- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT PURPOSE Randomized controlled trials have shown the efficacy of atropine for progressive myopia, and this treatment has become the preferred pattern for this condition in Taiwan.
This study explores the effectiveness of atropine 0.5% treatment for progressive high myopia and adherence to therapy in a non-Asian country. METHODS An effectiveness study was performed in
Rotterdam, the Netherlands. Overall 77 children (mean age 10.3 years±2.3), of European (_n_=53), Asian (_n_=18), and African (_n_=6) descent with progressive myopia were prescribed atropine
0.5% eye drops daily. Both parents and children filled in a questionnaire regarding adverse events and adherence to therapy. A standardized eye examination including cycloplegic refraction
and axial length was performed at baseline and 1, 4, and 12 months after initiation of therapy. RESULTS Mean spherical equivalent at baseline was −6.6D (±3.3). The majority (60/77, 78%) of
children adhered to atropine treatment for 12 months; 11 of the 17 children who discontinued therapy did so within 1 month after the start of therapy. The most prominent reported adverse
events were photophobia (72%), followed by reading problems (38%), and headaches (22%). The progression rate of spherical equivalent before treatment (−1.0D/year±0.7) diminished
substantially during treatment (−0.1D/year±0.7) compared to those who ceased therapy (−0.5D/year±0.6; _P_=0.03). CONCLUSIONS Despite the relatively high occurrence of adverse events, our
study shows that atropine can be an effective and sustainable treatment for progressive high myopia in Europeans. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFICACY OF 0.01% LOW DOSE ATROPINE
AND ITS CORRELATION WITH VARIOUS FACTORS IN MYOPIA CONTROL IN THE INDIAN POPULATION Article Open access 02 May 2022 A MULTICENTER SPANISH STUDY OF ATROPINE 0.01% IN CHILDHOOD MYOPIA
PROGRESSION Article Open access 05 November 2021 AGE-RELATED RESULTS OVER 2 YEARS OF THE MULTICENTER SPANISH STUDY OF ATROPINE 0.01% IN CHILDHOOD MYOPIA PROGRESSION Article Open access 28
September 2023 INTRODUCTION Worldwide, the prevalence of myopia has been rising dramatically, and it is estimated that 2.5 billion people will be affected by myopia by 2020.1 South-East Asia
is now facing a myopia frequency up to 95.5% in young academics,2, 3 but a rising trend has also been observed in recent European studies.4 The high rise also includes the prevalence of
high myopia (<−6D; axial length ≥26 mm), which in particular is associated with severe complications, such as myopic macular degeneration, retinal detachment, and glaucoma.2 The absolute
risk of severe visual impairment is 30% in individuals with axial length of 26 mm, and increases up to 95% in those with an axial length of 30 mm or more.5, 6 These dramatic figures create
the need for effective counteractions. Current treatment options for progressive myopia can be categorized in conservative and pharmacological interventions.7 The effects of the conservative
regimens, except for the orthokeratology, are relatively small.8 Pharmacological intervention has a much higher efficacy, in particular treatment with topically applied atropine eye drops.9
Atropine, a non-selective muscarinic receptor antagonist (M-antagonist), is the most studied pharmacological agent for the intervention of progressive myopia.10 In animals, topical atropine
showed an inhibitory effect on lens-induced and -deprived myopia.11 In humans, the use of atropine to reduce myopic progression was published decades ago,12 but it was not until the ATOM
study performed their large randomized clinical trial in 400 children of Asian ethnicity that atropine was acknowledged as an effective treatment for myopia progression.10 This 2-year study
found 75% reduction of myopic progression with atropine 1%, and did not report serious side effects. A systematic Cochrane review on atropine studies reported that myopia progression can be
reduced by 0.80–1.0D after a year of treatment of atropine 0.5 and 1%, respectively.7 Atropine is the preferred practice pattern for progressive myopia in Taiwan.13 As early as the year
2000, the Ophthalmological Society of Taiwan advised to use atropine to slow down myopia progression.13 This treatment is prescribed to nearly 50% of Taiwanese children with progressive
myopia.13 Although topical use of atropine is known to cause photophobia and accommodation lag, these adverse events do not appear to hamper its implementation in Taiwanese children. By
contrast, the lighter iris color in Europeans is generally considered as a barrier for its use in the Western world.14 Moreover, some studies have suggested that atropine is less effective
in persons of non-Asian descent.15 The aim of this study was to investigate the effect of atropine for progressive myopia under ‘real-world’ conditions in a non-Asian country. We compared
rates of myopia progression in consecutive children before and after therapy, assessed common complaints, evaluated reasons for discontinuation, and developed practice guidelines. METHODS
STUDY DESIGN, POPULATION, AND INTERVENTION The design was an effectiveness study, and was prospective and clinic-based. The setting was Erasmus Medical Center and Sophia Children’s Hospital
in Rotterdam, the Netherlands, and all consecutive children younger than 18 years of age presenting with progressive myopia were eligible for the study. Inclusion criteria were spherical
equivalent (SE) ≤−3D and SE progression rate ≥1D/year under cycloplegic conditions; exclusion criteria were myopia related to retinal dystrophies or collagen syndromes, and developmental
disorders. Eligible children and parents received a patient information leaflet followed by oral consultation. After providing written informed parental consent (parents or legal guardians
for children ≤12 years; also including children for ages 12+ years), participants received a prescription of atropine eye drops 0.5% (FNA Dutch pharmacists). Both eyes were treated by
atropine eye drops once daily before bedtime by the parent. The study and protocol adhered to the tenets of the Declaration of Helsinki, and were approved by the Medical Ethics Committee of
the Erasmus Medical Centre. EYE EXAMINATION A standardized ophthalmological examination was performed at baseline, 1 month, 4 months, and 12 months after initiation of atropine treatment.
Best corrected visual acuity was performed with a decimal equivalent (minutes) visual acuity chart at 6 m distance. Binocular reading visual acuity was performed with the LogMAR-based Dutch
Radner reading chart at 25 or 40 cm.16 Pupil size was measured with Richmond Products Clear Pupilometer (Albuquerque, NM, USA). At baseline, full cycloplegia was obtained 45 min after
administration of 1% cyclopentolate eye drops. At follow up, cycloplegia was already present at examination due to the use of atropine; this was confirmed by the investigators with dynamic
retinoscopy, and was therefore considered a measure of compliance. Subsequently, the refractive error was measured with a Topcon auto refractor KR8900 (Topcon, Tokyo, Japan); in younger
children with a Nikon Retinomax 2 auto refractor (Nikon, Tokyo, Japan), and in very young or uncooperative children refractive error was determined by an experienced orthoptist (JRP)
performing retinoscopy with a Heine beta 200 retinoscope (Heine Optotechnik, Herrsching, Germany) and lenses according to standard protocols. The same devices were used throughout the study
period. Spherical equivalent was calculated using the standard formula: (SE=sphere+1/2 cylinder). Axial length was measured with the IOL Master 500 (Carl Zeiss MEDITEC IOL-master, Jena,
Germany) at each visit. RISK FACTORS AND ADVERSE EVENTS At baseline, and after 4 and 12 months after the start of atropine, as well as 1 month after cessation of therapy, parents and
children filled in a questionnaire evaluating adverse events. The questionnaires were filled in independent of each other at different locations; children <8 years of age received help of
the investigator. The questions for the parents concerned risk factors for myopia, adverse events, and adherence to therapy; the questions for the children concerned only the latter two,
and were simplified versions of the same questions for parents. STATISTICAL ANALYSIS All data were entered into a database as nominal or ordinal variables. Proportions were calculated, and
data before and after start of atropine treatment were compared with Fisher’s exact test. Biometric measures of the eye were analyzed using Mann–Whitney _U_ non-parametric test. Throughout
the study, _P_=0.05 was used as border of significance. The annual progression rate before treatment was calculated by subtracting the SE at baseline from the SE estimated 1 year before
treatment for each participant. We calculated the progression rate during treatment by subtracting the SE at 1 year follow up (−6.8D±3.6) from the SE estimated at baseline (−6.7D±3.6). The
rate was analyzed with Wilcoxon signed ranks test to identify short term differences during the 1 year of treatment. Risk of adverse events and adherence to therapy were estimated using
logistic and linear regression analysis. Multivariate logistic regression analysis was used to determine the risk of discontinuation of therapy with age, gender, baseline SE, and ethnicity
in the model. All statistical tests were performed by using IBM SPSS Statistics for Windows, Version 21.0. (IBM Corp., Armonk, NY, USA). RESULTS From March 2011 to July 2013, a total of 84
consecutive progressive myopic children visited our clinic and were considered eligible for this study. Of these, 78 (92.9%) consented to participation and 6 (7.1%) refused. Of those
consented, 1 (1.3%) child was lost to follow up during the course of the study. The remaining 77 children completed 12 months of follow up. Demographics of the study population are
summarized in Table 1. Gender was evenly distributed; the mean age was 10.3 (±3.2) years, and two-third of the children had European ancestry. The mean refractive error 1.1 (±0.6) year
before treatment was −5.6D (±3.9). At baseline, mean refractive error was −6.63D (±3.31), resulting in a mean progression rate of −1.0 (0.7). Half (50.6%) of the children were already highly
myopic (SE >−6D, ranging from −6.13D to −18.63D). Mean pupil diameter before treatment was 4.4 mm (95% CI: 3.3–5.5). The majority (84.7%) reported at least one myopic parent. Five
children had been adopted, and had no information on the refractive error of the biological parents. Of the 77 children, 60 (78%) adhered to therapy for the complete follow up of 1 year.
Annual progression rates showed an advantage for the children who stayed on therapy (0.1D/year) _vs_ the children who discontinued therapy (0.5D/year) (_P_ 0.03) (Table 2). Mean change in SE
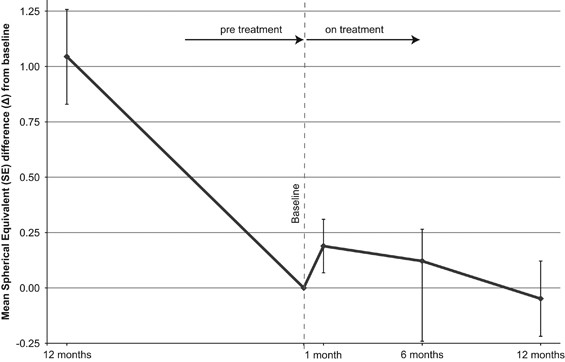
from baseline to 1 year before and during the year of treatment is presented in Figure 1. The SE difference from baseline to the first month of treatment appeared to improve by 0.19D
(±0.41) compared to baseline, but this temporary inverse progression of myopia was not sustained thereafter. The SE difference from baseline to 6 and 12 months was significantly lower than
before therapy and approached almost 0 (0.12 and −0.05D, _P_<0.01). Age modified the treatment effect significantly (_P_=0.01): children younger than 9 years of age had the lowest
treatment effect (annual progression rate −0.49D, CI: −0.90 to −0.08); 9–12 year olds had more effect (annual progression rate −0.06D, CI: −0.47 to +0.35), and older children had the highest
treatment effect (annual progression rate 0.02D, CI: −0.27 to +0.3). Ethnicity (_P_=0.58) nor gender (_P_=0.76) significantly influenced annual progression rate during treatment. More than
half (36/60; 60%) of the children who adhered to therapy did not report any skips in therapy administration. None showed more than 0.5D accommodation on dynamic retinoscopy. The mean pupil
diameter was 7.0 mm (±0.63) during the follow up visits. The most frequent reason for skips was forgetfulness. Overall 61.7% of children who commenced with atropine, 17 stopped treatment, of
whom 11 (64.7%) within the first 4 weeks (Table 3). Adverse events were reported as the primary reason (82.4%). The frequency of dropouts was higher in those aged 12 years and over (13.0%
in age <12 years _vs_ 44.4% in 12+ years; _P_<0.01). The questionnaires addressing treatment response and adverse events showed remarkable similarity between parents and their
children, although children reported complaints at slightly higher frequencies. Adverse events occurred often, 63 (82.9%) reported these by both parents and children. Photophobia (72.4%) and
reading problems (37.7%) were reported most frequently; 22.4% reported headaches; and systemic flushes occurred only in a minority. Those who discontinued therapy reported reading problems
significantly more often than those who maintained therapy (12/15 (80%) _vs_ 13/54 (24.1%), _P_<0.01) (Table 4). Other reported events were rare: pain in the eye, irritated eyes, overflow
of tears, trouble with depth perception, cosmetically disfiguring pupils, and an unpleasant taste in mouth (all reported only in one patient). DISCUSSION Our study shows that atropine 0.5%
can be an effective treatment for progressive myopia in a European setting. The mean progression rate before the year of intervention was −1.0D (±0.7)/year. Atropine 0.5% reduced this to
−0.1D (±0.7)/year during treatment. Despite a high frequency of adverse events, most children managed to prolong therapy for the entire study period. Those children who prolonged therapy had
a significant advantage over those who stopped (_P_=0.03). The effect of treatment was dependent on age, and was most prominent in teenagers. Although not powered to test for ethnicity and
gender, these did not appear to influence treatment outcome in our study. We deliberately chose a pragmatic study design to make a translation from findings of efficacy studies to daily
practice. Numerous studies including randomized controlled trials have reported on the efficacy of atropine treatment for progressive myopia. An effectiveness study such as ours more closely
reflects daily practice as it consisted of a heterogeneous patient population with a large range in refractive errors and age, and inclusion of multiple ethnicities. Other strengths of our
study are the standardized measurements of cycloplegic refraction, and the cross evaluation of parents and children by questionnaire to improve the validity of data on adherence and adverse
events. Among the limitations are the relatively short follow up, and the absence of a flexible dosing regimen which would have allowed tailored therapy for each subject. Higher
concentration atropine eye drops are known for their frequent occurrence of adverse events, and our study confirms this. Most commonly reported adverse events were photophobia and reading
problems. Headaches occurred in approximately one-fifth of the patients, but were reported to be mild and transient. Flushes of the cheeks were observed in only three children, but were not
a reason to discontinue therapy. Cessation of therapy most often occurred shortly after the start of therapy. Children who managed to adhere to therapy for 4 weeks were more likely to
prolong therapy thereafter. Most important startup difficulties were adaptation to the bright light and coping with reading problems. Following from this observation, we therefore recommend
to prescribe transitional multifocal glasses at the initiation of therapy. We also experienced that comprehensive instruction of the parent and child through information brochures and oral
clarification was greatly appreciated, and may improve motivation. After cessation of therapy, a rebound, or catch-up, growth spurt has been described.17 Tong _et al_18 found that the
positive effect of atropine lasted up to 3 years before being caught up by the rebound effect. Maintaining therapy for a longer period of time and tapering with lower concentrations after
achieving stability are suggested to prevent this rebound effect.19 Atropine is the standard of care for myopia progression in Taiwan.13 The reasons for not prescribing atropine for
progressive myopia in western countries is as yet unclear. One reason may be the report of a higher efficacy of treatment in Asians than in Europeans. Although our power to study differences
herein was low, our study could not confirm any differences between ethnicities. Another reason may be fear for serious and irreversible complications after prolonged use, but this is not
substantiated by literature. Long-term effects of atropine treatment have been investigated in both animal as well as human studies,20, 21 and photochemical damage to the retina due to
enlarged pupil for a longer period of time under daylight conditions has not been reported.22, 23 Therefore, daily atropine appears to be a safe treatment, even if used for several years.12,
24, 25 A remarkable finding was that the refractive error showed a hyperopic shift after 4 weeks, which disappeared after 4 months. This effect could be caused by the stronger cycloplegic
effect of atropine over classical cycloplegic agents used in the clinic, such as cyclogyl.26 The reduction in refractive error could also be the result of a temporary thickening of the
choroid, a phenomenon observed in animal studies.27 How atropine manages to interfere with myopia progression has not been well established, neither is there agreement on the site of
action.28 This may be the retina, because amacrine cells can express muscarinic receptors on their cell membrane.29 Binding of atropine to the muscarinic receptors of amacrine cells has been
hypothesized to increase the release of dopamine, which fits well with the view that dopamine is an inhibitory chemical mediator for eye growth.30 Reduction of γ-aminobutyric acid levels is
also a possible mechanism, as this neurotransmitter was shown to be downregulated following atropine treatment in myopia-induced mice.31 Other explanations include an effect of atropine via
the sclera. Scleral fibroblast cells carry all five muscarinic receptors on their cell membrane and binding to atropine may interfere with scleral remodeling.32 The inhibitory effect of
atropine is not likely executed through an accommodative mechanism, because the inhibitory effect of atropine on eye growth is also observed in chicks, and these animals activate the ciliary
muscle via nicotine receptors rather than the muscarinic receptor.30 Taken our findings together with the existing literature, we suggest the following guidelines for doctors treating
myopes at risk for high myopia in everyday clinical practice: first, identify and discuss the risk profile of the patient and provide lifestyle advice, such as increase of the time spent
outdoors. Second, start intervention with atropine 0.5% and prescribe transitional multifocal glasses. Third, perform regular follow up examinations including visual acuity, reading acuity,
cycloplegic refraction, and axial length. Fourth, adjust treatment regimen. In contrast, when SE and axial length have remained stable for a period of 12 months, gradually taper the atropine
concentration to naught. In conclusion, our study provides external validity of findings from randomized controlled trials and shows that atropine can be effective for progressive myopia in
daily clinical practice. Atropine should be considered a treatment option in children at risk of high myopia anywhere in the world. REFERENCES * Morgan IG, Ohno-Matsui K, Saw SM . Myopia.
_Lancet_ 2012; 379 (9827): 1739–1748. Article PubMed Google Scholar * Pan CW, Ramamurthy D, Saw SM . Worldwide prevalence and risk factors for myopia. _Ophthalmic Physiol Opt_ 2012; 32
(1): 3–16. Article PubMed Google Scholar * Sun J, Zhou J, Zhao P, Lian J, Zhu H, Zhou Y _et al_. High prevalence of myopia and high myopia in 5060 Chinese university students in Shanghai.
_Invest Ophthalmol Vis Sci_ 2012; 53 (12): 7504–7509. Article PubMed Google Scholar * Williams KM, Bertelsen G, Cumberland P, Wolfram C, Verhoeven VJ, Anastasopoulos E _et al_.
Increasing prevalence of myopia in Europe and the impact of education. _Ophthalmology_ 2015; 122 (7): 1489–1497. Article PubMed Google Scholar * Vongphanit J, Mitchell P, Wang JJ .
Prevalence and progression of myopic retinopathy in an older population. _Ophthalmology_ 2002; 109 (4): 704–711. Article PubMed Google Scholar * Verhoeven VJ, Wong KT, Buitendijk GH,
Hofman A, Vingerling JR, Klaver CC . Visual consequences of refractive errors in the general population. _Ophthalmology_ 2015; 122 (1): 101–109. Article PubMed Google Scholar * Walline
JJ, Lindsley K, Vedula SS, Cotter SA, Mutti DO, Twelker JD . Interventions to slow progression of myopia in children. _Cochrane Database Syst Rev_ 2011; (12): CD004916. * Sun Y, Xu F, Zhang
T, Liu M, Wang D, Chen Y _et al_. Orthokeratology to control myopia progression: a meta-analysis. _PLoS One_ 2015; 10 (4): e0124535. Article PubMed PubMed Central Google Scholar *
Gwiazda J . Treatment options for myopia. _Optom Vis Sci_ 2009; 86 (6): 624–628. Article PubMed PubMed Central Google Scholar * Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL
_et al_. Atropine for the treatment of childhood myopia. _Ophthalmology_ 2006; 113 (12): 2285–2291. Article PubMed Google Scholar * Schmid KL, Wildsoet CF . Inhibitory effects of
apomorphine and atropine and their combination on myopia in chicks. _Optom Vis Sci_ 2004; 81 (2): 137–147. Article PubMed Google Scholar * Brodstein RS, Brodstein DE, Olson RJ, Hunt SC,
Williams RR . The treatment of myopia with atropine and bifocals. A long-term prospective study. _Ophthalmology_ 1984; 91 (11): 1373–1379. Article CAS PubMed Google Scholar * Fang YT,
Chou YJ, Pu C, Lin PJ, Liu TL, Huang N _et al_. Prescription of atropine eye drops among children diagnosed with myopia in Taiwan from 2000 to 2007: a nationwide study. _Eye (Lond)_ 2013; 27
(3): 418–424. Article CAS Google Scholar * Shih YF, Hsiao CK, Chen CJ, Chang CW, Hung PT, Lin LL . An intervention trial on efficacy of atropine and multi-focal glasses in controlling
myopic progression. _Acta Ophthalmol Scand_ 2001; 79 (3): 233–236. Article CAS PubMed Google Scholar * Li SM, Wu SS, Kang MT, Liu Y, Jia SM, Li SY _et al_. Atropine slows myopia
progression more in Asian than white children by meta-analysis. _Optom Vis Sci_ 2014; 91 (3): 342–350. PubMed Google Scholar * Maaijwee KJ, Meulendijks CF, Radner W, van Meurs JC, Hoyng CB
. [The Dutch version of the Radner Reading Chart for assessing vision function]. _Ned Tijdschr Geneeskd_ 2007; 151 (45): 2494–2497. CAS PubMed Google Scholar * Lin L, Lan W, Liao Y, Zhao
F, Chen C, Yang Z . Treatment outcomes of myopic anisometropia with 1% atropine: a pilot study. _Optom Vis Sci_ 2013; 90 (12): 1486–1492. Article PubMed Google Scholar * Tong L, Huang
XL, Koh AL, Zhang X, Tan DT, Chua WH . Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. _Ophthalmology_ 2009; 116 (3): 572–579.
Article PubMed Google Scholar * Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D . Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. _Am
J Ophthalmol_ 2014; 157 (2): 451–457 e1. Article CAS PubMed Google Scholar * Lawwill T, Crockett S, Currier G . Retinal damage secondary to chronic light exposure, thresholds and
mechanisms. _Doc Ophthalmol_ 1977; 44 (2):379–402. Article CAS PubMed Google Scholar * Noell WK, Walker VS, Kang BS, Berman S . Retinal damage by light in rats. _Invest Ophthalmol_ 1966;
5 (5): 450–473. CAS PubMed Google Scholar * Wu J, Seregard S, Algvere PV . Photochemical damage of the retina. _Surv Ophthalmol_ 2006; 51 (5): 461–481. Article PubMed Google Scholar *
Luu CD, Lau AM, Koh AH, Tan D . Multifocal electroretinogram in children on atropine treatment for myopia. _Br J Ophthalmol_ 2005; 89 (2): 151–153. Article CAS PubMed PubMed Central
Google Scholar * Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A _et al_. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine
for the treatment of myopia 2). _Ophthalmology_ 2012; 119 (2): 347–354. Article PubMed Google Scholar * Kennedy RH, Dyer JA, Kennedy MA, Parulkar S, Kurland LT, Herman DC _et al_.
Reducing the progression of myopia with atropine: a long term cohort study of Olmsted County students. _Binocul Vis Strabismus Q_ 2000; 15 (3 Suppl): 281–304. CAS PubMed Google Scholar *
Rosenbaum AL, Bateman JB, Bremer DL, Liu PY . Cycloplegic refraction in esotropic children. Cyclopentolate versus atropine. _Ophthalmology_ 1981; 88 (10): 1031–1034. Article CAS PubMed
Google Scholar * Nickla DL, Zhu X, Wallman J . Effects of muscarinic agents on chick choroids in intact eyes and eyecups: evidence for a muscarinic mechanism in choroidal thinning.
_Ophthalmic Physiol Opt_ 2013; 33 (3): 245–256. Article PubMed PubMed Central Google Scholar * McBrien NA, Stell WK, Carr B . How does atropine exert its anti-myopia effects? _Ophthalmic
Physiol Opt_ 2013; 33 (3): 373–378. Article PubMed Google Scholar * Arumugam B, McBrien NA . Muscarinic antagonist control of myopia: evidence for M4 and M1 receptor-based pathways in
the inhibition of experimentally-induced axial myopia in the tree shrew. _Invest Ophthalmol Vis Sci_ 2012; 53 (9): 5827–5837. Article CAS PubMed Google Scholar * McBrien NA, Moghaddam
HO, Reeder AP . Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. _Invest Ophthalmol Vis Sci_ 1993; 34 (1): 205–215. CAS PubMed Google Scholar *
Barathi VA, Chaurasia SS, Poidinger M, Koh SK, Tian D, Ho C _et al_. Involvement of GABA transporters in atropine-treated myopic retina as revealed by iTRAQ quantitative proteomics. _J
Proteome Res_ 2014; 13 (11): 4647–4658. Article CAS PubMed PubMed Central Google Scholar * Gallego P, Martinez-Garcia C, Perez-Merino P, Ibares-Frias L, Mayo-Iscar A, Merayo-Lloves J .
Scleral changes induced by atropine in chicks as an experimental model of myopia. _Ophthalmic Physiol Opt_ 2012; 32 (6): 478–484. Article PubMed Google Scholar Download references AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Ophthalmology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands J R Polling, R G W Kok, J W L Tideman, B Meskat & C
C W Klaver * Department of Optometry and Orthoptics, Faculty of Health, University of Applied Sciences, Utrecht, The Netherlands J R Polling * Department of Epidemiology, Erasmus MC,
University Medical Center, Rotterdam, The Netherlands J W L Tideman & C C W Klaver Authors * J R Polling View author publications You can also search for this author inPubMed Google
Scholar * R G W Kok View author publications You can also search for this author inPubMed Google Scholar * J W L Tideman View author publications You can also search for this author inPubMed
Google Scholar * B Meskat View author publications You can also search for this author inPubMed Google Scholar * C C W Klaver View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to C C W Klaver. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. RIGHTS AND PERMISSIONS This
work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s
Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the
license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Polling, J., Kok, R., Tideman, J. _et al._ Effectiveness study of atropine for progressive myopia in Europeans. _Eye_ 30, 998–1004 (2016). https://doi.org/10.1038/eye.2016.78
Download citation * Received: 30 June 2015 * Accepted: 10 February 2016 * Published: 22 April 2016 * Issue Date: July 2016 * DOI: https://doi.org/10.1038/eye.2016.78 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative