- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Genetic predisposition of elevated nighttime blood pressure (BP) in patients with coronary heart disease is unknown. We evaluated genetic predisposition and the relationship between
elevated nighttime BP and cardiovascular complications over a median of 8.6 years of observation of hypertensive subjects with coronary atherosclerosis confirmed by coronary angiography.
Genetic Risk Score (GRS19) was constructed to evaluate the additive effect of single-nucleotide polymorphisms for daytime and nighttime BP. The Receiver Operating Characteristic was used for
determination of cutoff points for daytime BP (systolic BP (SBP) 133 mm Hg and diastolic BP (DBP) 77 mm Hg) and nighttime BP (SBP 122 mm Hg and DBP 73 mm Hg). The curves of cumulative
incidence revealed an increased risk of major advanced cardiovascular events in subjects with elevated nighttime BP compared with those without elevated nighttime BP during the follow-up
period. Subjects with normal daytime and elevated nighttime BP exhibited increased GRS19 compared with those with normal daytime and nighttime BPs (8.6±3.0 _vs._ 7.9±3.0, _P_<0.01). After
adjustment for cardiovascular risk factors, GRS19 determined nighttime SBP (_β_ 0.4, 95% confidence interval (CI) 0.3–0.5, _P_<0.01). Our study confirmed that elevated nighttime SBP was
genetically determined and related to an increased risk of major adverse coronary events in patients with confirmed coronary atherosclerosis. SIMILAR CONTENT BEING VIEWED BY OTHERS NIGHTTIME
BLOOD PRESSURE AND GLUCOSE CONTROL IMPACTS ON LEFT VENTRICULAR HYPERTROPHY: THE JAPAN MORNING SURGE HOME BLOOD PRESSURE (J-HOP) STUDY Article 30 October 2023 MAXIMUM AMBULATORY DAYTIME
BLOOD PRESSURE AND RISK OF STROKE IN INDIVIDUALS WITH HIGHER AMBULATORY ARTERIAL STIFFNESS INDEX: THE JAMP STUDY Article 16 October 2022 ASSESSING THE RELATIONSHIP BETWEEN LIPOPROTEIN(A)
LEVELS AND BLOOD PRESSURE AMONG HYPERTENSIVE PATIENTS BEYOND CONVENTIONAL MEASURES. AN OBSERVATIONAL STUDY Article Open access 23 June 2024 INTRODUCTION Several studies in different
populations have confirmed that nighttime blood pressure (BP) is a stronger predictor of cardiovascular events than daytime BP.1, 2, 3, 4 Previous studies have suggested that nighttime BP is
elevated in prediabetic and diabetic patients, patients with chronic kidney disease and those with lupus.5, 6, 7, 8 However, nighttime hypertension should be distinguished from a blunted
nighttime BP decrease (referred to as 'non-dipping status'), but nighttime hypertension is more common in non-dippers.9 From a pathophysiological point of view, there is a myriad
of nighttime BP control mechanisms, for example, arterial baroreceptors and chemoreceptors, autonomic and central nervous systems, the renin–angiotensin–aldosterone system and pressure
natriuresis.10 Recently, Obayashi _et al._11 confirmed that renal function is an essential parameter related to elevated nighttime BP in patients with prediabetes. Our recently published
study confirmed that non-dipping status is genetically determined in patients with coronary artery disease (CAD).12 A previously published meta-analysis concluded that nighttime BP is
superior to daytime BP in predicting cardiovascular events and total mortality.13 However, little information is known about the genetic determinants of elevated nighttime BP in this group
of patients. Therefore, we aim to investigate the relationship between genetic risk factors and elevated nighttime BP. METHODS SUBJECTS The present study recruited 1345 individuals (between
August 2003 and August 2006) with signs of myocardial ischemia identified by ECG stress test, dobutamine stress echocardiography or myocardial perfusion scintigraphy stress test. Subjects
with supraventricular and ventricular arrhythmias, NYHA class III or IV congestive heart failure, significant valvular heart disease (or valvular heart disease qualifying the patient for
cardiosurgery), renal insufficiency with a creatinine level ⩾2.0 mg dl−1, changes in pharmacotherapy of hypertension within 6 months before 24-h ambulatory BP monitoring and other chronic
diseases leading to limited life expectancy were excluded.12 BP MEASUREMENTS To avoid the influence on BP of either hospital conditions or the need for reduced physical activity, 24-h
ambulatory BP monitoring was obtained over a period of 2–4 weeks after coronary angiography (SpaceLabs 90210, SpaceLabs, Redmond, WA, USA) with BP readings set at 20-min intervals (0600–1800
hours) and at 30-min intervals (1800–0600 hours). The non-dominant arm was used for measurement with cuff size adjusted to arm circumference (adult cuff 27–34 cm or large adult cuff 35–44
cm). All BP recordings were obtained on working days. Patients were instructed to maintain their usual activities but to refrain from strenuous exercise and emotional burden. Patients were
instructed to hold their arm still by their side during BP measurement and to return to the hospital 24 h later. Participants had no access to their ambulatory BP values. BP measurements
recorded between 0800 and 2200 hours were considered daytime BP values, and BP measurements recorded between 0000 and 0600 hours were considered as nighttime BP values. The percentage
decrease in mean systolic BP (SBP) during the nighttime period was calculated as 100 × (daytime SBP mean–nighttime SBP mean)/daytime SBP mean. Similarly, the percentage decrease in mean
diastolic BP (DBP) during the nighttime period was calculated as 100 × (daytime DBP mean–nighttime DBP mean)/daytime DBP mean. Using this percentage ratio, subjects were classified as
dippers or non-dippers (nighttime relative SBP or DBP decline ⩾ and <10%, respectively).14 This classification was performed for SBP and DBP for each included patient. Office BP
measurements were performed immediately before ambulatory BP measurements using a validated oscillometric device (OMRON 705 IT, OMRON, Greenspoint Parkway, IL, USA) with the cuff fitted to
arm circumference. BP was measured on the non-dominant arm.12 LABORATORY TESTS On admission day before a coronary angiography, fasting blood samples were collected to measure creatinine
level, fasting glucose level, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol and triglyceride levels. Additionally, blood samples were collected
to analyze DNA separation. SINGLE-NUCLEOTIDE POLYMORPHISM (SNP) SELECTION AND GENOTYPING SNPs were selected from genome-wide association studies in which genome-wide association exceeded
the threshold of _P_<5 × 10−8. Selected SNPs were reported to be associated with CAD risk and are presented in Table 1.15, 16, 17, 18, 19, 20 DNA was obtained from whole blood. SNPs were
genotyped using an IPLEX reaction on a MassARRAY platform (Sequenom, San Diego, CA, USA) according to the manufacturer’s standard protocols at Lund University. Only SNPs with a genotyping
success rate >90% and minor allele frequency >5% were analyzed. Regarding quality control, we re-genotyped a random sample of 20% of the successfully genotyped samples for all
genotypes, and the concordance was 99.9%. GENETIC RISK SCORE Genetic Risk Score (GRS19) was constructed to evaluate the additive effect of 19 SNPs for elevated nighttime BP. This multilocus
score was created by summing the number of risk alleles (0/1/2) for each of the 19 SNPs (1 and 2 for heterozygous and homozygous risk allele, respectively, and 0 for homozygous non-risk
allele). GRS19 was assessed for each participant. ASSESSMENT OF CORONARY ATHEROSCLEROSIS Coronary angiography was performed in the Department of Invasive Cardiology, and angiograms were
evaluated independently by two experienced invasive cardiologists. Coronary angiography was used to confirm CAD. FOLLOW-UP PERIOD Subjects were observed from the date of coronary angiography
until 31 December 2013. Follow-up was performed during visits to the clinic. If patients were unable to attend, they were contacted by phone. Stroke diagnosis was performed according to
European Stroke Organization guidelines, and acute coronary syndromes were diagnosed according to European Society of Cardiology guidelines. Cardiovascular mortality involved events due to
acute coronary syndrome (myocardial infarction or unstable angina), heart failure and stroke. Major adverse coronary events included cardiovascular and total mortality and cardiovascular
events (stroke, acute coronary syndromes and new onset of heart failure). STATISTICAL ANALYSIS The main goal of the analysis was to create a relationship between SNPs and elevated nighttime
BP in coronary heart disease patients. A multiple regression model was used for this purpose. An analysis of the Receiver Operating Characteristic was used for predicting daytime and
nighttime SBP and DBP. To determine the ability of daytime and nighttime BPs, cutoff points used to correctly identify subjects with or without major adverse coronary events'
sensitivity and specificity across sites were compared. BP cutoff points were determined by interpolation from the point of intersection between the lines of specificity and sensitivity
(where sensitivity equaled specificity). The point of intersection between the lines of specificity and sensitivity identified the highest numbers of subjects with and without major adverse
coronary events. We also calculated positive and negative predictive values of BP cutoff points for major adverse coronary events. To compare continuous variables, Student's _t_-test or
Mann–Whitney _U_-test was used as appropriate. For categorical variables an χ2 test was used. Log-rank tests were used for comparison of the probability of major adverse coronary events in
the studied groups. Continuous variables were reported as the mean±s.d., and categorical variables were reported as percentages. _P_<0.05 was considered as the level of statistical
significance. Statistical analyses were performed using STATISTICA version 10 (STATISTICA, Tulsa, OK, USA) version 3.0.1. The study protocol was approved by the Ethics Committee of the
Medical University of Gdansk. RESULTS After considering the inclusion and exclusion criteria, 1345 subjects were included in the study. Daytime BP cutoff points that correctly identified the
greatest number of patients with major adverse coronary events were 133 mm Hg for SBP and 77 mm Hg for DBP. Nighttime BP cutoff points that correctly identified the greatest number of
patients with major adverse coronary events were 122 mm Hg for SBP and 73 mm Hg for DBP. According to daytime and nighttime BP cutoff points, patients were divided into four subgroups (Table
2). The baseline characteristics of the study group are summarized in Table 2. In patients with diabetes, we revealed a significant relationship between glycated hemoglobin and both
nighttime SBP (_r_=0.16, _P_<0.01) and nighttime DBP (_r_=0.10, _P_<0.01 ). However, there was no relationship between glycated hemoglobin and daytime SBP and daytime DBP. The BP
values and daytime–nighttime BP variability are presented in Table 3. Analysis of BP dipping revealed the following nighttime SBP drop in groups: daytime BP<133 and <77 mm Hg and
nighttime BP<122 and <73 mm Hg (7.0±5.8%), and daytime BP⩾133 and ⩾77 mm Hg and nighttime BP<122 and <73 mm Hg (13.0±4.8%), daytime BP<133 and <77 mm Hg and nighttime
BP⩾122 and ⩾73 mm Hg (3.0±5.3%), daytime BP⩾133 and ⩾77 mm Hg and nighttime BP⩾122 and ⩾73 mm Hg (4.1±6.6%). Analysis of BP dipping revealed the following nighttime DBP drop in groups:
daytime BP<133 and <77 mm Hg and nighttime BP<122 and <73 mm Hg (11.3±6.5%), daytime BP⩾133 and ⩾77 mm Hg and nighttime BP<122 and <73 mm Hg (17.5±5.5%), daytime BP<133
and <77 mm Hg and nighttime BP⩾122 and ⩾73 mm Hg (2.0±5.6%), and daytime BP⩾133 and ⩾77 mm Hg and nighttime BP⩾122 and ⩾73 mm Hg (8.1±6.6%). The relationships between daytime and
nighttime BPs and GRS19 are presented in Table 4. The median follow-up period was 8.6 years. During 11 567 person-years of follow-up, 245 participants died (2.1 per 1000 person-years),
including 114 of participants from cardiovascular causes (0.98 per 1000 person-years). After taking into consideration cardiovascular events during the follow-up period, 441 (32.7%) subjects
experienced major adverse coronary events. Patients with elevated nighttime and normal daytime BP exhibited an increased risk of major adverse coronary events compared with those with
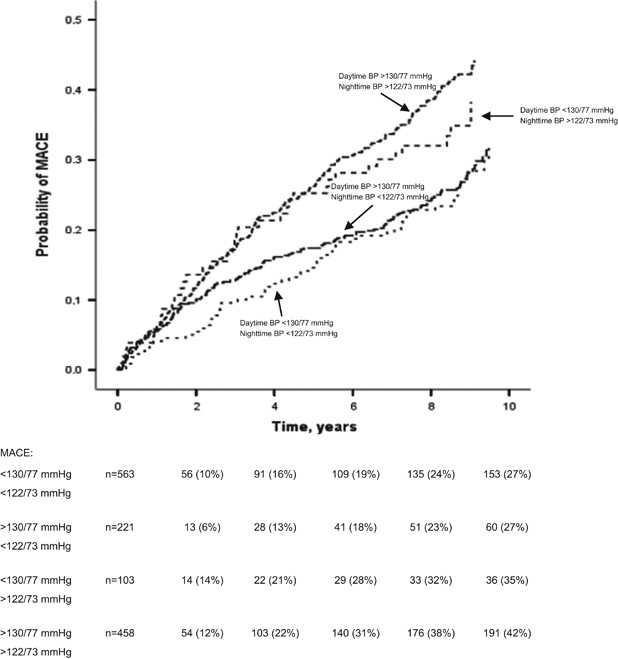
normal nighttime and elevated daytime BP. The probability of major adverse coronary events in the studied subgroups of patients is presented in Figure 1. Significant differences were noted
between the probability of major adverse coronary events in patients with daytime BP⩾133 and ⩾77 mm Hg and nighttime BP⩾122 and ⩾73 mm Hg and patients with daytime BP⩾133 and ⩾77 mm Hg and
nighttime BP<122 and <73 mm Hg (_P_<0.03). We also observed significant differences between the probability of major adverse coronary events in patients with daytime BP⩾133 and ⩾77
mm Hg and nighttime BP⩾122 and ⩾73 mm Hg and patients with daytime BP<133 and <77 mm Hg and nighttime BP<122 and <73 mm Hg (_P_<0.02). Significant differences were noted
between probability of major adverse coronary events in patients with daytime BP<133 and <77 mm Hg and nighttime BP⩾122 and ⩾73 mm Hg and patients with daytime BP⩾133 and ⩾77 mm Hg and
nighttime BP<122 and <73 mm Hg (_P_<0.05). We also observed significant differences between the probability of major adverse coronary events in patients with daytime BP<133 and
<77 mm Hg and nighttime BP⩾122 and ⩾73 mm Hg and patients with daytime BP<133 and <77 mm Hg and nighttime BP<122 and <73 mm Hg (_P_<0.04). DISCUSSION The main finding of
our study is the confirmation that elevated nighttime BP is genetically determined and nighttime DBP is related to GRS19 in CAD patients. We also found that elevated nighttime BP is
associated with risk of major adverse coronary events in CAD patients. Persuasive evidence suggests that nighttime BP is superior to daytime BP in predicting outcome.21 Given the limited
reproducibility of daytime BP levels due to the dependence of daily physical activity, cardiovascular risk assessment might be more accurate if based on nighttime BP. Therefore, it is
essential to understand the potential mechanisms that underlie BP regulation at night. The Georgia Cardiovascular Twin Study demonstrated that, apart from genes that also influence daytime
BP, specific genetic determinants explained 44% and 67% of nighttime SBP and DBP heritabilities, respectively.22 In addition to genes that influence both daytime and nighttime BP, a large
portion of heritability is explained by genes that specifically influence BP at night.23 In the present study, we detected and examined the relationship between nighttime BP and SNPs in
patients with CAD for the first time. Although a single SNP determines cardiovascular risk to a very small extent, Ripatti _et al._24 revealed that a GRS based on the additive effects of
SNPs is more associated with cardiovascular risk. Using the concept of the additive effect of SNPs, we constructed GRS based on 19 SNPs significantly associated with cardiovascular risk, and
we revealed that GRS19 was related to nighttime DBP. Moreover, we confirmed that elevated nighttime BP was related to an increased risk of major adverse coronary events. Therefore, GRS19
may be taken into consideration in cardiovascular risk assessment for patients with inappropriate daytime–nighttime BP variability. Leu _et al._25 confirmed genetic association with diurnal
BP changes among subjects with young-onset hypertension. In contrast to our study, Leu _et al._25 did not assess coronary atherosclerosis in coronary angiography. Previously performed
clinical trials confirmed that both elevated daytime BP and nighttime BP were related to cardiovascular risk in CAD patients.26 Moreover, blunted nighttime BP dipping was associated with
advanced CAD and cardiovascular complications that arise from this condition.27 Similarly, in a small group of men, Mousa _et al._28 confirmed the association between blunted nighttime BP
values and coronary atherosclerosis extent in coronary angiography. Brotman _et al._29 demonstrated that non-dipping status was a risk factor for all-cause mortality. Similar to the study by
Brotman _et al._,29 greater than half of the patients were non-dippers in our study. However, in the study by Brotman _et al._,29 non-dipping status was defined as a reduction in mean
nocturnal SBP<10% compared with mean daytime values. In our study, non-dipping status was defined as a reduction in mean SBP<10% and/or a reduction in mean DBP<10% compared with
respective daytime values. Pierdomenico _et al._30 confirmed that circadian BP changes were independently associated with increased cardiovascular risk in elderly subjects. In contrast to
our study, they did not take into consideration coexisting CAD confirmed by coronary angiography. Of note, in our study, elevated nighttime BPs were observed more often in patients with
diabetes, and glycemic control was worse in diabetics with elevated nighttime BPs. Diabetes mellitus is associated with markedly increased cardiovascular risk, and the predictive role of
nighttime BP has already been established in patients with diabetes.3, 31 Previously performed clinical trials confirmed that nocturnal hypertension is more frequent in diabetics, partially
due to autonomic dysfunction.32, 33 However, our study confirmed that elevated nighttime BP is more frequent in diabetics with less effectively controlled glycemia. Accordingly, diabetes,
especially when uncontrolled, may predispose a patient to elevated nighttime BP that may subsequently be related to high cardiovascular risk. A modification as simple and inexpensive as
switching the ingestion time of one or more hypertensive medications from morning to evening or bedtime may be the only action necessary to achieve proper control of nighttime BP. Recently
published results from the MAPEC study confirmed that elevated asleep BP was associated with cardiovascular morbidity and mortality, and a bedtime antihypertensive treatment strategy
significantly reduced cardiovascular risk.34 Our study confirmed that elevated nighttime BP was related to an increased probability of major adverse coronary events in patients with
confirmed CAD. Moreover, the highest risk of major adverse coronary events was observed in patients with elevated nighttime and daytime BPs, but patients with elevated nighttime BP and
normal daytime BP had increased risk of major adverse coronary events compared with subjects with normal nighttime BP and elevated daytime BP. To date, our studies have confirmed that
nighttime BP contribute to increased cardiovascular risk in patients with coronary atherosclerosis confirmed by coronary angiography.35 The systematic review by O'Flynn _et al._36
attempted to explain the reasons for high cardiovascular mortality related to isolated nighttime hypertension. However, analyzed data did not produce a clear answer concerning the
relationship between elevated nighttime BP and target organ damage associated with cardiovascular mortality.36 Haruhara _et al._37 suggested that isolated nocturnal hypertension was related
to renal interstitial fibrosis or tubular atrophy. Furthermore, in the RESIST-POL study, nighttime SBP was independently related to concentric heart hypertrophy.38 Sherwood _et al._39
confirmed that CAD and advanced age are accompanied by blunted nighttime BP dipping, which may increase the risk of adverse cardiovascular events. Some limitations of our study are worth
mentioning. Given that this study was retrospective, longitudinal assessment of hypertension control and changes in antihypertensive therapy were beyond the scope of the investigation. In
addition, multiple assessments of ambulatory BP were not performed, thereby eliminating our ability to determine whether BP values were stable over time or impacted by antihypertensive
treatment. Cutoff points of BP values were established on the basis of BP values measured over 24 h in patients with confirmed CAD. CONCLUSION In conclusion, our study confirmed an important
role of nighttime BP in the development of cardiovascular complications in CAD patients. Subjects with elevated nighttime BP and normal daytime BP exhibit an increased risk of
cardiovascular complications compared with those with normal nighttime and elevated daytime BP. Nighttime SBP is genetically determined and related to the additive effect of SNPs. Future
studies should address the application of GRSs in clinical practice. REFERENCES * Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P,
Parati G, Tuomilehto J, Webster J . Predicting cardiovascular risk using conventional _vs_. ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in
Europe Trial Investigators. _JAMA_ 1999; 282: 539–546. Article CAS Google Scholar * Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA,
O'Brien E . Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. _Hypertension_ 2005; 46: 156–161. Article CAS Google
Scholar * Hermida RC, Ayala DE, Mojon A, Fernandez JR . Sleep-time blood pressure as a therapeutic target for cardiovascular risk reduction in type 2 diabetes. _Am J Hypertens_ 2012; 25:
325–334. Article Google Scholar * Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G . Prognostic value of ambulatory and home blood pressures compared with office
blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. _Circulation_ 2005; 111: 1777–1783. Article Google
Scholar * Rossen NB, Knudsen ST, Fleischer J, Hvas AM, Ebbehoj E, Poulsen PL, Hansen KW . Targeting nocturnal hypertension in type 2 diabetes mellitus. _Hypertension_ 2014; 64: 1080–1087.
Article CAS Google Scholar * Ruiz-Hurtado G, Ruilope LM, de la Sierra A, Sarafidis P, de la Cruz JJ, Gorostidi M, Segura J, Vinyoles E, Banegas JR . Association between high and very high
albuminuria and nighttime blood pressure: influence of diabetes and chronic kidney disease. _Diabetes Care_ 2016; 39: 1729–1737. Article Google Scholar * Drawz PE, Alper AB, Anderson AH,
Brecklin CS, Charleston J, Chen J, Deo R, Fischer MJ, He J, Hsu CY, Huan Y, Keane MG, Kusek JW, Makos GK, Miller 3rd ER, Soliman EZ, Steigerwalt SP, Taliercio JJ, Townsend RR, Weir MR,
Wright Jr JT, Xie D, Rahman M . Masked hypertension and elevated nighttime blood pressure in CKD: prevalence and association with target organ damage. _Clin J Am Soc Nephrol_ 2016; 11:
642–652. Article CAS Google Scholar * Sabio JM, Martinez-Bordonado J, Sanchez-Berna I, Vargas-Hitos JA, Mediavilla JD, Navarrete-Navarrete N, Zamora-Pasadas M, Ruiz ME, Jimenez-Alonso J .
Nighttime blood pressure patterns and subclinical atherosclerosis in women with systemic lupus erythematosus. _J Rheumatol_ 2015; 42: 2310–2317. Article CAS Google Scholar * Sobiczewski
W, Wirtwein M, Gruchala M, Kocic I . Mortality in hypertensive patients with coronary heart disease depends on chronopharmacotherapy and dipping status. _Pharmacol Rep_ 2014; 66: 448–452.
Article CAS Google Scholar * Portaluppi F, Smolensky MH . Perspectives on the chronotherapy of hypertension based on the results of the MAPEC study. _Chronobiol Int_ 2010; 27: 1652–1667.
Article CAS Google Scholar * Obayashi K, Saeki K, Kurumatani N . Nighttime BP in elderly individuals with prediabetes/diabetes with and without CKD: The HEIJO-KYO Study. _Clin J Am Soc
Nephrol_ 2016; 11: 867–874. Article CAS Google Scholar * Wirtwein M, Melander O, Sjogren M, Hoffmann M, Narkiewicz K, Gruchala M, Sobiczewski W . The relationship between gene
polymorphisms and dipping profile in patients with coronary heart disease. _Am J Hypertens_ 2016; 29: 1094–1102. Article Google Scholar * Hansen TW, Li Y, Boggia J, Thijs L, Richart T,
Staessen JA . Predictive role of the nighttime blood pressure. _Hypertension_ 2011; 57: 3–10. Article CAS Google Scholar * O'Brien E, Sheridan J, O'Malley K . Dippers and
non-dippers. _Lancet_ 1988; 2: 397. Article CAS Google Scholar * Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H,
Erdmann J, Reilly MP, Rader DJ, Morgan T, Spertus JA, Stoll M, Girelli D, McKeown PP, Patterson CC, Siscovick DS, O'Donnell CJ, Elosua R, Peltonen L, Salomaa V, Schwartz SM, Melander O,
Altshuler D, Merlini PA, Berzuini C, Bernardinelli L, Peyvandi F, Tubaro M, Celli P, Ferrario M, Fetiveau R, Marziliano N, Casari G, Galli M, Ribichini F, Rossi M, Bernardi F, Zonzin P,
Piazza A, Yee J, Friedlander Y, Marrugat J, Lucas G, Subirana I, Sala J, Ramos R, Meigs JB, Williams G, Nathan DM, MacRae CA, Havulinna AS, Berglund G, Hirschhorn JN, Asselta R, Duga S,
Spreafico M, Daly MJ, Nemesh J, Korn JM, McCarroll SA, Surti A, Guiducci C, Gianniny L, Mirel D, Parkin M, Burtt N, Gabriel SB, Thompson JR, Braund PS, Wright BJ, Balmforth AJ, Ball SG, Hall
A, Linsel-Nitschke P, Lieb W, Ziegler A, Konig I, Hengstenberg C, Fischer M, Stark K, Grosshennig A, Preuss M, Wichmann HE, Schreiber S, Ouwehand W, Deloukas P, Scholz M, Cambien F, Li M,
Chen Z, Wilensky R, Matthai W, Qasim A, Hakonarson HH, Devaney J, Burnett MS, Pichard AD, Kent KM, Satler L, Lindsay JM, Waksman R, Knouff CW, Waterworth DM, Walker MC, Mooser V, Epstein SE,
Scheffold T, Berger K, Huge A, Martinelli N, Olivieri O, Corrocher R, McKeown P, Erdmann E, Konig IR, Holm H, Thorleifsson G, Thorsteinsdottir U, Stefansson K, Do R, Xie C, Siscovick D .
Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. _Nat Genet_ 2009; 41: 334–341. Article CAS Google Scholar *
Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de
Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M . Genetic variants associated with Lp(a) lipoprotein level and coronary disease. _N Engl J Med_ 2009; 361: 2518–2528. Article
CAS Google Scholar * Erdmann J, Grosshennig A, Braund PS, Konig IR, Hengstenberg C, Hall AS, Linsel-Nitschke P, Kathiresan S, Wright B, Tregouet DA, Cambien F, Bruse P, Aherrahrou Z,
Wagner AK, Stark K, Schwartz SM, Salomaa V, Elosua R, Melander O, Voight BF, O'Donnell CJ, Peltonen L, Siscovick DS, Altshuler D, Merlini PA, Peyvandi F, Bernardinelli L, Ardissino D,
Schillert A, Blankenberg S, Zeller T, Wild P, Schwarz DF, Tiret L, Perret C, Schreiber S, El Mokhtari NE, Schafer A, Marz W, Renner W, Bugert P, Kluter H, Schrezenmeir J, Rubin D, Ball SG,
Balmforth AJ, Wichmann HE, Meitinger T, Fischer M, Meisinger C, Baumert J, Peters A, Ouwehand WH, Deloukas P, Thompson JR, Ziegler A, Samani NJ, Schunkert H . New susceptibility locus for
coronary artery disease on chromosome 3q22.3. _Nat Genet_ 2009; 41: 280–282. Article CAS Google Scholar * Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M,
Stewart AF, Barbalic M, Gieger C, Absher D, Aherrahrou Z, Allayee H, Altshuler D, Anand SS, Andersen K, Anderson JL, Ardissino D, Ball SG, Balmforth AJ, Barnes TA, Becker DM, Becker LC,
Berger K, Bis JC, Boekholdt SM, Boerwinkle E, Braund PS, Brown MJ, Burnett MS, Buysschaert I, Carlquist JF, Chen L, Cichon S, Codd V, Davies RW, Dedoussis G, Dehghan A, Demissie S, Devaney
JM, Diemert P, Do R, Doering A, Eifert S, Mokhtari NE, Ellis SG, Elosua R, Engert JC, Epstein SE, de Faire U, Fischer M, Folsom AR, Freyer J, Gigante B, Girelli D, Gretarsdottir S, Gudnason
V, Gulcher JR, Halperin E, Hammond N, Hazen SL, Hofman A, Horne BD, Illig T, Iribarren C, Jones GT, Jukema JW, Kaiser MA, Kaplan LM, Kastelein JJ, Khaw KT, Knowles JW, Kolovou G, Kong A,
Laaksonen R, Lambrechts D, Leander K, Lettre G, Li M, Lieb W, Loley C, Lotery AJ, Mannucci PM, Maouche S, Martinelli N, McKeown PP, Meisinger C, Meitinger T, Melander O, Merlini PA, Mooser
V, Morgan T, Muhleisen TW, Muhlestein JB, Munzel T, Musunuru K, Nahrstaedt J, Nelson CP, Nothen MM, Olivieri O, Patel RS, Patterson CC, Peters A, Peyvandi F, Qu L, Quyyumi AA, Rader DJ,
Rallidis LS, Rice C, Rosendaal FR, Rubin D, Salomaa V, Sampietro ML, Sandhu MS, Schadt E, Schafer A, Schillert A, Schreiber S, Schrezenmeir J, Schwartz SM, Siscovick DS, Sivananthan M,
Sivapalaratnam S, Smith A, Smith TB, Snoep JD, Soranzo N, Spertus JA, Stark K, Stirrups K, Stoll M, Tang WH, Tennstedt S, Thorgeirsson G, Thorleifsson G, Tomaszewski M, Uitterlinden AG, van
Rij AM, Voight BF, Wareham NJ, Wells GA, Wichmann HE, Wild PS, Willenborg C, Witteman JC, Wright BJ, Ye S, Zeller T, Ziegler A, Cambien F, Goodall AH, Cupples LA, Quertermous T, Marz W,
Hengstenberg C, Blankenberg S, Ouwehand WH, Hall AS, Deloukas P, Thompson JR, Stefansson K, Roberts R, Thorsteinsdottir U, O'Donnell CJ, McPherson R, Erdmann J, Samani NJ . Large-scale
association analysis identifies 13 new susceptibility loci for coronary artery disease. _Nat Genet_ 2011; 43: 333–338. Article CAS Google Scholar * Roberts R, Stewart AF . Genes and
coronary artery disease: where are we? _J Am Coll Cardiol_ 2012; 60: 1715–1721. Article CAS Google Scholar * Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR,
Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, Konig IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna
AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikainen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL,
Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi-Boehm S, Cox D, Dimitriou M, Do R, Doney AS, El Mokhtari N, Eriksson P, Fischer K,
Fontanillas P, Franco-Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C,
Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Muller-Nurasyid M, Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S,
Rubin D, Rumpf MP, Schafer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJ, Wells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M,
Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrieres J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm
H, Iribarren C, Jang Y, Kahonen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous
T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Tregouet DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen
T, Syvanen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimaki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O'Donnell C,
Reilly MP, Marz W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ . Large-scale association analysis
identifies new risk loci for coronary artery disease. _Nat Genet_ 2013; 45: 25–33. Article CAS Google Scholar * Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Richart
T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O'Brien E, Staessen JA . Prognostic accuracy of day versus night ambulatory blood pressure: a
cohort study. _Lancet_ 2007; 370: 1219–1229. Article Google Scholar * Wang X, Ding X, Su S, Yan W, Harshfield G, Treiber F, Snieder H . Genetic influences on daytime and night-time blood
pressure: similarities and differences. _J Hypertens_ 2009; 27: 2358–2364. Article CAS Google Scholar * Xu X, Su S, Treiber FA, Vlietinck R, Fagard R, Derom C, Gielen M, Loos RJ, Snieder
H, Wang X . Specific genetic influences on nighttime blood pressure. _Am J Hypertens_ 2015; 28: 440–443. Article Google Scholar * Ripatti S, Tikkanen E, Orho-Melander M, Havulinna AS,
Silander K, Sharma A, Guiducci C, Perola M, Jula A, Sinisalo J, Lokki ML, Nieminen MS, Melander O, Salomaa V, Peltonen L, Kathiresan S . A multilocus genetic risk score for coronary heart
disease: case-control and prospective cohort analyses. _Lancet_ 2010; 376: 1393–1400. Article Google Scholar * Leu HB, Chung CM, Lin SJ, Chiang KM, Yang HC, Ho HY, Ting CT, Lin TH, Sheu
SH, Tsai WC, Chen JH, Yin WH, Chiu TY, Chen CI, Fann CS, Chen YT, Pan WH, Chen JW . Association of circadian genes with diurnal blood pressure changes and non-dipper essential hypertension:
a genetic association with young-onset hypertension. _Hypertens Res_ 2015; 38: 155–162. Article CAS Google Scholar * Sobiczewski W, Wirtwein M, Gruchala M . Is daytime blood pressure
adequate in cardiovascular risk assessment in patients with coronary atherosclerosis? _Blood Press_ 2014; 23: 96–101. Article Google Scholar * Wirtwein M, Gruchala M, Sobiczewski W .
Diurnal blood pressure profile and coronary atherosclerosis extent are related to cardiovascular complications. _Blood Press_ 2016; 26: 1–6. Google Scholar * Mousa T, el-Sayed MA, Motawea
AK, Salama MA, Elhendy A . Association of blunted nighttime blood pressure dipping with coronary artery stenosis in men. _Am J Hypertens_ 2004; 17: 977–980. Article Google Scholar *
Brotman DJ, Davidson MB, Boumitri M, Vidt DG . Impaired diurnal blood pressure variation and all-cause mortality. _Am J Hypertens_ 2008; 21: 92–97. Article Google Scholar * Pierdomenico
SD, Pierdomenico AM, Coccina F, Lapenna D, Porreca E . Circadian blood pressure changes and cardiovascular risk in elderly-treated hypertensive patients. _Hypertens Res_ 2016; 39: 805–811.
Article Google Scholar * Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson
A, Sattar N, White IR, Ray KK, Danesh J . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies.
_Lancet_ 2010; 375: 2215–2222. Article CAS Google Scholar * Tofe Povedano S, Garcia, De La Villa B . 24-Hour and nighttime blood pressures in type 2 diabetic hypertensive patients
following morning or evening administration of olmesartan. _J Clin Hypertens (Greenwich)_ 2009; 11: 426–431. Article Google Scholar * Hermida RC, Ayala DE, Mojon A, Fernandez JR .
Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. _Diabetes Care_ 2011; 34: 1270–1276. Article Google
Scholar * Hermida RC, Ayala DE, Smolensky MH, Fernandez JR, Mojon A, Portaluppi F . Chronotherapy with conventional blood pressure medications improves management of hypertension and
reduces cardiovascular and stroke risks. _Hypertens Res_ 2016; 39: 277–292. Article Google Scholar * Sobiczewski W, Wirtwein M, Gruchala M . Is nighttime blood pressure important in
cardiovascular risk assessment in coronary atherosclerosis? _J Hum Hypertens_ 2014; 28: 564–566. Article CAS Google Scholar * O'Flynn AM, Madden JM, Russell AJ, Curtin RJ, Kearney PM
. Isolated nocturnal hypertension and subclinical target organ damage: a systematic review of the literature. _Hypertens Res_ 2015; 38: 570–575. Article Google Scholar * Haruhara K,
Tsuboi N, Koike K, Fukui A, Miyazaki Y, Kawamura T, Ogura M, Yokoo T . Renal histopathological findings in relation to ambulatory blood pressure in chronic kidney disease patients.
_Hypertens Res_ 2015; 38: 116–122. Article Google Scholar * Dobrowolski P, Prejbisz A, Klisiewicz A, Florczak E, Rybicka J, Januszewicz A, Hoffman P . Determinants of concentric left
ventricular hypertrophy in patients with resistant hypertension: RESIST-POL study. _Hypertens Res_ 2015; 38: 545–550. Article CAS Google Scholar * Sherwood A, Bower JK, Routledge FS,
Blumenthal JA, McFetridge-Durdle JA, Newby LK, Hinderliter AL . Nighttime blood pressure dipping in postmenopausal women with coronary heart disease. _Am J Hypertens_ 2012; 25: 1077–1082.
Article Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pharmacology, Medical University of Gdansk, Gdansk, Poland Marcin Wirtwein *
Department of Clinical Sciences, Lund University, Malmö, Sweden Olle Melander & Marketa Sjőgren * Department of Hypertension and Diabetology, Medical University of Gdansk, Gdansk, Poland
Michal Hoffmann & Krzysztof Narkiewicz * I Department of Cardiology, Medical University of Gdansk, Gdansk, Poland Marcin Gruchala & Wojciech Sobiczewski Authors * Marcin Wirtwein
View author publications You can also search for this author inPubMed Google Scholar * Olle Melander View author publications You can also search for this author inPubMed Google Scholar *
Marketa Sjőgren View author publications You can also search for this author inPubMed Google Scholar * Michal Hoffmann View author publications You can also search for this author inPubMed
Google Scholar * Krzysztof Narkiewicz View author publications You can also search for this author inPubMed Google Scholar * Marcin Gruchala View author publications You can also search for
this author inPubMed Google Scholar * Wojciech Sobiczewski View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Marcin
Wirtwein. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wirtwein,
M., Melander, O., Sjőgren, M. _et al._ Genetic risk factors influence nighttime blood pressure and related cardiovascular complications in patients with coronary heart disease. _Hypertens
Res_ 41, 53–59 (2018). https://doi.org/10.1038/hr.2017.87 Download citation * Received: 07 January 2017 * Revised: 03 April 2017 * Accepted: 05 May 2017 * Published: 05 October 2017 * Issue
Date: January 2018 * DOI: https://doi.org/10.1038/hr.2017.87 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * coronary atherosclerosis *
coronary artery disease * dipping * DNA polymorphism * nighttime hypertension









