- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Hypertensive vascular disorders are characterized by endothelial dysfunction. Loss of renal autoregulation causes glomerular hypertension. However, the relationship between the
autoregulatory response and glomerular damage has not been well examined. We examined the contributions of uncoupled endothelial nitric oxide synthase (eNOS) in hypertensive renal disease,
and the relationship between the degree of autoregulation impairment and glomerular injury. We also investigated the effects of telmisartan on eNOS coupling and renal autoregulation. Male
Dahl salt-sensitive hypertensive (DS) rats (14-week old) fed an 8% salt diet were used to examine endothelial dysfunction and impaired renal autoregulation caused by glomerular hypertension.
Some DS rats were treated with telmisartan (3.0 mg kg−1 day−1), an angiotensin receptor blocker, for 2 weeks. Increased superoxide production and decreased nitric oxide production, as
detected by fluorescent indicator perfusion methods, were observed in the glomeruli and arterioles of hypertensive DS rats. Telmisartan improved the imbalance of superoxide and nitric oxide
in the glomeruli and arterioles. Decreased serum tetrahydrobiopterin levels and coupled eNOS seen in the DS rat kidney were improved with telmisartan treatment. The endothelial relaxation
reaction was impaired in DS rat aortic arteries. Autoregulatory capacity in response to step changes in perfusion pressure was also impaired in DS rat kidney. Treatment with telmisartan
improved these abnormalities. Endothelial dysfunction in the glomeruli and impaired renal autoregulation, which may cause glomerular sclerosis, were observed in DS rat kidney. Telmisartan
treatment improves these dysfunctions in hypertensive renal disease. SIMILAR CONTENT BEING VIEWED BY OTHERS NEBIVOLOL IS MORE EFFECTIVE THAN ATENOLOL FOR BLOOD PRESSURE VARIABILITY
ATTENUATION AND TARGET ORGAN DAMAGE PREVENTION IN L-NAME HYPERTENSIVE RATS Article 22 February 2021 NOBILETIN RESOLVES LEFT VENTRICULAR AND RENAL CHANGES IN 2K-1C HYPERTENSIVE RATS Article
Open access 03 June 2022 CHRONIC INFUSION OF ELABELA ALLEVIATES VASCULAR REMODELING IN SPONTANEOUSLY HYPERTENSIVE RATS VIA ANTI-INFLAMMATORY, ANTI-OXIDATIVE AND ANTI-PROLIFERATIVE EFFECTS
Article 08 March 2022 INTRODUCTION Hypertension is a well-known complication in chronic kidney disease and, at the same time, is one of the important risk factors for the development of
cardiovascular disease and for the progression of renal insufficiency.1, 2 Indeed, various studies have indicated that subjects with hypertension are at risk of developing end-stage renal
disease.3, 4, 5 Hypertensive vascular disorders are characterized by pathological changes in endothelial cells in the early stage of hypertension, and endothelial cell dysfunction causes an
imbalance between reactive oxygen species (ROS) and nitric oxide (NO) production.6 There are several overlapping mechanisms for endothelial dysfunction, including NO trapping by free
radicals and reduced endothelial NO synthase (eNOS) activity. The eNOS is a homodimeric enzyme that generates NO and L-citrulline from L-arginine (L-Arg). However, in certain circumstances,
eNOS may cause uncoupling that generates superoxide.7 Recent studies report the involvement of uncoupled eNOS during increased oxidative stress and endothelial dysfunction in association
with atherosclerosis8 and diabetes.9 Evidence also suggests that the absence of the substrate L-Arg or cofactor tetrahydrobiopterin (BH4) uncouples eNOS, thereby producing superoxide rather
than NO,10, 11, 12 which may contribute to the pathogenesis of endothelial dysfunction in arteries exposed to hypertension. However, little is known about the contributions of uncoupled eNOS
in the context of hypertensive glomerular injury. Pressure-mediated autoregulatory behavior operates by recognizing a change in renal perfusion pressure and initiating induction of
autoregulatory resistance changes intended to stabilize renal blood flow.13 The net result is that renal blood flow and glomerular filtration rates remain relatively stable over a wide range
of renal perfusion pressures. Autoregulatory behavior is significantly less efficient in pathological settings, such as in diabetes mellitus and in some forms of hypertension. Loss of
autoregulatory efficiency could be one contributing factor, which leads to glomerular stress, glomerular compensation and perhaps glomerular injury and failure. Evidence indicates that renal
autoregulation is impaired in some experimental animal models of hypertension.14, 15 However, the relationship between the degree of autoregulation impairment and glomerular injury have not
been well examined, and it is unclear whether blockade of renal autoregulation impairment would effectively inhibit hypertensive renal injury. In this study, we examined whether uncoupled
eNOS, which is a marker of endothelial dysfunction, and loss of renal autoregulation occur during hypertensive glomerular injury. We used Dahl salt-sensitive hypertensive (DS) rats to
examine the NOS coupling statement in the kidney using the dihydroethidium (DHE) assay16 and _in vivo_ NO and ROS imaging,17 and examined autoregulation capacity by measuring renal
microvascular responses to step changes in renal perfusion pressure. Moreover, to elucidate whether angiotensin receptor blockade (ARB) has a beneficial effect against eNOS uncoupling and
renal autoregulation, we examined DS rats treated with telmisartan, an angiotensin receptor blocker. METHODS EXPERIMENTAL PROTOCOL Male DS rats (8-week old) were purchased from Clea Japan
(Osaka, Japan). Animals were housed in a temperature- and humidity-controlled room with a 12:12 h light–dark cycle, and were fed standard laboratory animal chow with free access to tap
water. In the first 6 weeks, rats were fed an 8% salt diet to induce hypertension. Systolic blood pressure (SBP) was also measured weekly in each rat using the tail-cuff method using an
automatic sphygmomanometer (BP98A; Softron, Tokyo, Japan). After confirming the induction of hypertension, rats were randomly divided into three groups: the DS-H group (_n_=15) consisting of
untreated DS rats; the DS-H-Hyd group (_n_=15) consisting of DS rats treated with hydralazine (5.0 mg per rat per day); and the DS-H-Tel group (_n_=15) consisting of DS rats treated with
telmisartan (3.0 mg kg−1 day−1). As a control, we used another group of DS normotensive rats fed a 0.9% salt diet (DS-L, _n_=8). The experimental protocol (No. 07–060) was approved in
advance by the Ethics Review Committee for Animal Experimentation of Kawasaki Medical School, Kurashiki, Japan. During the experimental period, body weight was measured weekly for each rat.
All rats were killed after 2 weeks of treatment. Serum creatinine and blood urea nitrogen levels were measured after killing the rats. Before killing, 24-h urine samples were collected after
placing the rats in metabolic cages and urine protein concentrations were measured spectrophotometrically using the Bradford method (Bio-Rad Protein Assay; Bio-Rad Laboratories, Tokyo,
Japan). GLOMERULAR DAMAGE SCORE Half of the left kidneys were preserved in 4% paraformaldehyde for light microscopy. Paraffin-embedded tissue samples were cut into 4 μm-thick sections and
stained with periodic acid-Schiff for histological studies. In each animal, 100 glomeruli were evaluated for the presence of sclerotic lesions. The severity of glomerular lesions was graded
with a score of 0–3 based on the percentage of glomerular involvement, as described previously.18 FLUORESCENCE SPECTROMETRIC ASSAY OF O2- PRODUCTION IN THE RENAL CORTEX To assess the source
of ROS production, we performed fluorescence spectrometric assays. Fluorescence spectrometry of tissue O2- production was carried out using the fluorogenic oxidation of DHE to ethidium as a
measure of O2-, as previously described.16 The renal cortex of the right kidney obtained from each rat was cut into small pieces, and the glomeruli were isolated by the mechanical graded
sieving technique. After isolation, the purity of the final suspension was determined by light microscopic examination. On average, tubular contamination was <5%. Isolated glomeruli were
homogenized in ice-cold 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer containing 25 mM HEPES, 1 mM ethylenediaminetetraacetic acid (EDTA), and 0.1 mM phenylmethylsulfonyl
fluoride. After centrifugation of the homogenate, the supernatant was used for this DHE assay. A part of the supernatant was used for western blotting. Enzyme activities of different
pathways are expressed relative to the control. Following substrates or inhibitors were used in this study: nicotinamide adenine dinucleotide phosphate (NADPH) (0.1 mM) was used as a
substrate for NADPH oxidases; L-Arg (1 mM) was used as a substrate for NOS; NG-nitro-L-Arg methyl ester (1 mM) was used to block NOS activity; succinate (5 mM) was used as a substrate for
intramitochondrial O2- production; antimycin (0.05 mM) was used to block the normal reaction in the respiratory chain; and xanthine (0.1 mM) was used as a substrate for xanthine oxidase. The
effect of BH4 (0.01 mM) on L-Arg-induced O2- production was also examined. _IN SITU_ DETECTION OF NO AND ROS To visualize the endothelial production levels of NO and ROS resulting from NOS
coupling, _in situ_ NO and ROS were imaged by confocal laser microscopy after perfusion with fluorescence indicators, as previously described.17 In brief, an 18-gauge needle connected to an
infusion pump was inserted in the left ventricle. After the right atrium was cut, the whole body was perfused with PBS maintained at 37 °C. After exsanguination, the whole body was perfused
with diaminorhodamine–4 M acetoxymethyl ester (DAR–4 M AM; Daiichi Pure Chemicals, Tokyo, Japan) and 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA; Invitrogen, Tokyo, Japan) with L-Arg
and CaCl2. All unreacted DAR–4 M AM and DCFH-DA were removed by postperfusion with PBS. After fixation with 4% paraformaldehyde perfusion, the tissues were cut into 1 mm-thick sections and
placed on a slide glass. Fluorescent images of NO and ROS were obtained using confocal laser-scanning microscopy (TCS-NT, Leica-Microsystems Japan, Tokyo, Japan). The mean NO and ROS
fluorescence intensity of glomeruli (total of 100 glomeruli from five rats in each group) was analyzed using Leica TCS-NT system software (Leica-Microsystems). WESTERN IMMUNOBLOTTING Western
blots were performed to quantify total eNOS and its active dimer. Portions of the glomeruli samples (50 μg per lane) were subjected to SDS-PAGE. For immunoblot analysis of total eNOS, the
samples were heated at 95 °C for 5 min before electrophoresis. For immunoblot analysis of the dimeric form of eNOS, samples were not heated, and the temperature of the gel was maintained
below 15 °C during electrophoresis (low-temperature SDS-PAGE).17 Primary antibody 0.1 μg/ml rabbit anti-eNOS polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was applied
for 180 min at room temperature. The antibody was visualized using an enhanced chemiluminescence method (ECL plus; GE Healthcare Japan, Tokyo, Japan). The integrated density (density 3 area)
of the bands was quantified using Image-J software (http://rsbweb.nih.gov/ij/). DETERMINATION OF SERUM HYDROBIOPTERIN CONCENTRATIONS BH4 and dihydrobiopterin (BH2) concentrations were
determined by high-performance liquid chromatography, as described previously.17 Serum samples were mixed at a ratio of 1:1 with a solution of 0.5 M perchloric acid containing 0.1 mM
Na2-ethylenediaminetetraacetic acid and 0.1 mM Na2S2O3 for protein separation. After filtration, BH4 concentrations in the samples were measured by HPLC. Detection of BH4 was carried out
fluorometrically at wavelengths of 350 nm for excitation and of 440 nm for emission by postcolumn NaNO2 oxidation using a reversed-phase ion-pair LC system (LC-10 series; Shimadzu, Kyoto,
Japan). REAL-TIME QUANTITATIVE PCR The mRNA expression of guanosine-5′-triphosphate cyclohydrolase 1 (GTPCH1), which is the rate-limiting enzyme in BH4 synthesis, and purinergic receptor
P2X, ligand-gated ion channel 1 (P2rx1) were determined by real-time quantitative PCR. RNA isolation and real-time quantitative PCR for GTPCH1 and P2rx1 were performed using the ABI Prism
7700 sequence detection system (Applied Biosystems, Foster City, CA, USA), as described previously.19 Primers and probes for P2rx1 (NM_012997) were as follows: rat P2rx1 forward primer:
5′-CGTCATTGGGTGGGTGTT-3′; reverse primer: 5′-AGCTGGGTCACAGCCAAG-3′; TaqMan probe: 5′-FAM-TCAGCAGTGTGTCCGTGAAGCTCA-TAMRA-3′. Fold-change analysis was based on standardizing RNA levels by
normalizing to glyceraldehyde-3-phosphate dehydrogenase levels in the sample. ENDOTHELIUM-DEPENDENT VASCULAR RESPONSES Endothelium-dependent vascular responses were measured after
preparation of thoracic rat aortic rings, as reported previously.20 Briefly, cylindrical 3.0 mm-long segments were dissected from the aorta and bathed in Krebs bicarbonate saline (120 mM
NaCl, 5.2 mM KCl, 2.4 mM CaCl2, 1.2 mM MgSO4, 25 mM NaHCO3, 0.03 mM Na2-ethylenediaminetetraacetic acid and 11 mM dextrose (pH 7.4)) equilibrated with 95% O2 and 5% CO2, and maintained at 37
°C. The rings were suspended under 1 g of tension and preconstricted by adding 3 × 10−7 M norepinephrine. After the contraction force had reached a plateau, acetylcholine (10−9–10−5 M;
endothelium-dependent vasodilator) or sodium nitroprusside (10−9–10−5 M; endothelium-independent vasodilator) was added incrementally to the bath. The force of isometric contraction was
measured using a force-displacement transducer (Model MTOB-1Z; Labo Support, Osaka, Japan). Responses to acetylcholine were expressed as the percentage of the preconstricted tension induced
by norepinephrine. Nonlinear regression (GraphPad Prism5.0; GraphPad Software, La Jolla, CA, USA) was used to determine pEC50 and maximum responses (_R_max) to acetylcholine and
nitroprusside in each ring. RENAL MICROVASCULAR RESPONSES TO STEP CHANGES IN RENAL PERFUSION PRESSURE The effects of perfusion pressure on renal microvascular tone were examined by slight
modification of the isolated perfusion technique described by van Dokkum _et al._21 For perfusion of the kidneys, animals were anesthetized with sevoflurane, the left kidney was denervated
by stripping all visible nerves from the renal artery, the artery was coated with a 5% solution of phenol in ethanol, and the left renal artery was then cannulated by introducing a perfusion
cannula through the mesenteric artery and across the aorta. The perfusion medium consisted of Krebs–Ringer bicarbonate buffer containing 6.5 g/100 ml bovine serum albumin, 5 mM D-glucose
and a complement of amino acids. The perfusion pressure, monitored at the level of the renal artery, was altered by controlling the backpressure regulator. The renal artery was supplied with
a 1-mm Transonic flow probe connected to a Transonic T206 flowmeter (Transonic Systems, Ithaca, NY, USA) to monitor renal flow. Renal perfusion pressure was maintained at 100 mm Hg. The
pressure was then raised in a stepwise manner by 20-mm Hg increments to 160 mm Hg. Autoregulatory index (AI) between 100–160 mm Hg of pressure changes was calculated by the method of Semple
and de Wardener22 as follows: AI=[(renal flow (RF)2−RF1)/RF1]/[(renal perfusion pressure (RPP)2−RPP1)/RPP1]. STATISTICAL ANALYSIS Values are expressed as mean±s.e.m. Statistical comparisons
except the glomerular damage score were made using the one-factor analysis of variance with a Tukey–Kramer test for multiple comparisons. The glomerular damage score was evaluated by the
Kruskal–Wallis test with the Steel–Dwass test for multiple comparisons. A _P_-value <0.05 denoted a statistically significant difference. RESULTS PATHOPHYSIOLOGICAL DATA Systolic blood
pressure was significantly higher in DS-H rats than in DS-L rats (Table 1). A two-week treatment with telmisartan decreased systolic blood pressure in a manner similar to hydralazine. There
were no differences in serum creatinine and blood urea nitrogen levels among DS-H, DS-H-Hyd and DH-H-Tel rats. Urinary protein excretion in DS-H rats was greater than in DS-L rats
(_P_<0.05). Urinary protein excretion was significantly decreased in both DS-H-Hyd and DH-H-Tel rats compared with DS-H rats (_P_<0.05 each), and was much lower in DH-H-Tel rats than
in DS-H-Hyd rats (_P_<0.05). Glomerular damage, which was worse in DS-H rats than in DS-L rats, also did not worsen after treatment with telmisartan compared with hydralazine (data not
shown). Glomerular damage score was significantly improved in DH-H-Tel rats compared with DS-H and DS-H-Hyd rats (Table 1). SOURCE OF ROS PRODUCTION IN GLOMERULI OF SALT-SENSITIVE
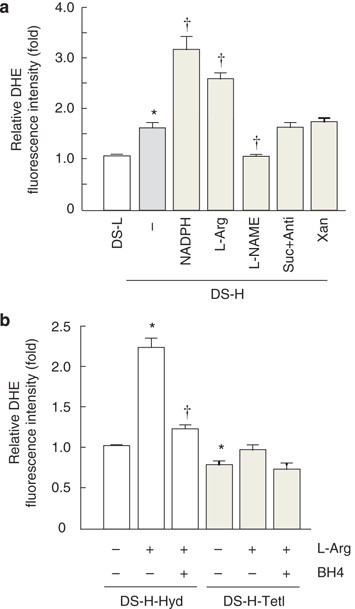
HYPERTENSIVE RATS The production of ROS was measured by dihydroethidium assay. The production of ROS was higher in the glomeruli of DS-H rats than DS-L rats (Figure 1a; _P_<0.05) and
increased in the former group after the addition of NADPH (3.2±0.4-fold; _P_<0.05 _vs_. DS-H (−)). Moreover, ROS production was accelerated by the addition of L-Arg (2.5±0.4-fold,
_P_<0.05 _vs_. DS-H (−)) and decreased by the addition of NG-nitro-L-Arg methyl ester (1.2±0.1-fold, _P_<0.05 _vs_. DS-H (−)). There were no changes observed with the addition of
succinate plus antimycin or xanthine. Furthermore, in DS-H-Hyd rats, ROS production was accelerated by the addition of L-Arg (Figure1b; 2.2±0.3-fold, _P_<0.05 _vs_. DS-H-Hyd (−)) and
diminished by incubation with BH4 (1.2±0.2-fold, _P_<0.05 _vs_. DS-H-Hyd+L-Arg). However, L-Arg did not increase ROS production in DS-H-Tel rats. _IN SITU_ DETECTION OF NOS COUPLING IN
GLOMERULI AND SMALL ARTERIES OF SALT-SENSITIVE HYPERTENSIVE RATS Figure 2 shows _in situ_ endothelial NO and ROS images for the four groups of rats. The NO detected by DAR–4 M AM appears red
(Figures 2a–d), and ROS detected by DCFH-DA appears green (Figures 2e–h). NO, but not ROS, was detected in the glomeruli and arterioles of DS-L rats. However, ROS production was increased
and NO production was decreased in the glomeruli and arterioles of DS-H rats compared with DS-L rats. The production of ROS was increased and NO production was reduced in the glomeruli and
arterioles of the Hyd group. Treatment with telmisartan normalized the production of NO and ROS in the glomeruli and arterioles. WESTERN BLOT ANALYSIS OF ENOS IN GLOMERULI OF SALT-SENSITIVE
HYPERTENSIVE RATS Total eNOS was examined by standard SDS–PAGE. Dimer/monomer eNOSs were examined by low-temperature SDS–PAGE. Total eNOS production was similar in the four groups (Figure
3a). However, the ratio of dimer to monomer eNOS was lower in DS-H rats than DS-L rats (Figure 3b, _P_<0.05). Treatment with telmisartan (_P_<0.05 _vs_. DS-H-Hyd), but not hydralazine,
ameliorated the decrease of the eNOS dimer/monomer ratio. BIOPTERIN PRODUCTION AND CONCENTRATION IN SALT-SENSITIVE HYPERTENSIVE RATS Tetrahydrobiopterin (BH4) is an essential cofactor
required for the production of NO by each of the NOS isoforms. Synthesis of BH4 involves a multistep process in which GTPCH1 is the rate-limiting enzyme required for the initial step in
conversion of guanosine-5′-triphosphate to BH4. The mRNA expression of GTPCH1, as measured by real-time PCR (Figure 3c), was increased in DS-H, DS-H-Hyd and DS-H-Tel rats compared with DS-L
rats, but there were no differences between the three groups. Serum BH4 levels were decreased in DS-H and DS-H-Hyd rats, but preserved in DS-H-Tel rats (Figure 3d). However, serum levels of
BH2, which is the oxidized form of BH4, were increased in DS-H and DS-H-Hyd rats, but preserved in DS-H-Tel rats (Figure 3e). AORTIC ARTERY ENDOTHELIAL FUNCTION IN SALT-SENSITIVE
HYPERTENSIVE RATS Endothelium-dependent relaxation of aortic artery rings in response to acetylcholine was significantly depressed in DS-H rats compared with DS-L rats (Figure 4a).
Acetylcholine caused concentration-dependent vasorelaxations in preconstricted control rings from DS-L rats (pEC50=7.2±0.1; _R_max=82±4%; _n_=5). In contrast, both the sensitivity and
maximum relaxations to acetylcholine enhanced in collared rings were depressed in rings from DS-L rats (pEC50=6.5±0.2; _R_max=27±3%; _n_=5; _P_<0.05). Telmisartan and hydralazine
significantly (_P_<0.05) improved both sensitivity (pEC50=6.9±0.1 and 6.9±0.1, respectively; _n_=6 each) and maximum relaxation (_R_max=60±1 and 37±2%, respectively; _n_=5 each) to
acetylcholine in aortic artery ring segments compared with the rings from DS-H rats. Regarding maximum relaxation, telmisartan treatment afforded more improvement (_P_<0.05) than
hydralazine treatment. However, endothelium-independent relaxation of the aortic artery rings in response to sodium nitroprusside did not differ in any group of rats (Figure 4b). To
determine whether endothelial cells were histologically damaged, expression of rat endothelial cell antigen 1 in the isolated aortic artery was examined. Endothelial cells were preserved in
DS-H rats (data not shown). RENAL FLOW AUTOREGULATION IN SALT-SENSITIVE HYPERTENSIVE RATS Alterations in renal flow during step changes in renal perfusion pressure are shown in Figure 5. The
capacity to autoregulate the steady-state renal flow in response to step changes was decreased in DS-H rats (Figure 5b) compared with DS-L rats (Figure 5a), as indicated by the difference
in the calculated AI between 100–160 mm Hg of pressure changes (AI=0.56±0.07 and 0.22±0.03, respectively, _P_<0.05). The renal flow autoregulation in response to step changes in perfusion
pressure was well improved in DS-H-Tel rats (Figure 5d; AI=0.34±0.05) but not in DS-H-Hyd rats (Figure 5c; AI=0.64±0.08, _P_<0.05 _vs_. DS-H-Tel). PURINERGIC RECEPTOR EXPRESSION IN
SALT-SENSITIVE HYPERTENSIVE RATS The mRNA expression levels of P2rx1 in renal cortex were examined by quantitative real-time PCR. The P2rx1 mRNA expression could be detected in the renal
cortex, and the expression was decreased in hypertensive rat glomeruli (Figure 6; 0.75±0.06-fold in DS-H compared with DS-L, _P_<0.05). Telmisartan treatment restored the P2rx1 expression
to control levels (0.96±0.12-fold). DISCUSSION In this study, we explored whether uncoupled eNOS parallel with endothelial dysfunction and deterioration of renal autoregulation were
involved in the progression of hypertensive renal disease. Our results demonstrated that NO/ROS imbalance, which is indicative of endothelial dysfunction, exists in DS rat glomerular and
pre-glomerular arterioles, and that telmisartan has beneficial effects against eNOS uncoupling, endothelial dysfunction and loss of renal autoregulation in a Dahl rat model for
salt-sensitive hypertensive renal disease. We reported previously that NADPH oxidase and uncoupled NOS are major sources of glomerular superoxide in streptozotocin-induced rats with diabetic
nephropathy,17 and NOS uncoupling has been reported to occur in the renal medulla of DS rats.23 In this study, we demonstrated that uncoupled eNOS also occurs in the glomeruli of DS rats.
The eNOS uncoupling was found to participate not only in kidney disease, but also in arteriosclerosis and endothelial dysfunction in large blood vessels of diabetics.8 Thus, eNOS uncoupling
seems to be a common mechanism of endothelial dysfunction, leading to reduced NO production and increased ROS production in the blood vessel wall. In DS rats, high salt intake induced the
activation of NADPH oxidase ,24 and in another study, such activation increased ROS production, with subsequent endothelial dysfunction.25 The production of ROS by NADPH oxidase oxidized BH4
to BH2 and caused eNOS uncoupling. In addition, ROS produced by NADPH oxidase reacted with NO immediately and produced peroxynitrite.26 Reaction of this peroxynitrite with the protein
promotes nitrotyrosine formation of the protein. Nitrotyrosine formation of eNOS may obstruct eNOS dimers. In addition, the resolution of GTPCH1, which is an enzyme involved in the
production of BH4, is promoted by proteasome-dependent degradation under the presence of peroxynitrite.27 Consequently, reduced production of BH4 may induce eNOS uncoupling. Treatment with
ARB clearly improved eNOS uncoupling. The mechanism of this action of ARB is thought to be mainly a reduction of ROS production by attenuation of glomerular hypertension and inhibition of
NADPH oxidase. There is a close link between NADPH oxidase activation and NOS uncoupling,28 and it has been reported that restraint of NADPH oxidase improved NOS uncoupling.29 In DS rats,
NADPH oxidase-induced superoxide production may trigger eNOS uncoupling, leading to impaired NO signaling and to endothelial dysfunction. Angiotensin receptor blockade (ARB) was demonstrated
to decrease NADPH oxidase activity.18 Therefore, reduction of NADPH oxidase activity by ARB seems the most efficient method to improve endothelial function through NOS recoupling. However,
the abnormal feature in DS rats is characterized by the dysfunction of NO system. Barton _et al._30 reported that DS rats failed to increase renal NOS activity in response to salt loading.
The ARB treatment may improve the NOS activity in response to salt loading. The inability of eNOS to produce NO on a regular basis leads to an imbalance between ROS and NO, and endothelial
dysfunction. In this study, we measured endothelium-dependent relaxation of the aortic artery rings in response to acetylcholine. The relaxation response significantly fell in DS rats with
disproportionate NO/ROS production. In addition, we measured the ability of autoregulatory responses, which was significantly lower in DS rat kidneys. The autoregulatory response is reported
to be significantly decreased in DS rats31 and remnant kidney rats, a model of progressive renal damage.32 The renal autoregulatory response is coordinated by two mechanisms: myogenic
response and tubulo-glomerular feedback.33 On the basis of the results of our experiments in DS rats, ARB seems to improve the renal autoregulatory response in parallel with endothelial
function. The endothelium has a central role in early functional adaptations to hypertension. Pressure-induced myogenic constriction is enhanced due to the augmented release of
endothelium-derived constrictor factors that modulate arteriolar smooth muscle sensitivity to Ca2+.34 In contrast, flow/shear stress-induced dilation of arterioles is reduced in
hypertension, due to the impaired mediation of the response by NO.35 It has also been proposed that high salt intake reduces endothelium-dependent dilation of mouse arterioles by enhancing
the release of ROS from NOS, which then interferes with the synthesis and/or action of endothelium-derived mediators.36 Treatment with ARB might maintain and improve endothelial function by
reducing superoxide production, which could lead to inhibition of vascular constrictive changes due to the improved mediation of the response by NO and consequently may maintain a normal
pressure-induced myogenic constriction. In general, angiotensin II promotes the autoregulatory response reaction in normal rats by increasing tubulo-glomerular feedback.37, 38 So, ARB may
dampen the strength of tubulo-glomerular feedback helping to restore the normal GFR response.39 Enhanced tubulo-glomerular feedback responses may be the result of an angiotensin II-induced
reduction in local levels of nitric oxide.40 Tubulo-glomerular feedback responses have been reported to be enhanced in spontaneously hypertensive rats,41 but there are few reports regarding
to tubulo-glomerular feedback in DS rats. It is well accepted that DS rats on high-salt diet exhibit a suppressed systemic RAS (rennin–angiotensin system) accompanied with inappropriately
activated renal RAS.42 Indeed, there is a possibility that the tubuloglomerular feedback response in the DS rats are reduced similar to deoxycorticosterone acetate (DOCA)– salt hypertensive
rat or Goldblatt hypertensive rats.43 So, ARB might have little effects on the renal autoregulatory mechanism via tubulo-glomerular feedback in DS rats. We have shown that the renal
autoregulation in the DS rat was impaired. Conclusive identification of the signaling molecules that mediate autoregulation remains controversial, but it does seem clear that extracellular
ATP and P2 purine receptor activation is required for autoregulatory control of renal hemodynamics.44, 45 Indeed, ATP is released from many different cell types in response to stretch,
osmotic stimuli46 and activation of P2rx1 receptors, which is an essential step in pressure-mediated afferent arteriolar vasoconstriction. Blockade of P2rx1 receptors or deletion of P2rx1
receptors in knockout mice eliminates pressure-mediated afferent arteriolar contraction.47 In hypertensive renal disease, pathological changes arise in small arteries in the early stage.48
So, this ATP–P2X axis might be damaged, which could subsequently lead to down regulation of pressure sensory ability and cause glomerular hypertensive injury. Actually, mRNA expression
levels of P2rx1 in the renal cortex of DS rats were decreased, which seem to be parallel with impairment of renal autoregulation. Telmisartan effectively prevented the deterioration of renal
autoregulation, which might be due to the preservation of P2rx1. The relationship between endothelial function and renal autoregulational ability is not clear from the results of this
study. Decreased endothelial function might induce the failure of autoregulation. We demonstrated that treatment with telmisartan improved both functions in DS rat kidney, but there are no
data to support the hypothesis that the decreased endothelial function lies behind the decreased autoregulation. Similarly, whether a causal relation exists between improved autoregulation
and a decrease in renal damage cannot be determined from this study. It is very difficult to design experiments that directly address these important questions, but this is what is needed if
we are to gain new insight into these questions in the future. In summary, we detected glomerular ROS/NO imbalance and impairment of renal autoregulatory response in rats with salt-induced
hypertension. We also showed that correction of the ROS/NO disproportion and endothelial dysfunction were improved by ARB treatment. CONFLICT OF INTEREST The authors declare no conflict of
interest. REFERENCES * Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J . Blood pressure and end-stage renal disease in men. _N Engl J Med_ 1996; 334:
13–18. Article CAS Google Scholar * Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S . Blood pressure predicts risk of developing end-stage renal disease in men and women.
_Hypertension_ 2003; 41: 1341–1345. Article CAS Google Scholar * The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of
ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric non-diabetic nephropathy. _Lancet_ 1997; 349: 1857–1863. Article Google Scholar * Walker
WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD . Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT
Research Group. _JAMA_ 1992; 268: 3085–3091. Article CAS Google Scholar * Feld LG, Van Liew JB, Galaske RG, Boylan JW . Selectivity of renal injury and proteinuria in the spontaneously
hypertensive rat. _Kidney Int_ 1977; 12: 332–343. Article CAS Google Scholar * Cai H, Harrison DG . Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. _Circ
Res_ 2000; 87: 840–844. Article CAS Google Scholar * Stuehr D, Pou S, Rosen GM . Oxygen reduction by nitric-oxide synthases. _J Biol Chem_ 2001; 276: 14533–14536. Article CAS Google
Scholar * Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Munzel T . Vasodilator-stimulated phosphoprotein serine 239
phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. _Circ Res_ 2000; 87: 999–1005. Article CAS Google Scholar * Hink U, Li H,
Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T . Mechanisms underlying endothelial
dysfunction in diabetes mellitus. _Circ Res_ 2001; 88: E14–E22. Article CAS Google Scholar * Xia Y, Tsai AL, Berka V, Zweier JL . Superoxide generation from endothelial nitric-oxide
synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. _J Biol Chem_ 1998; 273: 25804–25808. Article CAS Google Scholar * Vasquez-Vivar J, Kalyanaraman B,
Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard Jr KA . Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. _Proc Natl Acad Sci USA_ 1998;
95: 9220–9225. Article CAS Google Scholar * Alp NJ, Channon KM . Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. _Arterioscler Thromb Vasc
Biol_ 2004; 24: 413–420. Article CAS Google Scholar * Persson PB . Renal blood flow autoregulation in blood pressure control. _Curr Opin Nephrol Hypertens_ 2002; 11: 67–72. Article
Google Scholar * Inscho EW, Carmines PK, Cook AK, Navar LG . Afferent arteriolar responsiveness to altered perfusion pressure in renal hypertension. _Hypertension_ 1990; 15: 748–752.
Article CAS Google Scholar * Inscho EW, Imig JD, Deichmann PC, Cook AK . Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats. _J Am Soc
Nephrol_ 1999; 10 (Suppl 11): S178–S183. CAS PubMed Google Scholar * Haruna Y, Morita Y, Yada T, Satoh M, Fox DA, Kashihara N . Fluvastatin reverses endothelial dysfunction and increased
vascular oxidative stress in rat adjuvant-induced arthritis. _Arthritis Rheum_ 2007; 56: 1827–1835. Article CAS Google Scholar * Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, Komai
N, Sasaki T, Tsujioka K, Makino H, Kashihara N . NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic
nephropathy. _Am J Physiol Renal Physiol_ 2005; 288: F1144–F1152. Article CAS Google Scholar * Fujimoto S, Satoh M, Horike H, Hatta H, Haruna Y, Kobayashi S, Namikoshi T, Arakawa S,
Tomita N, Kashihara N . Olmesartan ameliorates progressive glomerular injury in subtotal nephrectomized rats through suppression of superoxide production. _Hypertens Res_ 2008; 31: 305–313.
Article CAS Google Scholar * Satoh M, Fujimoto S, Arakawa S, Yada T, Namikoshi T, Haruna Y, Horike H, Sasaki T, Kashihara N . Angiotensin II type 1 receptor blocker ameliorates uncoupled
endothelial nitric oxide synthase in rats with experimental diabetic nephropathy. _Nephrol Dial Transplant_ 2008; 23: 3806–3813. Article CAS Google Scholar * Namikoshi T, Tomita N, Satoh
M, Haruna Y, Kobayashi S, Komai N, Sasaki T, Kashihara N . Olmesartan ameliorates renovascular injury and oxidative stress in Zucker obese rats enhanced by dietary protein. _Am J Hypertens_
2007; 20: 1085–1091. Article CAS Google Scholar * van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ . Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded
rat. _Am J Physiol_ 1999; 276: R855–R863. CAS PubMed Google Scholar * Semple SJ, De Wardener HE . Effect of increased renal venous pressure on circulatory autoregulation of isolated dog
kidneys. _Circ Res_ 1959; 7: 643–648. Article CAS Google Scholar * Taylor NE, Maier KG, Roman RJ, Cowley Jr AW . NO synthase uncoupling in the kidney of Dahl S rats: role of
dihydrobiopterin. _Hypertension_ 2006; 48: 1066–1071. Article CAS Google Scholar * Fujii S, Zhang L, Igarashi J, Kosaka H . L-arginine reverses p47phox and gp91phox expression induced by
high salt in Dahl rats. _Hypertension_ 2003; 42: 1014–1020. Article CAS Google Scholar * Ray R, Shah AM . NADPH oxidase and endothelial cell function. _Clin Sci (Lond)_ 2005; 109:
217–226. Article CAS Google Scholar * Squadrito GL, Pryor WA . The formation of peroxynitrite _in vivo_ from nitric oxide and superoxide. _Chem Biol Interact_ 1995; 96: 203–206. Article
CAS Google Scholar * Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH . Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in
diabetes mellitus. _Circulation_ 2007; 116: 944–953. Article CAS Google Scholar * Xu J, Xie Z, Reece R, Pimental D, Zou MH . Uncoupling of endothelial nitric oxidase synthase by
hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. _Arterioscler Thromb Vasc Biol_ 2006; 26: 2688–2695. Article CAS Google Scholar * Oelze M, Daiber A,
Brandes RP, Hortmann M, Wenzel P, Hink U, Schulz E, Mollnau H, von Sandersleben A, Kleschyov AL, Mulsch A, Li H, Forstermann U, Munzel T . Nebivolol inhibits superoxide formation by NADPH
oxidase and endothelial dysfunction in angiotensin II-treated rats. _Hypertension_ 2006; 48: 677–684. Article CAS Google Scholar * Barton M, Vos I, Shaw S, Boer P, D′Uscio LV, Grone HJ,
Rabelink TJ, Lattmann T, Moreau P, Luscher TF . Dysfunctional renal nitric oxide synthase as a determinant of salt-sensitive hypertension: mechanisms of renal artery endothelial dysfunction
and role of endothelin for vascular hypertrophyandGlomerulosclerosis. _J Am Soc Nephrol_ 2000; 11: 835–845. CAS PubMed Google Scholar * Karlsen FM, Andersen CB, Leyssac PP,
Holstein-Rathlou NH . Dynamic autoregulation and renal injury in Dahl rats. _Hypertension_ 1997; 30: 975–983. Article CAS Google Scholar * Bidani AK, Hacioglu R, Abu-Amarah I, Williamson
GA, Loutzenhiser R, Griffin KA . ‘Step’ vs‘dynamic’ autoregulation: implications for susceptibility to hypertensive injury. _Am J Physiol Renal Physiol_ 2003; 285: F113–F120. Article CAS
Google Scholar * Cupples WA, Braam B . Assessment of renal autoregulation. _Am J Physiol Renal Physiol_ 2007; 292: F1105–F1123. Article CAS Google Scholar * Ungvari Z, Koller A .
Selected contribution: NO released to flow reduces myogenic tone of skeletal muscle arterioles by decreasing smooth muscle Ca(2+) sensitivity. _J Appl Physiol_ 2001; 91: 522–527 discussion
504-525. Article CAS Google Scholar * Huang A, Sun D, Kaley G, Koller A . Superoxide released to high intra-arteriolar pressure reduces nitric oxide-mediated shear stress- and
agonist-induced dilations. _Circ Res_ 1998; 83: 960–965. Article CAS Google Scholar * Nurkiewicz TR, Boegehold MA . High salt intake reduces endothelium-dependent dilation of mouse
arterioles via superoxide anion generated from nitric oxide synthase. _Am J Physiol Regul Integr Comp Physiol_ 2007; 292: R1550–R1556. Article CAS Google Scholar * Rahgozar M, Guan Z,
Matthias A, Gobe GC, Endre ZH . Angiotensin II facilitates autoregulation in the perfused mouse kidney: An optimized _in vitro_ model for assessment of renal vascular and tubular function.
_Nephrology (Carlton)_ 2004; 9: 288–296. Article CAS Google Scholar * Guan Z, Willgoss DA, Matthias A, Manley SW, Crozier S, Gobe G, Endre ZH . Facilitation of renal autoregulation by
angiotensin II is mediated through modulation of nitric oxide. _Acta Physiol Scand_ 2003; 179: 189–201. Article CAS Google Scholar * Mitchell KD, Navar LG . Enhanced tubuloglomerular
feedback during peritubular infusions of angiotensins I and II. _Am J Physiol_ 1988; 255: F383–F390. CAS PubMed Google Scholar * Ichihara A, Hayashi M, Koura Y, Tada Y, Sugaya T, Hirota
N, Saruta T . Blunted tubuloglomerular feedback by absence of angiotensin type 1A receptor involves neuronal NOS. _Hypertension_ 2002; 40: 934–939. Article CAS Google Scholar * Leyssac
PP, Holstein-Rathlou NH . Tubulo-glomerular feedback response: enhancement in adult spontaneously hypertensive rats and effects of anaesthetics. _Pflugers Arch_ 1989; 413: 267–272. Article
CAS Google Scholar * Rapp JP, Tan SY, Margolius HS . Plasma mineralocorticoids, plasma renin, and urinary kallikrein in salt-sensitive and salt-resistant rats. _Endocr Res Commun_ 1978; 5:
35–41. Article CAS Google Scholar * Muller-Suur R, Gutsche HU, Samwer KF, Oelkers W, Hierholzer K . Tubuloglomerular feedback in rat kidneys of different renin contents. _Pflugers Arch_
1975; 359: 33–56. Article CAS Google Scholar * Schnermann J, Levine DZ . Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. _Annu Rev Physiol_ 2003; 65:
501–529. Article CAS Google Scholar * Inscho EW . P2 receptors in regulation of renal microvascular function. _Am J Physiol Renal Physiol_ 2001; 280: F927–F944. Article CAS Google
Scholar * Schwiebert EM . ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. _Clin Exp Pharmacol Physiol_ 2001; 28: 340–350. Article CAS Google Scholar *
Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ . Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. _J Clin Invest_ 2003; 112: 1895–1905. Article CAS
Google Scholar * Mulvany MJ . Small artery remodelling in hypertension: causes, consequences and therapeutic implications. _Med Biol Eng Comput_ 2008; 46: 461–467. Article Google Scholar
Download references ACKNOWLEDGEMENTS We thank M Ono and S Tsujita for excellent technical assistance. This study was supported by grants from KAKENHI (No. 19590969) to NK and the Salt
Science Research Foundation (No. 0731) to MS. Telmisartan was kindly supplied by Astellas Pharma (Tokyo, Japan). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Nephrology,
Department of Internal Medicine, Kawasaki Medical School, Kurashiki, Okayama, Japan, Minoru Satoh, Yoshisuke Haruna, Sohachi Fujimoto, Tamaki Sasaki & Naoki Kashihara Authors * Minoru
Satoh View author publications You can also search for this author inPubMed Google Scholar * Yoshisuke Haruna View author publications You can also search for this author inPubMed Google
Scholar * Sohachi Fujimoto View author publications You can also search for this author inPubMed Google Scholar * Tamaki Sasaki View author publications You can also search for this author
inPubMed Google Scholar * Naoki Kashihara View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Minoru Satoh. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Satoh, M., Haruna, Y., Fujimoto, S. _et al._ Telmisartan improves endothelial dysfunction and renal autoregulation
in Dahl salt-sensitive rats. _Hypertens Res_ 33, 135–142 (2010). https://doi.org/10.1038/hr.2009.190 Download citation * Received: 27 May 2009 * Revised: 12 September 2009 * Accepted: 21
October 2009 * Published: 20 November 2009 * Issue Date: February 2010 * DOI: https://doi.org/10.1038/hr.2009.190 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative KEYWORDS * angiotensin receptor blocker * eNOS uncoupling * nitric oxide * superoxide






