrophic transketolase that could participate in formaldehyde fixation via the ribulose monophosphate pathway
(Trotsenko et al., 1986). In combination, these data suggest that the Inferno vent plume harbored type I methanotrophs that assimilated carbon from CH4 using pMMO, methanol dehydrogenase and
the ribulose monophosphate pathway (Figure 5). It is clear from the organisms and enzymes identified in vent plumes that reduced compounds emitted from the deep subsurface have the
potential to impact carbon cycling in the deep ocean when they mix with seawater and stimulate growth of a subset of free-living marine bacteria and archaea. Sulfur oxidizers, methylotrophs
and iron oxidizers expressed key proteins associated with carbon fixation. Broader spatial and temporal studies of functions expressed in hydrothermal vent plumes will provide further
insights into carbon cycling in the dark ocean and connectivity between the deep subsurface and deep ocean biospheres. ACCESSION CODES ACCESSIONS GENBANK/EMBL/DDBJ * AJ563935 * KC522839 *
KC522949 REFERENCES * Anantharaman K, Breier JA, Sheik CS, Dick GJ . (2013). Evidence for hydrogen oxidation and metabolic plasticity in widespread deep-sea sulfur-oxidizing bacteria. _Proc
Natl Acad Sci USA_ 110: 330–335. Article CAS Google Scholar * Anderson RE, Beltrán MT, Hallam SJ, Baross JA . (2013). Microbial community structure across fluid gradients in the Juan de
Fuca Ridge hydrothermal system. _FEMS Microbiol Ecol_ 83: 324–339. Article CAS Google Scholar * Baker ET, Massoth GJ, Walker SL, Embley RW . (1993). A method for quantitatively estimating
diffuse and discrete hydrothermal discharge. _Earth Planet Sci Lett_ 118: 235–249. Article Google Scholar * Bano N, Hollibaugh JT . (2002). Phylogenetic composition of bacterioplankton
assemblages from the Arctic Ocean. _Appl Environ Microbiol_ 68: 505–518. Article CAS Google Scholar * Caress DW, Clague DA, Paduan JB, Martin JF, Dreyer BM, Chadwick WW _et al_ (2012).
Repeat bathymetric surveys at 1-metre resolution of lava flows erupted at Axial Seamount in April 2011. _Nat Geosci_ 5: 483–488. Article CAS Google Scholar * Cavanaugh CM, Levering PR,
Maki JS, Mitchell R, Lidstrom ME . (1987). Symbiosis of methylotrophic bacteria and deep-sea mussels. _Nature_ 325: 346–348. Article Google Scholar * Chadwick WW, Nooner SL, Butterfield
DA, Lilley MD . (2012). Seafloor deformation and forecasts of the April 2011 eruption at Axial Seamount. _Nat Geosci_ 5: 474–477. Article CAS Google Scholar * Childress JJ, Fisher CR,
Favuzzi JA, Sanders NK . (1991). Sulfide and carbon dioxide uptake by the hydrothermal vent clam, _Calyptogena magnifica_, and its chemoautotrophic symbionts. _Physiol Zool_ 64: 1444–1470.
Article CAS Google Scholar * Dick GJ, Tebo BM . (2010). Microbial diversity and biogeochemistry of the Guaymas Basin deep-sea hydrothermal plume. _Environ Microbiol_ 12: 1334–1347.
Article CAS Google Scholar * Dickson AG, Sabine CL, Christian JR (eds). (2007) _Guide to Best Practices for Ocean CO2 Measurements_, 191 pp. * Distel DL, Lane DJ, Olsen GJ, Giovannoni SJ,
Pace B, Pace NR _et al_ (1988). Sulfur-oxidizing bacterial endosymbionts: analysis of phylogeny and specificity by 16_S_ rRNA sequences. _J Bacteriol_ 170: 2506–2510. Article CAS Google
Scholar * Dziak RP, Haxel JH, Bohnenstiehl DR, Chadwick WW, Nooner SL, Fowler MJ _et al_ (2012). Seismic precursors and magma ascent before the April 2011 eruption at Axial Seamount. _Nat
Geosci_ 5: 478–482. Article CAS Google Scholar * Eng JK, McCormack AL, Yates JR . (1994). An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a
protein database. _J Am Soc Mass Spectrom_ 5: 976–989. Article CAS Google Scholar * Eng JK, Fischer B, Grossmann J, MacCoss MJ . (2008). A fast SEQUEST cross correlation algorithm. _J
Proteome Res_ 7: 4598–4602. Article CAS Google Scholar * Friedrich CG, Rother D, Bardischewsky F, Quentmeier A, Fischer J . (2001). Oxidation of reduced inorganic sulfur compounds by
bacteria: emergence of a common mechanism? _Appl Environ Microbiol_ 67: 2873–2882. Article CAS Google Scholar * Friedrich CG, Bardischewsky F, Rother D, Quentmeier A, Fischer J . (2005).
Prokaryotic sulfur oxidation. _Curr Opin Microbiol_ 8: 253–259. Article CAS Google Scholar * Fuchs BM, Woebken D, Zubkov MV, Burkill P, Amann R . (2005). Molecular identification of
picoplankton populations in contrasting waters of the Arabian Sea. _Aquat Microb Ecol_ 39: 145–157. Article Google Scholar * Ghosh W, Dam B . (2009). Biochemistry and molecular biology of
lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. _FEMS Microbiol Rev_ 33: 999–1043. Article CAS Google Scholar * Hansell DA . (2005).
Dissolved organic carbon reference material program. _EOS_ 86: 318. Article Google Scholar * Hensen D, Sperling D, Trüper HG, Brune DC, Dahl C . (2006). Thiosulphate oxidation in the
phototrophic sulphur bacterium _Allochromatium vinosum_. _Mol Microbiol_ 62: 794–810. Article CAS Google Scholar * Huang X, Madan A . (1999). CAP3: a DNA sequence assembly program.
_Genome Res_ 9: 868–877. Article CAS Google Scholar * Huber JA, Butterfield DA, Baross JA . (2003). Bacterial diversity in a subseafloor habitat following a deep-sea volcanic eruption.
_FEMS Microbiol Ecol_ 43: 393–409. Article CAS Google Scholar * Huber JA, Johnson HP, Butterfield DA, Baross JA . (2006). Microbial life in ridge flank crustal fluids. _Environ Microbiol_
8: 88–99. Article CAS Google Scholar * Huber JA, Mark Welch DB, Morrison HG, Huse SM, Neal PR, Butterfield DA _et al_ (2007). Microbial population structures in the deep marine
biosphere. _Science_ 318: 97–100. Article CAS Google Scholar * Iverson V, Morris RM, Frazar CD, Berthiaume CT, Morales RL, Armbrust EV . (2012). Untangling genomes from metagenomes:
revealing an uncultured class of marine Euryarchaeota. _Science_ 335: 587–590. Article CAS Google Scholar * Kato S, Hara K, Kasai H, Teramura T, Sunamura M, Ishibashi J-i _et al_ (2009).
Spatial distribution, diversity and composition of bacterial communities in sub-seafloor fluids at a deep-sea hydrothermal field of the Suiyo Seamount. _Deep Sea Res Pt I_ 56: 1844–1855.
Article CAS Google Scholar * Lavik G, Stuhrmann T, Bruchert V, Van der Plas A, Mohrholz V, Lam P _et al_ (2009). Detoxification of sulphidic African shelf waters by blooming
chemolithotrophs. _Nature_ 457: 581–584. Article CAS Google Scholar * Lesniewski RA, Jain S, Anantharaman K, Schloss PD, Dick GJ . (2012). The metatranscriptome of a deep-sea hydrothermal
plume is dominated by water column methanotrophs and lithotrophs. _ISME J_ 6: 2257–2268. Article CAS Google Scholar * Lieberman RL, Rosenzweig AC . (2005). Crystal structure of a
membrane-bound metalloenzyme that catalyses the biological oxidation of methane. _Nature_ 434: 177–182. Article CAS Google Scholar * Lilley MD, de Angelis MA, Gordon LI . (1982). CH4, H2,
CO and N2O in submarine hydrothermal vent waters. _Nature_ 300: 48–50. Article CAS Google Scholar * Lilley MD, Feely RA, Trefry JH . (1995) _Seafloor Hydrothermal Systems: Physical,
Chemical, Biological, and Geological Interactions_ VOL. 91. AGU: Washington, DC. Google Scholar * Lupton JE, Delaney JR, Johnson HP, Tivey MK . (1985). Entrainment and vertical transport of
deep-ocean water by buoyant hydrothermal plumes. _Nature_ 316: 621–623. Article Google Scholar * Marshall KT, Morris RM . (2013). Isolation of an aerobic sulfur oxidizer from the
SUP05/Arctic96BD-19 clade. _ISME J_ 7: 452–455. Article CAS Google Scholar * McCune B, Grace JB . (2002) _Analysis of Ecological Communities_. MjM Software Design: Gleneden Beach, OR.
Google Scholar * Morris RM, Rappe MS, Connon SA, Vergin KL, Siebold WA, Carlson CA _et al_ (2002). SAR11 clade dominates ocean surface bacterioplankton communities. _Nature_ 420: 806–810.
Article CAS Google Scholar * Morris RM, Nunn BL, Frazar C, Goodlett DR, Ting YS, Rocap G . (2010). Comparative metaproteomics reveals ocean-scale shifts in microbial nutrient utilization
and energy transduction. _ISME J_ 4: 673–685. Article CAS Google Scholar * Morris RM, Frazar CD, Carlson CA . (2012). Basin-scale patterns in the abundance of SAR11 subclades, marine
Actinobacteria (OM1), members of the Roseobacter clade and OCS116 in the South Atlantic. _Environ Microbiol_ 14: 1133–1144. Article CAS Google Scholar * Murray AE, Grzymski JJ . (2007).
Diversity and genomics of Antarctic marine micro-organisms. _Philos Trans R Soc Lond B Biol Sci_ 362: 2259–2271. Article CAS Google Scholar * Opatkiewicz AD, Butterfield DA, Baross JA .
(2009). Individual hydrothermal vents at Axial Seamount harbor distinct subseafloor microbial communities. _FEMS Microbiol Ecol_ 70: 413–424. Article CAS Google Scholar * Panchaud A,
Scherl A, Shaffer SA, von Haller PD, Kulasekara HD, Miller SI _et al_ (2009). Precursor ACquisition Independent From Ion Count: how to dive deeper into the proteomics ocean. _Anal Chem_ 81:
6481–6488. Article CAS Google Scholar * Panchaud A, Jung S, Shaffer SA, Aitchison JD, Goodlett DR . (2011). Faster, quantitative, and accurate precursor acquisition independent from ion
count. _Anal Chem_ 83: 2250–2257. Article CAS Google Scholar * Proskurowski G, Lilley MD, Olson EJ . (2008). Stable isotopic evidence in support of active microbial methane cycling in
low-temperature diffuse flow vents at 9°50′N East Pacific Rise. _Geochim Cosmochim Acta_ 72: 2005–2023. Article CAS Google Scholar * Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W,
Peplies J _et al_ (2007). SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. _Nucleic Acids Res_ 35: 7188–7196. Article
CAS Google Scholar * Reinthaler T, van Aken HM, Herndl GJ . (2010). Major contribution of autotrophy to microbial carbon cycling in the deep North Atlantic’s interior. _Deep Sea Res Pt II_
57: 1572–1580. Article CAS Google Scholar * Robinson JJ, Cavanaugh CM . (1995). Expression of form I and form II RuBisCo in chemoautotrophic symbioses: implications for the
interpretation of stable carbon isotope values. _Limnol Oceanogr_ 40: 1496–1502. Article CAS Google Scholar * Stothard P . (2000). The Sequence Manipulation Suite: JavaScript programs for
analyzing and formatting protein and DNA sequences. _Biotechniques_ 28: 1102–1104. Article CAS Google Scholar * Sunamura M, Higashi Y, Miyako C, Ishibashi J-i, Maruyama A . (2004). Two
bacteria phylotypes are predominant in the Suiyo Seamount hydrothermal plume. _Appl Environ Microbiol_ 70: 1190–1198. Article CAS Google Scholar * Swan BK, Martinez-Garcia M, Preston CM,
Sczyrba A, Woyke T, Lamy D _et al_ (2011). Potential for chemolithoautotrophy among ubiquitous bacteria lineages in the dark ocean. _Science_ 333: 1296–1300. Article CAS Google Scholar *
Sylvan JB, Toner BM, Edwards KJ . (2012). Life and death of deep-sea vents: bacterial diversity and ecosystem succession on inactive hydrothermal sulfides. _MBio_ 3: 1–10. Article Google
Scholar * Trotsenko YA, Doronina NV, Govorukhina NI . (1986). Metabolism of non-motile obligately methylotrophic bacteria. _FEMS Microbiol Lett_ 33: 293–297. Article CAS Google Scholar *
Walsh DA, Zaikova E, Howes CG, Song YC, Wright JJ, Tringe SG _et al_ (2009). Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. _Science_ 326: 578–582. Article
CAS Google Scholar * Wang Q, Garrity GM, Tiedje JM, Cole JR . (2007). Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. _Appl Environ
Microbiol_ 73: 5261–5267. Article CAS Google Scholar * Xie W, Wang F, Guo L, Chen Z, Sievert SM, Meng J _et al_ (2011). Comparative metagenomics of microbial communities inhabiting
deep-sea hydrothermal vent chimneys with contrasting chemistries. _ISME J_ 5: 414–426. Article Google Scholar * Zaikova E, Walsh DA, Stilwell CP, Mohn WW, Tortell PD, Hallam SJ . (2010).
Microbial community dynamics in a seasonally anoxic fjord: Saanich Inlet, British Columbia. _Environ Microbiol_ 12: 172–191. Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We thank chief scientists J Delaney and D Kelley and officers and crew of the _R/V Thomas G Thompson_. This study was supported by grants from the National Science
Foundation OCE-1232840 (RM Morris) and OCE-0825790 (BL Nunn) and National Institutes of Health 5P30ES007033-12 and 1S10RR023044. DAH was supported by OCE-1153930. TEM was supported by a
University of Iowa Career Development Award. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Civil and Environmental Engineering, University of Iowa, Iowa City, IA, USA Timothy E
Mattes * Department of Medicinal Chemistry, University of Washington, Seattle, WA, USA Brook L Nunn * School of Oceanography, University of Washington, Seattle, WA, USA Katharine T
Marshall, Giora Proskurowski, Deborah S Kelley, Orest E Kawka & Robert M Morris * Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, MD, USA
David R Goodlett * Rosenstiel School of Marine and Atmospheric Science, University of Miami, Miami, FL, USA Dennis A Hansell Authors * Timothy E Mattes View author publications You can also
search for this author inPubMed Google Scholar * Brook L Nunn View author publications You can also search for this author inPubMed Google Scholar * Katharine T Marshall View author
publications You can also search for this author inPubMed Google Scholar * Giora Proskurowski View author publications You can also search for this author inPubMed Google Scholar * Deborah S
Kelley View author publications You can also search for this author inPubMed Google Scholar * Orest E Kawka View author publications You can also search for this author inPubMed Google
Scholar * David R Goodlett View author publications You can also search for this author inPubMed Google Scholar * Dennis A Hansell View author publications You can also search for this
author inPubMed Google Scholar * Robert M Morris View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Robert M Morris.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies this paper on The ISME Journal website
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE S1 (PDF 1639 KB) SUPPLEMENTARY FIGURE S2 (JPG 306 KB) SUPPLEMENTARY FIGURE S3 (JPG 55 KB) SUPPLEMENTARY TABLES (XLS 368 KB) SUPPLEMENTARY
INFORMATION (DOC 32 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mattes, T., Nunn, B., Marshall, K. _et al._ Sulfur oxidizers dominate carbon
fixation at a biogeochemical hot spot in the dark ocean. _ISME J_ 7, 2349–2360 (2013). https://doi.org/10.1038/ismej.2013.113 Download citation * Received: 08 March 2013 * Revised: 25 May
2013 * Accepted: 31 May 2013 * Published: 11 July 2013 * Issue Date: December 2013 * DOI: https://doi.org/10.1038/ismej.2013.113 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * Axial * hydrothermal * proteomics * bacteria * SUP05 * Arctic96BD-19 * methylotroph