- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT PURPOSE To evaluate the neovascular age-related macular degeneration (nAMD) in patients who were morphologically poor responders to intravitreal ranibizumab (IVR) treatment using
indocyanine green angiography (ICGA) for further investigation. METHODS This was a cross-sectional, retrospective study. The patients with an initial diagnosis of nAMD who made through the
clinical examination, optical coherence tomography, and fluorescein angiography imaging, and were treated with at least three monthly IVR injections that resulted with a morphological poor
response, were included. ICGA was obtained from the patients and evaluated in regard to differential diagnosis of other macular diseases, which might mimic nAMD. RESULTS The study included
132 eyes of 117 patients. The mean age was 67.4±9.4 years. After ICGA imaging, 13 eyes (9.8%) were diagnosed as true nAMD, 74 eyes (56.1%) as polypoidal choroidal vasculopathy (PCV), 35 eyes
(26.5%) as chronic central serous chorioretinopathy (CSC), 3 eyes (2.3%) as retinal angiomatous proliferation (RAP), 3 eyes (2.3%) as choroidal neovascularization secondary to CSC, 2 eyes
(1.5%) as adult-onset vitelliform macular dystrophy, and 2 eyes (1.5%) as drusenoid pigment epithelial detachment with vitelliform material, respectively. The duration between the initial
diagnosis and the revised diagnosis was 15.6±10.5 months in the non-AMD group, and the mean injection number of these patients was 6.6±4.4. CONCLUSIONS Most of the nAMD patients who were
thought to be morphologically poor responders to IVR were diagnosed as having non-AMD diseases via ICGA. A detailed differential diagnostic work-up is needed before considering these
patients as poor responders. SIMILAR CONTENT BEING VIEWED BY OTHERS MACULAR NEOVASCULARIZATION AND POLYPOIDAL CHOROIDAL VASCULOPATHY: PHENOTYPIC VARIATIONS, PATHOGENIC MECHANISMS AND
IMPLICATIONS IN MANAGEMENT Article Open access 06 October 2023 MACULAR NEOVASCULARIZATION IN AMD, CSC AND BEST VITELLIFORM MACULAR DYSTROPHY: QUANTITATIVE OCTA DETECTS DISTINCT CLINICAL
ENTITIES Article 25 January 2021 COMPARISON OF VISUAL OUTCOMES BETWEEN THERAPY CHOICES AND SUBTYPES OF POLYPOIDAL CHOROIDAL VASCULOPATHY (PCV) IN TAIWAN: A REAL-WORLD STUDY Article Open
access 11 January 2021 INTRODUCTION Neovascular age-related macular degeneration (nAMD) is a major cause of visual loss among elderly population in developed countries.1, 2 Before the era of
intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents, only prevention of visual loss might have been achieved in a limited number of patients despite the use of different
treatment modalities.3, 4, 5, 6, 7, 8 Bevacizumab, ranibizumab, and finally aflibercept have led to the conservation of the baseline visual acuity (VA) in the vast majority of the patients
and have given the chance of increasing VA significantly in approximately one third of the patients.9, 10, 11, 12 Multicenter studies have shown that ranibizumab is effective in the
prevention of VA loss in up to 95% of the patients, and an improvement in VA can be achieved in up to 40% of the patients.13, 14 However, there was still a subgroup of patients who did not
respond well to the IVR treatment. A new debate has begun since then, and some other treatment strategies were evaluated for this group of poor-responding patients, such as switching the
drugs, shortening the injection intervals, increasing the drug dose, and using combination therapy.15 Although some of these patients did well with the alternative treatment regimens, others
were still poor responders. Also, their diagnosis was questioned by several authors and various studies were designed to evaluate deeply the true diagnosis of these patients.15, 16, 17, 18,
19, 20 Enhanced depth imaging optical coherence tomography (EDI-OCT), fundus autofluorescence (FAF) imaging, and indocyanine green angiography (ICGA) were used as additional diagnostic
tools in some of these studies.15, 16, 17, 18, 19, 20 Macular diseases such as polypoidal choroidal vasculopathy (PCV), central serous chorioretinopathy (CSC), and retinal angiomatous
proliferation (RAP) may sometimes mimic nAMD and thus create diagnostic challenges. Polypoidal choroidal vasculopathy and RAP are usually considered as variants of nAMD; however, some
authors consider them as different entities than nAMD. Likewise, although some of the PCV and RAP patients respond well to anti-VEGF monotherapy, a substantial number of these patients are
indeed anti-VEGF poor responders. Only a few studies have investigated specific diseases such as PCV or chronic CSC via ICGA in anti-VEGF poor responders, and none of these studies evaluated
purely the morphological poor-responding patients.16, 17, 18, 19, 20 Therefore, in this study we aimed to evaluate the patients who had a diagnosis of nAMD with a morphological poor
response to IVR treatment via multimodal imaging—especially ICGA—for further differential diagnosis from all other macular diseases that mimic nAMD. MATERIALS AND METHODS In this
cross-sectional, retrospective, and observational study, we reviewed the records of the nAMD patients who were treated with IVR in our clinic on an as-needed treatment regimen basis between
January 2014 and December 2014. A written informed consent was obtained from all patients before the treatment and the study adhered to the tenets of the Declaration of Helsinki. To be
included in the study, each patient was required to have all of the following criteria, age ≥50 years, to be initially diagnosed as nAMD, to have received at least three IVR injections, and
an incomplete morphological response as defined below. Patients were not included in the study if they had a known retinal disease other than nAMD. All patients received three initiating
doses of monthly IVR injections (0.5 mg/0.05 ml) initially. Then the patients were followed monthly. A single injection of IVR was repeated when the VA had decreased by one or more Early
Treatment Diabetic Retinopathy Study (ETDRS) lines from the last visit, or when the patients had developed new onset macular hemorrhage, or evidence of subretinal fluid on OCT. The
morphologically poor responder status was defined if there was persistent subretinal and/or intraretinal fluid. If the subretinal or intraretinal fluid had not disappeared after the initial
three loading doses of IVR or if the subretinal fluid had persisted after three consecutive injections in any time during the follow-up period, then the patient was then categorized as a
morphologically poor responder and ICGA was obtained at the same visit. Data collected from the patients’ records included age, gender, and the revised diagnosis after ICGA imaging, the time
interval between the initial and revised diagnosis via ICGA, and the number of anti-VEGF injections within this time period. All patients underwent a standardized examination including
measurement of best-corrected VA (BCVA) via the ETDRS chart at 4 m, slit-lamp biomicroscopy, measurement of intraocular pressure (IOP) via applanation tonometry, and biomicroscopic fundus
examination. Fundus photography, fluorescein angiography (FA) and indocyanine green angiography (HRA-2; Heidelberg Engineering, Heidelberg, Germany), and OCT imaging (Spectralis; Heidelberg
Engineering, Heidelberg, Germany) were also performed. Two independent retina specialists (AO, CA) who were blinded to the clinical data simultaneously evaluated the images. The initial
diagnosis of neovascular AMD was made by eight different retina specialists who worked in our clinic using a combination of clinical examination, OCT, and FA findings of the disease. The
diagnosis of neovascular AMD was made according to the following criteria, well-known clinical findings,1, 2, 3, 4 a stippled hyperfluorescence in FA representing like an occult choroidal
neovascularization (CNV), subretinal fluid associated with an adjacent hyper-reflective area representing like a fibrovascular pigment epithelial detachment (PED).1, 7, 13 The final
differential diagnosis was made according to known specific FA, OCT, FAF, and especially ICGA findings of the diseases. The initial diagnosis of the eyes was usually occult CNV secondary to
nAMD. Only a few patients had classical CNVs, which were later diagnosed as classic CNV secondary to chronic CSC. The differential diagnosis was described in detail in the following
paragraph. Neovascular AMD with occult CNV was diagnosed when a significant hot plaque was detected in the late frames of ICGA, or when dilated choroidal vessels were not seen as a sign of
CSC, or when any hot spot that may be a polyp was not seen (Figure 1).21 In fact neovascular AMD was like an exclusion diagnosis in this study. Polypoidal choroidal vasculopathy was
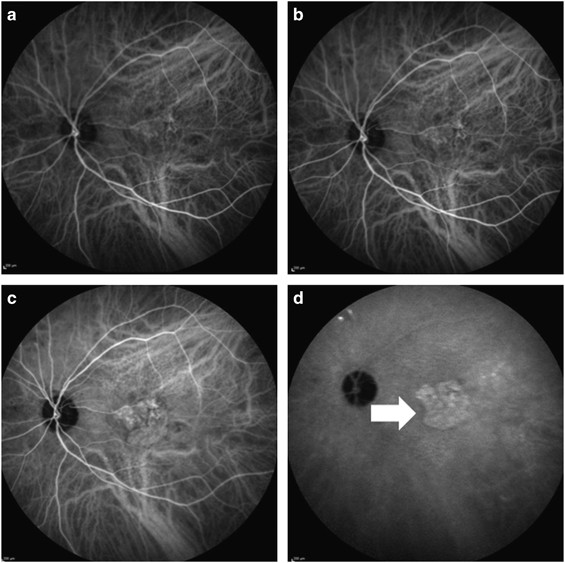
diagnosed when polyps and/or branching vascular network were detected in ICGA (Figure 2).16 Choroidal thickness measurements via EDI-OCT was not routinely obtained by a quantitative method;
however, OCT images were usually evaluated in regard of choroidal thickness with eye examination as a routine practice. Chronic CSC was diagnosed when enlarged choroidal vessels and leakage
from these vessels and hyperfluorescence were detected in the early frames of ICGA, and when wash-out pattern of the leakage and hypofluorescence was detected in the late frames (Figure
2).22 Also in a detailed manner, we evaluated all of the ICGA, FA, and OCT images of the patients for the other signs of CSC, such as diffuse hyperfluorescence in FA, gravitational tracts,
and pigmentary changes.22 RAP was diagnosed when a large and high retinal pigment epithelial detachment with a notch on the surface was detected and/or when angioma _per se_ was detected in
ICGA as a hot spot (Figure 3).16 CNV associated with chronic CSC was diagnosed when a classic CNV was detected with the previously described features of chronic CSC in ICGA. The patients
were diagnosed as adult-onset vitelliform macular dystrophy when the specific features of pattern dystrophy were detected in FAF and OCT (Figure 4) imaging, and when a CNV was not detected
in ICGA.16 The FAF patterns of adult-onset vitelliform macular dystrophy were, a diffuse round increased autofluorescence, or partial increased autofluorescence, or decrease in
autofluorescence if there was no prominent yellow accumulation on examination.23 Drusenoid retinal PED associated with vitelliform-like material diagnosis was made according to the typical
OCT findings, which were seen as a focal elevation of the retinal pigment epithelium contour associated with fluid but without showing a CNV on ICGA.24 Of course, there were some confusing
cases that we were not able to make a definite diagnosis. In such cases, two experienced retinal specialists (AO and CA) jointly adjudicated and arrived at the most probable diagnosis.
Primary outcome measures of this study were the initial and revised diagnosis of nAMD eyes with a morphologic poor response to IVR treatment, the relative distribution of the mimicking
diseases, the time interval from the initial diagnosis to the revised diagnosis, and the number of injections received before the revised diagnosis. STATISTICAL ANALYSIS Statistical analyses
were performed using the Statistical Package for the Social Sciences (SPSS) software (version 16.0, SPSS Inc., Chicago, IL, USA). Data were first analyzed for normality using
Kolmogorov–Smirnov test. The continuous variables were expressed as means±SD. The categorical variables were expressed as number (_n_) and percentages (%). RESULTS One hundred and thirty two
out of 1024 eyes were found to be morphologically poor responders to IVR treatment and included in the study. The mean age of the patients was 67.4±9.4 years (ranging from 52 to 88).
Fifty-five patients (47%) were women and 62 patients (53%) were men. Seventeen of the eyes (12.8%) were diagnosed early poor responders after the initial three injections, and 115 eyes
(87.2%) were late poor responders. After ICGA imaging and a detailed examination done by using other multimodal imaging tools, 13 eyes (9.8%) were diagnosed as having true nAMD, and 119 eyes
(90.2%) were diagnosed as having non-AMD diseases. The revised diagnosis of the non-AMD eyes were as follows, 74 eyes (56.1%) had PCV, 35 eyes (26.5%) had chronic CSC, 3 eyes (2.3%) had
RAP, 3 eyes (2.3%) had CNV secondary to CSC, 2 eyes (1.5%) had adult-onset vitelliform macular dystrophy, and 2 eyes (1.5%) had drusenoid PED associated with vitelliform-like material (Table
1). The mean duration between the initial and the revised diagnosis was 15.6±10.5 months (ranging between 4 and 48 months) in the non-AMD eyes, and the mean injection number of these eyes
was 6.6±4.4 (ranging between 3 and 24). DISCUSSION The treatment response to anti-VEGF agents in nAMD patients has been an evolving subject in the medical retina era. Several studies have
been conducted in this important topic and the terminology for anti-VEGF treatment response has been defined in detail.15, 16, 25 The response to anti-VEGF agents has been categorized either
as functional or morphological response.15 Amoaku _et al_15 have recently proposed to classify patients with nAMD into four categories with regard to their functional response based on the
change in VA, which were as follows, good responders (>5 ETDRS letter gain from the baseline), suboptimal responders (gain of 0–5 letters from the baseline), poor responders (stable or
<4 letters loss from the baseline), and nonresponders (>5 letters loss from the baseline). Morphological responses of the patients were defined according to their disease activity and
divided into two categories, good responders in whom no disease activity was detected, and suboptimal or poor responders in whom only a partial regression of disease activity was evident.
The authors have also evaluated the treatment response according to treatment phases, which were addressed as primary and secondary responses.15 Primary response was usually determined after
the three initial loading doses at month 4, and secondary response was determined at any time during the follow-up period after the first 4 months.15 In the pivotal multicenter studies, ~5%
of the patients were found to be unresponsive to anti-VEGF treatment, and showed sustained VA loss despite treatment; these patients have been called as nonresponders.11, 13, 14, 26 The
sustained VA loss has been attributed to several factors, such as fibrous scar formation, pigmentary anomalies, geographic atrophy, RPE tear, retinal thinning, and thickening.26 Also some of
the baseline characteristics were found to be associated with sustained VA loss, which were presence of non-foveal geographic atrophy, larger CNV area, poor baseline VA, <20/50 baseline
VA in the fellow eye, and intraretinal fluid not at the foveal center.26 The patients’ clinical characteristics, lesion type, and misdiagnosis have been evaluated thoroughly and constitute
now the main discussed topics in anti-VEGF nonresponse literature.15, 16, 26 Factors affecting drug pharmacodynamics and pharmacokinetics such as receptor downregulation, autoantibody
formation, change in drug distribution, and absorption have also been shown to be related to nonresponse.15, 25, 26 Suboptimal treatment of the patients with less frequent treatment regimens
is another important clinical factor which can affect the treatment success and underlie nonresponse. Real-life studies showed that many of the patients were undertreated especially in the
PRN treatment regimens.25 Also disease chronicity, change of cytokine profile during the treatment, and chronic inflammation were shown to be important clinical factors.15, 26 It is worth to
recall here that some lesion types of nAMD are less responsive to anti-VEGF treatment. Polypoidal choroidal vasculopathy and RAP are usually considered as subtypes of nAMD; however,
nowadays they are being considered by some as different clinical entities. Some of the PCV and RAP lesions respond well to anti-VEGF monotherapy, but in considerable number of the patients
different treatment modalities such as photodynamic therapy and combination therapy are required. Taken together, all these data refer to the important role of detailed differential
diagnostic work-up in the event of nonresponse. It is important to exclude all these factors by using available imaging tools in an individual patient before considering nonresponse.15, 16,
27, 28, 29 In a study by Manoj, poor-responding patients’ clinical diagnosis was reported to be revised in 46.2%. And 79.2% of these patients were diagnosed as having PCV. The remaining
patients were diagnosed as having RAP and adult-onset vitelliform macular dystrophy.16 However, ICGA was not used in all of the patients in the study. The authors reported that ICGA and
autofluorescence imaging were done according to the clinicians’ discretion. In our study, we obtained ICGA from all of our anti-VEGF poor-responding patients. As ICGA has been shown to be a
valuable imaging modality in this group of patients;15, 18, 19, 20 autofluorescence and EDI-OCT were obtained only when needed. ICGA is particularly useful in the differential diagnosis of
PCV, chronic CSC, and RAP, which are usually misdiagnosed as nAMD.15 Our study demonstrate that with the use of ICGA imaging, only 9.8% of our morphologically poor-responding patients were
found to exhibit nAMD, whereas 90.2% of them were misdiagnosed. The revised diagnosis were PCV in 56.1% of the eyes, chronic CSC in 26.5 of the eyes, RAP in 2.3% of the eyes, and CNV
secondary to CSC in 2.3% of the eyes. Interestingly, the patients which required additional photodynamic therapy represented 97% of the misdiagnosed patients. The true number of
morphologically poor-responding eyes was 13, which represented only 1.2% of the 1024 ranibizumab-treated eyes during the study period. Indeed, the morphological poor response that was
132/1024 (12.8%) was found to be 13/1024 (1.2%) after the use of ICGA, which led to a tenfolds decrease in the misdiagnosis of these non-AMD patients. In another study by Hatz and Prünte the
ranibizumab poor-responding patients who required at least eight injections were evaluated via ICGA. In that study the prevalence of PCV and RAP was found to be 16.8% and 11.4%,
respectively. In the Hatz and Prunte study, the prevalence of PCV was lower than our study, because of the difference the poor-response criterion. The authors described the poor-response
status as ‘need for frequent intravitreal injections during OCT-guided dosing’. However our criterion was persistent intraretinal and/or subretinal fluid 1 month after the last intravitreal
injection. In this study, we evaluated specifically the morphologically poor-responding patients. The functional poor responders were not included in the study, which was the most important
limitation. Also, in fact using a three-injection threshold for defining poor response may not be suitable because some of the patients may show late responses to anti-VEGF treatment.15
Indeed, ICGA is not used as a standard imaging technique in our country. The dye is off-label and the cost of dye is not covered by social insurance. Therefore, ICGA imaging cannot be
performed in all of the nAMD patients and is preserved for confusing cases such as the ones that were included in this study. Our policy is to start anti-VEGF treatment for all of nAMD
confirmed with FFA and OCT first, obtain ICGA in the event of poor or nonresponse, and in patients with a possible diagnosis of PCV, chronic CSC, or RAP. In this vein, we rather prefer being
more cautious about the cases who do not have a good response to three injections of anti-VEGFs and use a lower threshold for poor response in order not to delay the diagnosis of an
underlying mimicking disease. The retrospective study design was also a limitation and the treatment outcomes of the patients after the revised diagnosis were not reported. However, we are
reviewing the records of these patients regarding the follow-up period after the ICGA imaging and we have planned to conduct two separate studies in which we will evaluate the treatment
outcomes of misdiagnosed PCV and chronic CSC patients. On a positive side, this study was conducted in a single center and included a considerable number of nAMD patients. The imaging
evaluation was done by two experienced retina specialists and ICGA was obtained from all of the patients. In conclusion, we shown that ~90% of the morphologically ranibizumab poor-responding
patients would have been classified inappropriately without using ICGA. More importantly, 97% of these patients was diagnosed as having PCV, chronic CSC, and RAP and required a different
treatment modality such as photodynamic therapy. The real morphological poor-response rate was indeed around 1%. In the light of these findings we propose that all ranibizumab
poor-responding patients should undergo a detailed differential diagnostic work-up including ICGA before being considered as a ‘true poor responder’ and ICGA imaging might be one of the
important key diagnostic tools in the evaluation of anti-VEGF nonesponse. REFERENCES * Soubrane G, Coscas G, Français C, Koenig F . Occult subretinal new vessels in age-related macular
degeneration. Natural history and early laser treatment. _Ophthalmology_ 1990; 97: 649–657. Article CAS Google Scholar * Roy M, Kaiser-Kupfer M . Second eye involvement in age-related
macular degeneration: a four-year prospective study. _Eye (Lond)_ 1990; 4: 813–818. Article Google Scholar * Singerman LJ, Kalski RS . Tunable dye laser photocoagulation for choroidal
neovascularization complicating age-related macular degeneration. _Retina_ 1989; 9: 247–257. Article CAS Google Scholar * Tornambe PE, Poliner LS, Hovey LJ, Taren D . Scatter macular
photocoagulation for subfoveal neovascular membranes in age-related macular degeneration. A pilot study. _Retina_ 1992; 12: 305–314. Article CAS Google Scholar * Kapran Z, Ozkaya A, Uyar
OM . Hemorrhagic age-related macular degeneration managed with vitrectomy, subretinal injection of tissue plasminogen activator, gas tamponade, and upright positioning. _Ophthalmic Surg
Lasers Imaging Retina_ 2013; 44: 471–476. Article Google Scholar * Takeuchi K, Kachi S, Iwata E, Ishikawa K, Terasaki H . Visual function 5 years or more after macular translocation
surgery for myopic choroidal neovascularisation and age-related macular degeneration. _Eye (Lond)_ 2012; 26: 51–60. Article CAS Google Scholar * García-Finana M, Murjaneh S, Mahmood S,
Harding SP . Baseline clinical measures and early response predict success in verteporfin photodynamic therapy for neovascular age-related macular degeneration. _Eye (Lond)_ 2010; 24:
1213–1219. Article Google Scholar * Hogan AC, Kilmartin DJ . Low power vs standard power transpupillary thermotherapy in patients with age-related macular degeneration and subfoveal
choroidal neovascularization ineligible for photodynamic therapy. _Eye (Lond)_ 2006; 20: 649–654. Article CAS Google Scholar * Subramanian ML, Abedi G, Ness S, Ahmed E, Fenberg M, Daly MK
_et al_. Bevacizumab vs ranibizumab for age-related macular degeneration: 1-year outcomes of a prospective, double-masked randomised clinical trial. _Eye (Lond)_ 2010; 24: 1708–1715.
Article CAS Google Scholar * Ozkaya A, Alkin Z, Yazici AT, Demirok A . Comparison of intravitreal ranibizumab in phakic and pseudophakic neovascular age-related macular degeneration
patients with good baseline visual acuity. _Retina_ 2014; 34: 853–859. Article CAS Google Scholar * Brown DM, Kaiser PK, Michels M, Heier JS, Sy JP, Ianchulev T . ANCHOR Study Group.
Ranibizumab versus verteporfin for neovascular age-related macular degeneration. _N Engl J Med_ 2006; 355: 1432–1444. Article CAS Google Scholar * Grewal DS, Gill MK, Sarezky D, Lyon AT,
Mirza RG . Visual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month results. _Eye (Lond)_ 2014; 28:
895–899. Article CAS Google Scholar * Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY _et al_. MARINA Study Group. Ranibizumab for neovascular age-related macular
degeneration. _N Engl J Med_ 2006; 355: 1419–1431. Article CAS Google Scholar * CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL _et al_. Ranibizumab and
bevacizumab for neovascular age-related macular degeneration. _N Engl J Med_ 2011; 364: 1897–1908. Article Google Scholar * Amoaku WM, Chakravarthy U, Gale R, Gavin M, Ghanchi F, Gibson J
_et al_. Defining response to anti-VEGF therapies in neovascular AMD. _Eye (Lond)_ 2015; 29: 721–731. Article CAS Google Scholar * Manoj S . Why does anti VEGF treatment fail in age
related macular degeneration (AMD). _Kerala J Ophthalmol_ 2011; 3: 282–286. Google Scholar * Stangos AN, Gandhi JS, Nair-Sahni J, Heimann H, Pournaras CJ, Harding SP . Polypoidal choroidal
vasculopathy masquerading as neovascular age-related macular degeneration refractory to ranibizumab. _Am J Ophthalmol_ 2010; 150: 666–673. Article CAS Google Scholar * Ilginis T, Ottosen
S, Harbo Bundsgaard K, Uggerhøj Andersen C, Vorum H . Polypoidal choroidal vasculopathy in patients diagnosed with neovascular age-related macular degeneration in Denmark. _Acta Ophthalmol_
2012; 90: e487–e488. Article Google Scholar * Hatz K, Prünte C . Polypoidal choroidal vasculopathy in Caucasian patients with presumed neovascular age-related macular degeneration and poor
ranibizumab response. _Br J Ophthalmol_ 2014; 98: 188–194. Article Google Scholar * Fung AT, Yannuzzi LA, Freund KB . Type 1 (sub-retinal pigment epithelial) neovascularization in central
serous chorioretinopathy masquerading as neovascular age-related macular degeneration. _Retina_ 2012; 32: 1829–1837. Article Google Scholar * Lee JE, Kim HW, Lee SJ, Lee JE . Changes of
choroidal neovascularization in indocyanine green angiography after intravitreal ranibizumab injection. _Retina_ 2015; 35: 999–1006. Article CAS Google Scholar * Alkin Z, Ozkaya A, Agca
A, Yazici AT, Demirok A . Early visual and morphologic changes after half-fluence photodynamic therapy in chronic central serous chorioretinopathy. _J Ocul Pharmacol Ther_ 2014; 30: 359–365.
Article CAS Google Scholar * Grob S, Yonekawa Y, Eliott D . Multimodal imaging of adult-onset foveomacular vitelliform dystrophy. _Saudi J Ophthalmol_ 2014; 28: 104–110. Article Google
Scholar * Alexandre de Amorim Garcia Filho C, Yehoshua Z, Gregori G, Farah ME, Feuer W, Rosenfeld PJ . Spectral-domain optical coherence tomography imaging of drusenoid pigment epithelial
detachments. _Retina_ 2013; 33: 1558–1566. Article Google Scholar * Cazet-Supervielle A, Gozlan J, Cabasson S, Boissonnot M, Manic H, Leveziel N . Intravitreal ranibizumab in daily
clinical practice for age-related macular degeneration: treatment of exudative age-related macular degeneration in real life. _Ophthalmologica_ 2015; 234: 26–32. Article CAS Google Scholar
* Ying GS, Kim BJ, Maguire MG, Huang J, Daniel E, Jaffe GJ _et al_. CATT Research Group. Sustained visual acuity loss in the comparison of age-related macular degeneration treatment
trials. _JAMA Ophthalmol_ 2014; 132: 915–921. Article Google Scholar * Mrejen S, Sarraf D, Mukkamala SK, Freund KB . Multimodal imaging of pigment epithelial detachment: a guide to
evaluation. _Retina_ 2013; 33: 1735–1762. Article Google Scholar * Gomi F, Oshima Y, Mori R, Kano M, Saito M, Yamashita A _et al_. Initial versus delayed photodynamic therapy in
combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: the Fujisan Study. _Retina_ 2015; 35: 1569–1576. Article CAS Google Scholar * Engelbert M, Zweifel SA,
Freund KB . ‘‘Treat and extend’’ dosing of intravitreal endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. _Retina_ 2009; 29: 1424–1431.
Article Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Beyoglu Eye Training and Research Hospital, Istanbul, Turkey A Ozkaya, C Alagoz, R Garip, Z Alkin,
I Perente, A T Yazici & M Taskapili Authors * A Ozkaya View author publications You can also search for this author inPubMed Google Scholar * C Alagoz View author publications You can
also search for this author inPubMed Google Scholar * R Garip View author publications You can also search for this author inPubMed Google Scholar * Z Alkin View author publications You can
also search for this author inPubMed Google Scholar * I Perente View author publications You can also search for this author inPubMed Google Scholar * A T Yazici View author publications You
can also search for this author inPubMed Google Scholar * M Taskapili View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to A Ozkaya. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION AUTHOR CONTRIBUTIONS Involved in design and conduct of
the study: AO, CA, RG, ZA, IP, ATY, MT; preparation and review of the study: AO, IP, ATY, MT; data collection: AO, CA, RG, ATY; and statistical analysis: AO, ZA. RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ozkaya, A., Alagoz, C., Garip, R. _et al._ The role of indocyanine green angiography imaging in further differential diagnosis
of patients with nAMD who are morphologically poor responders to ranibizumab in a real-life setting. _Eye_ 30, 958–965 (2016). https://doi.org/10.1038/eye.2016.71 Download citation *
Received: 16 November 2015 * Accepted: 15 February 2016 * Published: 15 April 2016 * Issue Date: July 2016 * DOI: https://doi.org/10.1038/eye.2016.71 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative



