- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The aim of this study was to determine whether pulse wave velocity (PWV), a marker of vascular endothelial impairment and arteriosclerosis, predicts future ischemic stroke in
patients who developed acute lacunar infarction. Patients with a first-ever ischemic stroke due to acute lacunar infarction were enrolled in this study. An oscillometric device (Form
PWV/ABI; Omron Colin, Tokyo, Japan) was used to measure brachial–ankle PWV 1 week after stroke onset. Patients were followed for at least 5 years. The main end point of the study was
recurrent ischemic stroke. Event-free survival was analyzed using Kaplan–Meier plots and log-rank tests. The risk of recurrent ischemic stroke was estimated using the Cox
proportional-hazards model. Of the 156 patients (61% male, mean age: 69.2±11.3 years) assessed in this study, 29 developed recurrent ischemic stroke. The median brachial–ankle PWV value was
20.4 m s−1. Patients with high PWV values had a greater risk of recurrent ischemic stroke than patients with low PWV values (28% _vs_. 15%, _P_=0.08). Kaplan–Meier curve analysis showed that
patients with high PWV values had a less favorable (that is, free of recurrent ischemic stroke) survival time (_P_=0.015). A multivariate Cox proportional-hazards model identified high PWV
as an independent predictor of recurrent ischemic stroke after adjusting for age, sex and blood pressure (hazard ratio 2.35, 95% confidence interval, 1.02–5.70, _P_=0.044). In patients with
acute lacunar infarction, a high PWV predicts a twofold greater risk of future ischemic stroke, independent of patient age, sex and blood pressure levels. SIMILAR CONTENT BEING VIEWED BY
OTHERS ASSOCIATION BETWEEN BASELINE BRACHIAL–ANKLE PULSE WAVE VELOCITY AND SHORT-TERM RISK OF FIRST STROKE AMONG CHINESE HYPERTENSIVE ADULTS Article 15 November 2021 BRACHIAL-ANKLE PULSE
WAVE VELOCITY AND PROGNOSIS IN PATIENTS WITH ATHEROSCLEROTIC CARDIOVASCULAR DISEASE: A SYSTEMATIC REVIEW AND META-ANALYSIS Article 14 June 2021 EARLY VASCULAR AGING DETERMINED BY
BRACHIAL-ANKLE PULSE WAVE VELOCITY AND ITS IMPACT ON ISCHEMIC STROKE OUTCOME: A RETROSPECTIVE OBSERVATIONAL STUDY Article Open access 13 June 2024 INTRODUCTION Arterial stiffness is an
independent determinant of cardiovascular and cerebrovascular risks; it is also a marker of vascular endothelial impairment, arteriosclerosis and subclinical organ damage.1, 2, 3 Arterial
stiffness has been monitored using pulse wave velocity (PWV) parameters, such as carotid–femoral PWV,4, 5 brachial–ankle PWV6, 7, 8, 9 and cardio-ankle vascular index.10, 11 Although some
methodological differences exist between these surrogate markers,12, 13 the significance of PWV assessment has been clarified.1, 2, 3, 14, 15, 16 Evidence of PWV indicates microvessel
arteriosclerosis presenting with vascular endothelial dysfunction and can lead to cardiovascular disease,1, 3, 16, 17, 18, 19, 20 stroke2, 21 and death.1, 3, 22 Furthermore, cerebral small
vessel diseases, such as silent lacunar infarction and white matter hyperintensities, pose a risk of cerebrovascular disease by affecting arterial stiffness.3, 14, 17, 23, 24, 25
Specifically, vascular narrowing due to atherosclerosis and worsening vascular stiffness can accelerate pulse waves14 and may also increase the risk of ischemic stroke. We have previously
demonstrated the cross-sectional relationship between PWV and early neurological outcomes after ischemic stroke in patients who developed acute lacunar infarction, which is attributed to
cerebral small vessel disease.7 Although our findings align with those of previous studies,5, 26 the longitudinal neurological outcome (that is, whether PWV predicts recurrent ischemic
stroke) has not been clarified. The aim of this study was to determine whether PWV predicts future ischemic stroke in patients who have developed acute lacunar infarction attributed to
cerebral small vessel disease. METHODS PATIENTS Between October 2003 and March 2010, we enrolled consecutive patients with clinical lacunar stroke syndrome (within 48 h after stroke onset)
who were admitted to the Department of Neurology, Hyogo Brain and Heart Center at Himeji (HBHC), Himeji, Japan. All patients had experienced a first-ever ischemic stroke owing to acute
lacunar infarction. The cross-sectional analysis of these patients is described elsewhere.7 This longitudinal observational study complied with the Declaration of Helsinki, and it was
approved by the Institutional Review Board at the HBHC. Informed consent was obtained from all patients. This study is registered with the UMIN Clinical Trials Registry (UMIN000023239).
BASELINE ASSESSMENT We assessed the following clinical parameters: (1) demographic data, such as age and sex, (2) vascular risk factors, (3) laboratory data, (4) brain imaging, (5)
arteriosclerosis assessment, and (6) clinical outcomes. Using an oscillometric device (Form PWV/ABI; Omron Colin, Tokyo, Japan), we measured brachial–ankle PWV 1 week after the onset of
stroke.7 We measured the common carotid artery intima-media thickness using high-resolution B-mode ultrasonography, with a 7.5-MHz linear array transducer (Aplio XG SSA-790A, Toshiba
Medical, Tokyo, Japan).9, 11 Trained neurologists assessed the National Institutes of Health Stroke Scale scores upon patient admission and the modified Rankin Scale scores at discharge.
Detailed information is provided in the Supplementary File and described elsewhere.7 LONGITUDINAL ASSESSMENT Patients were followed for at least 5 years after the onset of stroke at their
primary care physician’s offices and/or at the HBHC. The study end points were all-cause mortality and recurrent ischemic stroke. Recurrent ischemic stroke subtypes were categorized as
atherothrombotic (large-artery atherosclerosis), cardioembolism, lacunar (small-artery occlusion) and other causes attributed to undetermined etiology or other determined etiology.27
Patients were thoroughly assessed using multiple concurrent approaches, including reviewing the HBHC hospital medical records, contacting the patients and their families for telephone
interviews and contacting their primary care physicians using a standardized interview form. The longitudinal assessment ended in March 2015. STATISTICAL ANALYSIS Continuous, ordinal and
categorical variables were compared using unpaired Student’s _t_-tests, Wilcoxon rank-sum tests and χ2 tests, respectively. First, we compared the PWV values of the patients who developed
recurrent ischemic stroke and those who did not. Second, we divided the patients into two groups, according to their PWV values (high _vs_. low compared with the median PWV value), and we
compared their clinical characteristics. Third, an event-free survival analysis was performed using Kaplan–Meier plots and log-rank tests to compare the two groups. Fourth, a Cox
proportional-hazards model was used to estimate the risks of recurrent ischemic stroke and all-cause mortality associated with PWV (unadjusted, adjusted for age, sex, blood pressure and the
presence of cerebral small vessel disease). Hazard ratios (HRs) are presented along with 95% confidence intervals (CIs). All comparisons were two-tailed, and a _P_ value<0.05 was
considered to be being statistically significant. All data were analyzed using the JMP 11.0 software package (SAS Institute, Cary, NC, USA). RESULTS PATIENT CHARACTERISTICS Of the 1503
consecutive patients with acute ischemic stroke, we assessed 156 eligible patients (61% males, mean age: 69.2±11.3 years).7 The median brachial–ankle PWV value was 20.4 m s−1. The end points
were reached by 29 recurrent ischemic stroke patients and 28 all-cause mortality patients. The distribution of recurrent ischemic stroke subtypes was as follows: lacunar 16, other causes 7,
and atherothrombotic 6. The median follow-up time was 5.9 years. RECURRENT ISCHEMIC STROKE Among the patients who developed recurrent ischemic stroke, more than half developed lacunar
stroke (16 of the 29 patients). Patients who developed atherothrombotic stroke had higher PWV values, which were measured during the acute phase of first-ever stroke, compared with patients
who developed lacunar stroke (median value, 28.2 _vs_. 21.2 m s−1, _P_=0.036). Conversely, the patients who did not develop recurrent ischemic stroke had lower PWV values compared with those
who did develop recurrent ischemic stroke (median value, 19.5 _vs_. 22.7 m s−1, _P_=0.035). The PWV cutoff value used to predict recurrent ischemic stroke was 19.9 m s−1, with 72%
sensitivity and 52% specificity. HIGH _VS_. LOW PWV Compared with patients with low PWV values, patients with high PWV values were more likely to be female and older. They were also more
likely to have chronic kidney disease, silent lacunar infarct, white matter hyperintensities and a severe neurological deficit upon admission, as well as poor functional outcomes at
discharge (Table 1). Patients with high PWV values were more likely to have a high risk of all-cause mortality and recurrent ischemic stroke (23% _vs_. 13%, _P_=0.143 and 26% _vs_. 12%,
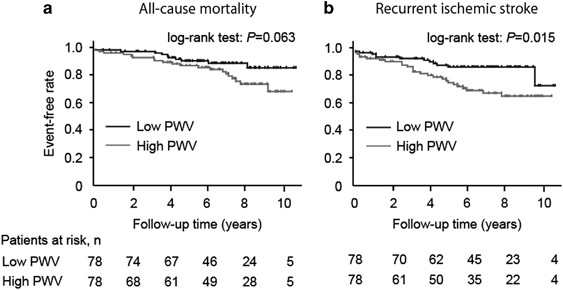
_P_=0.038, respectively). LONGITUDINAL ANALYSES Kaplan–Meier curve analyses showed that patients with high PWV values had less favorable survival times free of all-cause mortality (log-rank
test, _P_=0.063) and recurrent ischemic stroke (log-rank test, _P_=0.015) than those with low PWV values (Figure 1). The multivariate Cox proportional-hazards model revealed that high PWV
was an independent predictor of recurrent ischemic stroke, after adjusting for age, sex and systolic blood pressure (Table 2). DISCUSSION Our study showed that high PWV doubles the risk of
future ischemic stroke in patients who developed acute lacunar infarction, independent of age, sex and blood pressure levels. Measuring PWV during the acute phase of ischemic stroke is
useful to assess the risk of future ischemic stroke. The progressive neurological deficit that develops in patients with acute lacunar infarction7 might share several multifactorial
mechanisms with recurrent ischemic stroke. The vascular endothelial impairment indicated by PWV is associated with blood–brain barrier failure and damages the cerebral parenchyma.25, 28 The
related arteriosclerotic microcirculatory impairment may also increase the risk of ischemic stroke.7 Our hypothesis regarding a possible ‘tsunami effect’ of high PWV on microcirculatory
cerebral parenchyma damage14 may partially explain this association. Additionally, lacunar stroke was a major subtype of recurrent ischemic stroke in the present study. This finding also
supports our hypothesis. Consequently, these multifactorial mechanisms could increase the risk of future ischemic stroke. The results of this study highlight the utility of measuring PWV to
predict future ischemic stroke after the onset of first-ever ischemic stroke. To date, numerous studies have demonstrated the relationships between PWV and mortality,1, 3, 22 cardiovascular
diseases1, 3, 16, 17, 18, 19, 20 and cerebrovascular diseases,2, 16, 21 including cerebral small vessel diseases,4, 8, 9, 23 in hypertensive adults. Furthermore, some studies have assessed
PWV during the acute phase of stroke.5, 6, 7, 15, 24, 26, 29 Although high PWV has been previously shown to predict mortality6 and functional outcomes5, 7, 26 in patients with acute ischemic
stroke, the causal relationship between PWV and recurrent ischemic stroke has not been clarified. Our findings extend the clinical significance of PWV to include it as a predictor of the
risk of future ischemic stroke in acute ischemic stroke patients. Our study has several limitations. First, the study sample size was small. A study with a relatively small number of
patients may be at risk of being statistically underpowered. Second, we did not assess cerebral microbleeds because T2*-weighted magnetic resonance imaging was only used after the present
study had started. Third, cardiovascular events were also not assessed because of the small number of such patients (_n_=9). A full assessment of the relationship between PWV and recurrent
ischemic stroke will require the inclusion of patients with cerebral microbleeds and cardiovascular events. Fourth, we did not thoroughly assess PWV after patient discharge. The PWV changes
may provide further information to help understand the pathophysiology associated with predicting future ischemic stroke. In conclusion, high PWV values in patients who developed acute
lacunar infarction double the risk of future ischemic stroke, independent of patient age, sex and blood pressure. Measuring PWV during the acute phase of ischemic stroke is useful to assess
the risk of future ischemic stroke in such patients. REFERENCES * Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A . Aortic stiffness is an
independent predictor of all-cause and cardiovascular mortality in hypertensive patients. _Hypertension_ 2001; 37: 1236–1241. Article CAS Google Scholar * Laurent S, Katsahian S, Fassot
C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P . Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. _Stroke_ 2003; 34: 1203–1206. Article Google
Scholar * Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C . Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity
index: a systematic review and meta-analysis. _Hypertension_ 2012; 60: 556–562. Article CAS Google Scholar * Poels MM, Zaccai K, Verwoert GC, Vernooij MW, Hofman A, van der Lugt A,
Witteman JCM, Breteler MM, Mattace-Raso FU, Ikram MA . Arterial stiffness and cerebral small vessel disease: the Rotterdam Scan Study. _Stroke_ 2012; 43: 2637–2642. Article Google Scholar
* Gasecki D, Rojek A, Kwarciany M, Kowalczyk K, Boutouyrie P, Nyka W, Laurent S, Narkiewicz K . Pulse wave velocity is associated with early clinical outcome after ischemic stroke.
_Atherosclerosis_ 2012; 225: 348–352. Article CAS Google Scholar * Kim J, Song TJ, Song D, Lee KJ, Kim EH, Lee HS, Nam CM, Nam HS, Kim YD, Heo JH . Brachial-ankle pulse wave velocity is a
strong predictor for mortality in patients with acute stroke. _Hypertension_ 2014; 64: 240–246. Article CAS Google Scholar * Saji N, Kimura K, Kawarai T, Shimizu H, Kita Y . Arterial
stiffness and progressive neurological deficit in patients with acute deep subcortical infarction. _Stroke_ 2012; 43: 3088–3090. Article Google Scholar * Saji N, Kimura K, Shimizu H, Kita
Y . Association between silent brain infarct and arterial stiffness indicated by brachial-ankle pulse wave velocity. _Intern Med_ 2012; 51: 1003–1008. Article Google Scholar * Saji N,
Shimizu H, Kawarai T, Tadano M, Kita Y, Yokono K . Increased brachial-ankle pulse wave velocity is independently associated with white matter hyperintensities. _Neuroepidemiology_ 2011; 36:
252–257. Article Google Scholar * Schillaci G, Battista F, Settimi L, Anastasio F, Pucci G . Cardio-ankle vascular index and subclinical heart disease. _Hypertens Res_ 2015; 38: 68–73.
Article Google Scholar * Saji N, Kimura K, Shimizu H, Kita Y . Silent brain infarct is independently associated with arterial stiffness indicated by cardio-ankle vascular index (CAVI).
_Hypertens Res_ 2012; 35: 756–760. Article Google Scholar * Hickson SS, Nichols WW, Yasmin, McDonnell BJ, Cockcroft JR, Wilkinson IB, McEniery CM . Influence of the central-to-peripheral
arterial stiffness gradient on the timing and amplitude of wave reflections. _Hypertens Res_ 2016; 39: 723–729. Article Google Scholar * Ye C, Pan Y, Xu X, Su S, Snieder H, Treiber F,
Kapuku G, Wang X . Pulse wave velocity in elastic and muscular arteries: tracking stability and association with anthropometric and hemodynamic measurements. _Hypertens Res_ 2016; 39:
786–791. Article Google Scholar * Saji N, Toba K, Sakurai T . Cerebral small vessel disease and arterial stiffness: tsunami effect in the brain? _Pulse_ 2016; 3: 182–189. Article Google
Scholar * Saji N, Kimura K, Yagita Y, Kawarai T, Shimizu H, Kita Y . Comparison of arteriosclerotic indicators in patients with ischemic stroke: ankle-brachial index, brachial-ankle pulse
wave velocity, and cardio-ankle vascular index. _Hypertens Res_ 2015; 38: 323–328. Article Google Scholar * Ben—Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie
P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K,
Verbeke F, Wang KL, Webb DJ, Willum-Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB . Aortic pulse wave velocity improves cardiovascular event prediction: an individual
participant meta-analysis of prospective observational data from 17635 subjects. _J Am Coll Cardiol_ 2014; 63: 636–646. Article Google Scholar * Mitchell GF, Hwang SJ, Vasan RS, Larson MG,
Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ . Arterial stiffness and cardiovascular events: the Framingham Heart Study. _Circulation_ 2010; 121: 505–511. Article Google Scholar *
Takashima N, Turin TC, Matsui K, Rumana N, Nakamura Y, Kadota A, Saito Y, Sugihara H, Morita Y, Ichikawa M, Hirose K, Kawakani K, Hamajima N, Miura K, Ueshima H, Kita Y . The relationship
of brachial-ankle pulse wave velocity to future cardiovascular disease events in the general Japanese population: the Takashima Study. _J Hum Hypertens_ 2014; 28: 323–327. Article CAS
Google Scholar * Ninomiya T, Kojima I, Doi Y, Fukuhara M, Hirakawa Y, Hata J, Kitazono T, Kiyohara Y . Brachial-ankle pulse wave velocity predicts the development of cardiovascular disease
in a general Japanese population: the Hisayama Study. _J Hypertens_ 2013; 31: 477–483. Article CAS Google Scholar * Ohishi M, Tatara Y, Ito N, Takeya Y, Onishi M, Maekawa Y, Kato N,
Kamide K, Rakugi H . The combination of chronic kidney disease and increased arterial stiffness is a predictor for stroke and cardiovascular disease in hypertensive patients. _Hypertens Res_
2011; 34: 1209–1215. Article Google Scholar * Song Y, Xu B, Xu R, Tung R, Frank E, Tromble W, Fu T, Zhang W, Yu T, Zhang C, Fan F, Zhang Y, Li J, Bao H, Cheng X, Qin X, Tang G, Chen Y,
Yang T, Sun N, Li X, Zhao L, Hou FF, Ge J, Dong Q, Wang B, Xu X, Huo Y . Independent and joint effect of brachial-ankle pulse wave velocity and blood pressure control on incident stroke in
hypertensive adults. _Hypertension_ 2016; 68: 46–53. Article CAS Google Scholar * Turin TC, Kita Y, Rumana N, Takashima N, Kadota A, Matsui K, Sugihara H, Morita Y, Nakamura Y, Miura K,
Ueshima H . Brachial-ankle pulse wave velocity predicts all-cause mortality in the general population: findings from the Takashima study, Japan. _Hypertens Res_ 2010; 33: 922–925. Article
Google Scholar * Henskens LH, Kroon AA, Van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, Lodder J, De Leeuw PW . Increased aortic pulse wave velocity is associated with
silent cerebral small-vessel disease in hypertensive patients. _Hypertension_ 2008; 52: 1120–1126. Article CAS Google Scholar * Tuttolomondo A, Di Sciacca R, Di Raimondo D, Serio A,
D’Aguanno G, Pinto A, Licata G . Arterial stiffness indexes in acute ischemic stroke: relationship with stroke subtype. _Atherosclerosis_ 2010; 211: 187–194. Article CAS Google Scholar *
Pantoni L . Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. _Lancet Neurol_ 2010; 9: 689–701. Article Google Scholar * Lee YB, Park
JH, Kim E, Kang CK, Park HM . Arterial stiffness and functional outcome in acute ischemic stroke. _J Cerebrovasc Endovasc Neurosurg_ 2014; 16: 11–19. Article Google Scholar * Adams HP,
Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd . Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial
of Org 10172 in Acute Stroke Treatment. _Stroke_ 1993; 24: 35–41. Article Google Scholar * Wiseman S, Marlborough F, Doubal F, Webb DJ, Wardlaw J . Blood markers of coagulation,
fibrinolysis, endothelial dysfunction and inflammation in lacunar stroke versus non-lacunar stroke and non-stroke. systematic review and meta-analysis. _Cerebrovasc Dis_ 2014; 37: 64–75.
Article CAS Google Scholar * Kwarciany M, Gasecki D, Kowalczyk K, Rojek A, Laurent S, Boutouyrie P, Skrzypek-Czerko M, Nyka WM, Narkiewicz K, Karaszewski B . Acute hypertensive response
in ischemic stroke is associated with increased aortic stiffness. _Atherosclerosis_ 2016; 251: 1–5. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Naoko Akazawa,
Tomoko Yunoki, Hiroko Ohta and Mari Okamoto for their secretarial assistance. This study was partially funded by the KAWASAKI Foundation for Medical Science & Medical Welfare, Research
Funding of Longevity Sciences (25-6, 28-15) from the National Center for Geriatrics and Gerontology and Grants-in-Aid for Scientific Research (no 26870765) from the Japan Society for the
Promotion of Science. Dr NS was the recipient of the research funding from the KAWASAKI Foundation for Medical Science & Medical Welfare, Research Funding for Comprehensive Research on
Aging and Health from the Japan Agency for Medical Research and Development, Research Funding of Longevity Sciences (25-6, 28-15) from the National Center for Geriatrics and Gerontology,
Grants-in-Aid for Scientific Research (no 26870765) from the Japan Society for the Promotion of Science and the Japan Foundation for Aging and Health. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Neurology, Hyogo Brain and Heart Center at Himeji, Hyogo, Japan Naoki Saji, Hirotaka Shimizu, Toshiyuki Uehara & Yasushi Kita * Center for Comprehensive Care
and Research on Memory Disorders, National Center for Geriatrics and Gerontology, Aichi, Japan Naoki Saji, Kenji Toba & Takashi Sakurai * Department of Stroke Medicine, Kawasaki Medical
School, Okayama, Japan Naoki Saji * Division of Biostatistics, Center for Clinical Research, Aichi Medical University, Aichi, Japan Kenta Murotani Authors * Naoki Saji View author
publications You can also search for this author inPubMed Google Scholar * Kenta Murotani View author publications You can also search for this author inPubMed Google Scholar * Hirotaka
Shimizu View author publications You can also search for this author inPubMed Google Scholar * Toshiyuki Uehara View author publications You can also search for this author inPubMed Google
Scholar * Yasushi Kita View author publications You can also search for this author inPubMed Google Scholar * Kenji Toba View author publications You can also search for this author inPubMed
Google Scholar * Takashi Sakurai View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Naoki Saji. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on Hypertension Research website SUPPLEMENTARY
INFORMATION SUPPLEMENTARY MATERIAL (DOC 40 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Saji, N., Murotani, K., Shimizu, H. _et al._ Increased
pulse wave velocity in patients with acute lacunar infarction doubled the risk of future ischemic stroke. _Hypertens Res_ 40, 371–375 (2017). https://doi.org/10.1038/hr.2016.157 Download
citation * Received: 21 July 2016 * Revised: 18 September 2016 * Accepted: 03 October 2016 * Published: 17 November 2016 * Issue Date: April 2017 * DOI: https://doi.org/10.1038/hr.2016.157
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * arterial stiffness * lacunar infarction * pulse wave velocity * recurrent stroke





