- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The recent finding that the neuronal cadherin gene _CDH2_ confers a highly significant risk for canine compulsive disorder led us to investigate whether missense variants within the
human ortholog _CDH2_ are associated with altered susceptibility to obsessive-compulsive disorder (OCD), Tourette disorder (TD) and related disorders. Exon resequencing of _CDH2_ in 320
individuals identified four non-synonymous single-nucleotide variants, which were subsequently genotyped in OCD probands, Tourette disorder probands and relatives, and healthy controls
(total _N_=1161). None of the four variants was significantly associated with either OCD or TD. One variant, N706S, was found only in the OCD/TD groups, but not in controls. By examining
clinical data, we found there were significant TD-related phenotype differences between those OCD probands with and without the N845S variant with regard to the co-occurrence of TD (Fisher’s
exact test _P_=0.014, OR=6.03). Both N706S and N845S variants conferred reduced CDH2 protein expression in transfected cells. Although our data provide no overall support for association of
CDH2 rare variants in these disorders considered as single entities, the clinical features and severity of probands carrying the uncommon non-synonymous variants suggest that _CDH2_, along
with other cadherin and cell adhesion genes, is an interesting gene to pursue as a plausible contributor to OCD, TD and related disorders with repetitive behaviors, including autism spectrum
disorders. SIMILAR CONTENT BEING VIEWED BY OTHERS A BURDEN OF RARE COPY NUMBER VARIANTS IN OBSESSIVE-COMPULSIVE DISORDER Article Open access 27 October 2024 EXOME SEQUENCING IN
OBSESSIVE–COMPULSIVE DISORDER REVEALS A BURDEN OF RARE DAMAGING CODING VARIANTS Article 28 June 2021 A DIMENSIONAL PERSPECTIVE ON THE GENETICS OF OBSESSIVE-COMPULSIVE DISORDER Article Open
access 21 July 2021 INTRODUCTION Obsessive-compulsive disorder (OCD) and Tourette disorder (TD) are chronic, severe neuropsychiatric disorders, commonly having an early age of onset and a
significant genetic component as shown by family, twin, segregation and linkage studies.1, 2, 3, 4, 5, 6 Compulsive, repetitive and tic/TD-like behaviors in rodent models have been
associated with variants in single genes such as _Sapap3_ and _Slitrk5_.7, 8, 9, 10 Recently, single-nucleotide polymorphisms (SNPs) within the canine neuronal cadherin gene (_CDH2_) were
shown to confer a significant risk for canine compulsive disorder (CCD).11 CCD shares many similarities with OCD: (a) both are characterized by repetitive, time-consuming behaviors that
cause distress and functional impairment; (b) both have at least partially genetic heritabilities; and (c) symptoms in both humans and dogs can be alleviated by behavioral therapy,
administration of antidepressants or a combination of both therapies.12, 13 _CDH2_ belongs to the cadherin gene family of cell–cell adhesion molecules, which function in early brain
morphogenesis, synaptogenesis and synaptic plasticity, including synaptic vesicle trafficking in glutamatergic neurons.14, 15, 16, 17 Other cadherin genes, including _CDH8_, _CDH9_ and
_CDH10_, have recently been implicated in the etiology of autism spectrum disorders, which may also be characterized by repetitive and compulsive behaviors.18, 19, 20 We hypothesized that
variants in the human ortholog _CDH2_ could confer susceptibility to OCD and OCD spectrum disorders such as TD. To test this, we exon-sequenced _CDH2_ to identify non-synonymous SNPs in a
sample of 160 healthy controls and 160 OCD probands from our National Institute of Mental Health (NIMH) Intramural Research Program’s Laboratory DNA collection,21 and subsequently performed
genotyping of identified putatively functional SNPs in a total of 1161 individuals, including OCD probands (_N_=260), TD probands and their relatives (_N_=454), and healthy controls
(_N_=447). METHODS Unrelated OCD probands (_N_=260) were evaluated with the Structured Clinical Interview for DSM-IV-TR (SCID), the Yale-Brown Obsessive Compulsive Scale (YBOCS) ratings and
other measures as described previously.21, 22 The mean±SD of total YBOCS scores was 22.4±0.5, and there were no subgroup differences for the different OCD subgroups considered (including the
OCD subgroups with identified _CDH2_ variants). TD probands and relatives (_N_=454) were evaluated by an experienced child psychiatrist based upon TD-related rating scales, as described
elsewhere.23 Unrelated healthy volunteers (_N_=447) consisted of undergraduate students from a large public university who participated in a separate study of genes and personality in return
for partial course credit; they were administered self-report scales for personality measures. Although the control group completed a battery of self-report questionnaires, we cannot
completely rule out the occurrence of OCD or TD as they did not complete a formal diagnostic interview. Additional details on proband and control samples have been described previously.22,
23, 24 All studies were conducted under protocols approved by the Institutional Review Board at the NIMH Intramural Research Program (OCD probands), the Rutgers University Institutional
Review Board (TD probands and relatives) and by the Human Subjects Committee at Florida State University (healthy controls). Written informed consent was obtained from all adult participants
(or, at Rutgers, their legal guardians, with written assent for minors). Genomic DNA was extracted from whole blood obtained through venipuncture or from saliva samples (Oragene discs; DNA
Genotek, Ottawa, ON, Canada). Exon sequencing was carried out in an initial subsample of 160 healthy controls and 160 OCD probands by the National Institutes of Health (NIH) Intramural
Sequencing Center (NISC) as described previously.25 These samples plus the remaining OCD probands, TD probands and relatives, and healthy controls were subsequently genotyped for the four
non-synonymous _CDH2_ variants identified by sequencing. Genotyping was performed using 5′-exonuclease TaqMan predesigned or custom assays under standard conditions: a total volume of 20
_μ_l and 20 ng of genomic DNA were amplified in the presence of 1 × PCR Master mix (Qiagen, Valencia, CA, USA) and 1 × TaqMan Assay (Applied Biosystems, Foster City, CA, USA; assay
identification numbers and primer/probe sequences, as well as sequencing primer sequences are available from the corresponding author). Thermocycling conditions were as follows: 95 °C × 10
min, followed by 50 cycles (95 °C × 10 s, 60 °C × 30 s, fluorescence reading). The overall genotyping completion rate exceeded 97% for each assay. None of the SNPs deviated from
Hardy–Weinberg equilibrium in OCD probands, TD probands and relatives, or controls as determined by contingency-table statistics (nominal _P_>0.05; data not shown). Duplicate samples (at
least 10% of all samples, randomly chosen for each of the four SNPs) and no-template controls consistently yielded expected results. Statistical analyses were performed using Fisher’s exact
test with significance set at _P_<0.05 in two-sided analyses. To begin to evaluate the functionality of the two _CDH2_ variants of greatest interest, site-directed mutagenesis was used to
generate the corresponding mutants for N706S and N845S using QuickChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) in the pCMV6-XL6 vector expressing
human CDH2 (Origene, Rockville, MD, USA). For the N706 variant, 5′-TCCAACGGG-3′ was mutated to 5′-TCCAGCGGG-3′; for the N845S variant, 5′-GACAATGAC-3′ was mutated to 5′-GACAGTGAC-3′.
Bidirectional DNA sequence analysis was performed to confirm the mutagenesis procedure, as well as to discard any off effects on other regions of the constructs. HEK293 cells were grown and
transfected under standard conditions. At 48 h after transfection, cells were harvested and protein extracts were obtained for western blot evaluations. Anti-N-cadherin was prepared by
immunization of rabbits with the extracellular domain of N-cadherin, expressed and secreted into the media by HEK293 cells. Western blots were analyzed using ImageJ (NIH, Bethesda, MD, USA).
RESULTS In the initial sample of healthy controls (_N_=160) and OCD probands (_N_=160), all 16 _CDH2_ exons (Ref Seq NM_001792.3) were successfully sequenced, except for the first exon,
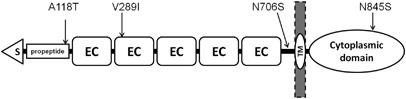
which could not be amplified despite several primer re-designs. Four non-synonymous SNPs, Ala118Thr (A118T), Val289Ile (V289I), Asn706Ser (N706S) and Asn845Ser (N845S), were identified in
_CDH2_, two of them (V289I and N706S) being novel variants (Table 1 and Figure 1). These four variants were chosen for follow-up genotyping in the ‘extended’ sample of OCD (_N_=260), TD
probands and relatives (_N_=454), and healthy controls (_N_=447). One of the novel variants, N706S, located between the extracellular domain EC5 and the transmembrane region of CDH2 (Figure
1), occurred in three individuals: an OCD proband; a TD proband; and a sibling of a different, unrelated TD proband. The latter individual had motor and phonic tics, but did not meet full TD
diagnostic criteria. Thus, N706S was found in 3/714 of the OCD/TD patients plus TD relatives sample and not in any of 447 controls; this difference was not statistically significant.
Interestingly, these three individuals had unusual clinical features, as summarized in the Supplementary Material. In particular, the OCD proband with the N706S variant had extremely severe
OCD (YBOCS rating of 32), rapid-cycling bipolar disorder as well as other distinctive features, including a family pedigree with multiple other neuropsychiatric problems, including
schizophrenia (Supplementary Figure 1). _In silico_ analysis using PMut predicted N706S as ‘pathologically relevant’.26 The other novel variant, V289I, located in the extracellular domain
EC2 of CDH2 (Figure 1), was found in three individuals: a single TD proband who also had attention deficit hyperactivity disorder (ADHD) and polysubstance abuse; a single, unrelated TD
proband who had OCD, ADHD and anorexia nervosa, and a single healthy control. No statistically significant differences were found for V289I. The frequency of the N845S variant, located in
the cytoplasmic domain of CDH2 (Figure 1), was generally similar across the OCD probands (4.6%), TD probands (5.6%) and healthy control (4.3%) populations (NS). We then compared the OCD/TD
probands with N845S to those without the variant. In the OCD subgroup (_N_=260) we found that of the 12 individuals (4.6%) with N845S, four (33.3%) had coexisting TD diagnoses; in contrast,
only 19 (7.7%) of those OCD probands without N845S (_N_=248) had comorbid TD (Fisher’s exact test _P_=0.014, OR=6.03). In considering the TD probands, 55% (5/9) of those with the N845S
variant had OCD. In comparison, only 41% (62/153) of TD probands without N845S had OCD; this was not statistically significant. The fourth variant, A118T, located in the propeptide region
(Figure 1), was found in 10.4% of OCD probands, 6.1% of TD probands and 7.6% of controls (NS). This variant was not associated with any SCID-assessed diagnostic group. In the total sample of
OCD probands, TD probands and relatives, as well as the healthy control group, there were no other differences in other comorbid disorders or demographic variables between those with or
without the four _CDH2_ variants. Overall, among the TD probands, comorbid OCD or ADHD was diagnosed in 41% or 48%, respectively. Among the TD relatives, rates of TD, OCD or ADHD were 14%,
21% or 12%, respectively. Among the OCD probands, TD was present in 12% of the sample overall. (ADHD was not diagnostically evaluated in the OCD probands as it is not a component of the SCID
adult evaluation.) In the initial evaluations of the impact of the variants on N-cadherin functionality/activity, both N706S and N845S variants showed consistently, markedly reduced protein
levels compared with wild-type CDH2 when transfected in HEK293 cells (47% and 42%, respectively), as shown in Figure 2. DISCUSSION This is the first report on the exon sequencing of the
neuronal cadherin gene _CDH2_, encoding N-cadherin in a large human sample and, to our knowledge, the first study of _CDH2_ in any human disorder, other than _in vitro_ studies of human
cancer cells. Sequencing of _CDH2_ confirmed the relatively low heterozygosity of two known missense variants, A118T and N845S. Further, we also identified two novel missense SNPs: N706S,
located between the fifth extracellular domain and the transmembrane domain, and V289I within the second extracellular domain (Figure 1a). None of the four missense variants was
significantly associated with OCD or TD diagnoses _per se_. Of interest, N706S occurred only in two unrelated OCD and TD probands and an unrelated TD proband’s sibling (with motor and phonic
tics) and not in any of 447 controls, while N845S appears to be associated with OCD/TD-related subgroups. Cadherins constitute a superfamily of adhesion molecules featuring an N-terminal
tandem series of ectodomains, followed by a single anchoring transmembrane domain and a C-terminal cytoplasmic region (∼150 amino acids) that links cadherins to the underlying cytoskeleton.
In the case of CDH2/N-cadherin, this is via sequential binding of _β_-catenin to _α_-catenin and then through intermediates to actin.15, 27, 28 N-cadherin is required for critical brain
processes, including long-term potentiation, pre- to post-synaptic adhesion, dendritic spine elongation – thereby regulating glutamate receptor trafficking and neuronal migration.29, 30, 31,
32 A bioinformatic prediction of the multiple functional associations for _CDH2_ is provided in Supplementary Figure 2.33 The CDH2 N845S variant lies in the highly conserved cytoplasmic
domain. Loss of integrity of this domain leads to loss of adhesive function.34, 35, 36, 37, 38, 39, 40 N845 is located in the ‘interaction region 2’ of the extended region through which
N-cadherin interacts with _β_-catenin.41 D846 forms a hydrogen bond with Y654 of _β_-catenin. Phosphorylation of Y654 by Src and other cytoplasmic kinases reduces the association of
cadherins with _β_-catenin, resulting in dissociation of the cadherin-catenin complex. Thus, the N845S mutation in N-cadherin appears well placed to modulate cadherin–_β_-catenin
interactions. However, there have been no site-directed mutagenesis studies before our initial study presented here, suggesting that a restricted amino-acid change resulting from the N845S
variant might result in impaired N-cadherin expression and/or stability. The N706S variant lies in the short region of CDH2 connecting the extracellular domains of CDH2 to the transmembrane
segment (Figure 1). Our initial mutagenesis study presented here indicates that the N706S variant reduces CDH2 expression and/or stability. This variant lies very close to the proposed
cleavage site of CDH2 by metalloproteinase ADAM10 (residues 714–715). The proteolytic cleavage by ADAM10 – as well as by PS1/_γ_-secretase – is critically important for the roles of CDH2 in
cell adhesion and cell signaling.42, 43 In addition, a prior study showed that induced single amino-acid changes that disrupted self-assembly of the transmembrame region reduced E-cadherin
cell–cell adhesiveness.41 Thus, N706S, found in one OCD proband, an unrelated TD proband, and an unrelated TD proband’s sibling with chronic tics, and in none of the 447 healthy controls in
this study, may represent a very rare variant related to the complex OCD, TD as well as perhaps bipolar and other neuropsychiatric disorder phenotypes found in these individuals and at least
one of their relatives. This finding is relevant in the case of TD, where bilineal transmission has been reported.6 Despite its lack of association, N706S seems an interesting variant to be
followed up in larger cohorts. The V289I variant lies in the EC2 ectodomain. Although EC1 has been documented to be critical to the adhesional/appositional functions of cadherins across
cell–cell connections such as synapses, less seems to be known about the functional role of EC2. As noted above, other members of the cadherin gene families have recently been found to be
associated with autism spectrum disorders, in which repetitive behaviors are frequently observed.18, 19, 20, 44, 45 Protocadherins and other cadherins have also been studied as candidate
risk genes, but generally in small samples (<100 patients) of schizophrenia, bipolar disorder and OCD patients.46, 47, 48, 49, 50, 51, 52, 53, 54, 55 In addition, some cadherins have been
specifically identified in genome-wide association scans of ADHD, addiction and neuroticism personality features.56, 57, 58 Of related interest, variants in _CDH2_ and other cadherins have
been widely found to be associated with various cancers16, 59, 60 and, specifically, upregulated _CDH2_ has been associated with transepithelial spreading of melanoma and pancreatic cancer
together with rapid recurrence of cancer.61, 62, 63 In summary, although _CDH2_ is an attractive candidate gene based on the CCD study findings,11 the present results suggest that these
_CDH2_ variants are not disease-causing by themselves. Further studies are needed to clarify if N706S and N845S, identified in OCD and TD subgroups, may or may not be risk factors of
interest in OCD/TD when investigated in larger cohorts. Also, future experiments are underway to better characterize the impact of N706S and N845S on N-cadherin functionality. There are
several limitations to this study. Our strategy was directed exclusively toward non-synonymous variants in _CDH2_, as they provide a ‘fast-track’ for functional characterization and
interpretation of findings. However, genetic variation leading to a disorder might not necessarily be located in protein coding regions; it is known that very distant regulatory elements
affecting gene expression can have a role in the etiology of disorders. Importantly, an overwhelming majority of human genetic variation comes from non-coding variants.64 The relatively
small sample size available for examination of the very rare _CDH2_ variants (N706S and V289I) and for N845S in OCD and TD subphenotypes calls for major replication studies before drawing
conclusions. Also, the healthy controls provided only allele frequencies and not complete phenotypic information. Finally, gene–gene and gene–environment interactions are relevant to
accurate genotype–phenotype associations. In particular, there is some evidence for environmental contributions to OCD onset, OCD severity and other features of OCD, including possible
contributions from psychological trauma, head trauma and autoimmune reactions.65 Further research using larger numbers of samples from rigorously phenotyped affected and control individuals
will be helpful to evaluate the validity of increased risk for compulsive and related disorders conferred by _CDH2_ and other cadherins in neuropsychiatric disorders. REFERENCES * Hettema
JM, Neale MC, Kendler KS : A review and meta-analysis of the genetic epidemiology of anxiety disorders. _Am J Psychiatry_ 2001; 158: 1568–1578. Article CAS Google Scholar * Pauls DL : The
genetics of obsessive-compulsive disorder: a review. _Dialogues Clin Neurosci_ 2010; 12: 149–163. PubMed PubMed Central Google Scholar * Rasmussen SA, Tsuang MT : The epidemiology of
obsessive compulsive disorder. _J Clin Psychiatry_ 1984; 45: 450–457. CAS PubMed Google Scholar * Worbe Y, Mallet L, Golmard JL _et al_: Repetitive behaviours in patients with Gilles de
la Tourette syndrome: tics, compulsions, or both? _PLoS One_ 2010; 5: e12959. Article Google Scholar * Felling RJ, Singer HS : Neurobiology of tourette syndrome: current status and need
for further investigation. _J Neurosci_ 2011; 31: 12387–12395. Article CAS Google Scholar * Mathews CA, Grados MA : Familiality of Tourette syndrome, obsessive-compulsive disorder, and
attention-deficit/hyperactivity disorder: heritability analysis in a large sib-pair sample. _J Am Acad Child Adolesc Psychiatry_ 2011; 50: 46–54. Article Google Scholar * Shmelkov SV,
Hormigo A, Jing D _et al_: Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. _Nat Med_ 2010; 16: 598–602, 591p following 602.
Article CAS Google Scholar * Welch JM, Lu J, Rodriguiz RM _et al_: Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. _Nature_ 2007; 448: 894–900. Article
CAS Google Scholar * Taylor JL, Rajbhandari AK, Berridge KC, Aldridge JW : Dopamine receptor modulation of repetitive grooming actions in the rat: potential relevance for Tourette
syndrome. _Brain Res_ 2011; 1322: 92–101. Article Google Scholar * Swerdlow NR, Sutherland AN : Preclinical models relevant to Tourette syndrome. _Adv Neurol_ 2006; 99: 69–88. PubMed
Google Scholar * Dodman NH, Karlsson EK, Moon-Fanelli A _et al_: A canine chromosome 7 locus confers compulsive disorder susceptibility. _Mol Psychiatry_ 2010; 15: 8–10. Article CAS
Google Scholar * Overall KL, Dunham AE : Clinical features and outcome in dogs and cats with obsessive-compulsive disorder: 126 cases (1989–2000). _J Am Vet Med Assoc_ 2002; 221: 1445–1452.
Article Google Scholar * Moon-Fanelli AA, Dodman NH, Famula TR, Cottam N : Characteristics of compulsive tail chasing and associated risk factors in Bull Terriers. _J Am Vet Med Assoc_
2011; 238: 883–889. Article Google Scholar * Goodwin M, Yap AS : Classical cadherin adhesion molecules: coordinating cell adhesion, signaling and the cytoskeleton. _J Mol Histol_ 2004; 35:
839–844. Article CAS Google Scholar * Shapiro L, Love J, Colman DR : Adhesion molecules in the nervous system: structural insights into function and diversity. _Annu Rev Neurosci_ 2007;
30: 451–474. Article CAS Google Scholar * Suriano G, Seixas S, Rocha J, Seruca R : A model to infer the pathogenic significance of CDH1 germline missense variants. _J Mol Med_ 2006; 84:
1023–1031. Article CAS Google Scholar * Reichardt LF : N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. _Neuron_ 2008; 60:
398–399. Article Google Scholar * Kroisel PM, Windpassinger C, Wagner K _et al_: De novo translocation t(5;18)(q33.1;q12.1) associated with autistic disorder. _Am J Med Genet A_ 2004;
129A: 98–100. Article Google Scholar * Pagnamenta AT, Khan H, Walker S _et al_: Rare familial 16q21 microdeletions under a linkage peak implicate cadherin 8 (CDH8) in susceptibility to
autism and learning disability. _J Med Genet_ 2010. * Wang K, Zhang H, Ma D _et al_: Common genetic variants on 5p14.1 associate with autism spectrum disorders. _Nature_ 2009; 459: 528–533.
Article CAS Google Scholar * Wendland JR, DeGuzman TB, McMahon F, Rudnick G, Detera-Wadleigh SD, Murphy DL : SERT Ileu425Val in autism, Asperger syndrome and obsessive-compulsive
disorder. _Psychiatr Genet_ 2008; 18: 31–39. Article Google Scholar * Wendland JR, Kruse MR, Cromer KR, Murphy DL : A large case–control study of common functional SLC6A4 and BDNF variants
in obsessive-compulsive disorder. _Neuropsychopharmacology_ 2007; 32: 2543–2551. Article CAS Google Scholar * Heiman GA, King RA, Tischfield JA : New Jersey Center for Tourette Syndrome
sharing repository: methods and sample description. _BMC Med Genomics_ 2008; 1: 58. Article Google Scholar * Wendland JR, Kruse MR, Murphy DL : Functional SLITRK1 var321, varCDfs and
SLC6A4 G56A variants and susceptibility to obsessive-compulsive disorder. _Mol Psychiatry_ 2006; 11: 802–804. Article CAS Google Scholar * Biesecker LG, Mullikin JC, Facio FM _et al_: The
ClinSeq Project: piloting large-scale genome sequencing for research in genomic medicine. _Genome Res_ 2009; 19: 1665–1674. Article CAS Google Scholar * Ferrer-Costa C, Gelpi JL,
Zamakola L, Parraga I, de la Cruz X, Orozco M : PMUT: a web-based tool for the annotation of pathological mutations on proteins. _Bioinformatics_ 2005; 21: 3176–3178. Article CAS Google
Scholar * Kobielak A, Fuchs E : Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. _Nat Rev Mol Cell Biol_ 2004; 5: 614–625. Article CAS Google Scholar *
Shapiro L, Weis WI : Structure and biochemistry of cadherins and catenins. _Cold Spring Harb Perspect Biol_ 2009; 1: a003053. Article Google Scholar * Bozdagi O, Wang XB, Nikitczuk JS _et
al_: Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. _J Neurosci_ 2010; 30: 9984–9989. Article CAS
Google Scholar * Kawauchi T, Sekine K, Shikanai M _et al_: Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. _Neuron_
2010; 67: 588–602. Article CAS Google Scholar * Tanaka H, Shan W, Phillips GR _et al_: Molecular modification of N-cadherin in response to synaptic activity. _Neuron_ 2000; 25: 93–107.
Article CAS Google Scholar * Nuriya M, Huganir RL : Regulation of AMPA receptor trafficking by N-cadherin. _J Neurochem_ 2006; 97: 652–661. Article CAS Google Scholar * Jensen LJ, Kuhn
M, Stark M _et al_: STRING 8 – a global view on proteins and their functional interactions in 630 organisms. _Nucleic Acids Res_ 2009; 37: D412–D416. Article CAS Google Scholar * Hirano
S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M : Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization. _Cell_ 1992; 70: 293–301.
Article CAS Google Scholar * Nagafuchi A, Takeichi M : Cell binding function of E-cadherin is regulated by the cytoplasmic domain. _EMBO J_ 1988; 7: 3679–3684. Article CAS Google
Scholar * Oyama T, Kanai Y, Ochiai A _et al_: A truncated beta-catenin disrupts the interaction between E-cadherin and alpha-catenin: a cause of loss of intercellular adhesiveness in human
cancer cell lines. _Cancer Res_ 1994; 54: 6282–6287. CAS PubMed Google Scholar * Ozawa M, Kemler R : Altered cell adhesion activity by pervanadate due to the dissociation of alpha-catenin
from the E-cadherin.catenin complex. _J Biol Chem_ 1998; 273: 6166–6170. Article CAS Google Scholar * Shimoyama Y, Nagafuchi A, Fujita S _et al_: Cadherin dysfunction in a human cancer
cell line: possible involvement of loss of alpha-catenin expression in reduced cell–cell adhesiveness. _Cancer Res_ 1992; 52: 5770–5774. CAS PubMed Google Scholar * Watabe M, Nagafuchi A,
Tsukita S, Takeichi M : Induction of polarized cell–cell association and retardation of growth by activation of the E-cadherin–catenin adhesion system in a dispersed carcinoma line. _J Cell
Biol_ 1994; 127: 247–256. Article CAS Google Scholar * Takeichi M : The cadherins: cell–cell adhesion molecules controlling animal morphogenesis. _Development_ 1988; 102: 639–655. CAS
PubMed Google Scholar * Huber O, Kemler R, Langosch D : Mutations affecting transmembrane segment interactions impair adhesiveness of E-cadherin. _J Cell Sci_ 1999; 112 (Part 23):
4415–4423. CAS PubMed Google Scholar * Reiss K, Maretzky T, Ludwig A _et al_: ADAM10 cleavage of N-cadherin and regulation of cell–cell adhesion and beta-catenin nuclear signalling. _EMBO
J_ 2005; 24: 742–752. Article CAS Google Scholar * Uemura K, Kihara T, Kuzuya A _et al_: Characterization of sequential N-cadherin cleavage by ADAM10 and PS1. _Neurosci Lett_ 2006; 402:
278–283. Article CAS Google Scholar * Dibbens LM, Tarpey PS, Hynes K _et al_: X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. _Nat Genet_ 2008;
40: 776–781. Article CAS Google Scholar * Morrow EM, Yoo SY, Flavell SW _et al_: Identifying autism loci and genes by tracing recent shared ancestry. _Science_ 2008; 321: 218–223.
Article CAS Google Scholar * Soronen P, Ollila HM, Antila M _et al_: Replication of GWAS of bipolar disorder: association of SNPs near CDH7 with bipolar disorder and visual processing.
_Mol Psychiatry_ 2010; 15: 4–6. Article CAS Google Scholar * Durand CM, Kappeler C, Betancur C _et al_: Expression and genetic variability of PCDH11Y, a gene specific to _Homo sapiens_
and candidate for susceptibility to psychiatric disorders. _Am J Med Genet B_ 2006; 141B: 67–70. Article CAS Google Scholar * Giouzeli M, Williams NA, Lonie LJ, DeLisi LE, Crow TJ :
ProtocadherinX/Y, a candidate gene-pair for schizophrenia and schizoaffective disorder: a DHPLC investigation of genomic sequence. _Am J Med Genet B_ 2004; 129B: 1–9. Article Google Scholar
* Lachman HM, Petroulo OA, Pedrosa E, Novak T, Nolan K, Stopkova P : Analysis of protocadherin alpha gene deletion variant in bipolar disorder and schizophrenia. _Psychiatr Genet_ 2008;
18: 110–115. Article Google Scholar * Pedrosa E, Stefanescu R, Margolis B _et al_: Analysis of protocadherin alpha gene enhancer polymorphism in bipolar disorder and schizophrenia.
_Schizophr Res_ 2008; 102: 210–219. Article Google Scholar * Vincent JB, Noor A, Windpassinger C _et al_: Characterization of a de novo translocation t(5;18)(q33.1;q12.1) in an autistic
boy identifies a breakpoint close to SH3TC2, ADRB2, and HTR4 on 5q, and within the desmocollin gene cluster on 18q. _Am J Med Genet B_ 2009; 150B: 817–826. Article Google Scholar * Cherlyn
SY, Woon PS, Liu JJ, Ong WY, Tsai GC, Sim K : Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: a decade of advance. _Neurosci Biobehav
Rev_ 2010; 34: 958–977. Article CAS Google Scholar * Ivleva EI, Morris DW, Moates AF, Suppes T, Thaker GK, Tamminga CA : Genetics and intermediate phenotypes of the schizophrenia –
bipolar disorder boundary. _Neurosci Biobehav Rev_ 2010; 34: 897–921. Article CAS Google Scholar * Johnson C, Drgon T, McMahon FJ, Uhl GR : Convergent genome wide association results for
bipolar disorder and substance dependence. _Am J Med Genet B_ 2009; 150B: 182–190. Article Google Scholar * Maier W : Common risk genes for affective and schizophrenic psychoses. _Eur Arch
Psychiatry Clin Neurosci_ 2008; 258 (Suppl 2): 37–40. Article Google Scholar * Lasky-Su J, Neale BM, Franke B _et al_: Genome-wide association scan of quantitative traits for attention
deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. _Am J Med Genet B_ 2008; 147B: 1345–1354. Article CAS Google Scholar * Terracciano
A, Sanna S, Uda M _et al_: Genome-wide association scan for five major dimensions of personality. _Mol Psychiatry_ 2010; 15: 647–656. Article CAS Google Scholar * Redies C, Hertel N,
Hubner CA : Cadherins and neuropsychiatric disorders. _Brain Res_ 2012; 1470: 130–144. Article CAS Google Scholar * Berx G, van Roy F : Involvement of members of the cadherin superfamily
in cancer. _Cold Spring Harb Perspect Biol_ 2009; 1: a003129. Article Google Scholar * Guilford P, Humar B, Blair V : Hereditary diffuse gastric cancer: translation of CDH1 germline
mutations into clinical practice. _Gastric Cancer_ 2010; 13: 1–10. Article CAS Google Scholar * Liu ZJ, Xiao M, Balint K _et al_: Notch1 signaling promotes primary melanoma progression by
activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. _Cancer Res_ 2006; 66: 4182–4190. Article CAS Google
Scholar * Qi J, Chen N, Wang J, Siu CH : Transendothelial migration of melanoma cells involves N-cadherin-mediated adhesion and activation of the beta-catenin signaling pathway. _Mol Biol
Cell_ 2005; 16: 4386–4397. Article CAS Google Scholar * Nakajima S, Doi R, Toyoda E _et al_: N-cadherin expression and epithelial–mesenchymal transition in pancreatic carcinoma. _Clin
Cancer Res_ 2004; 10: 4125–4133. Article CAS Google Scholar * Altshuler D, Durbin RM, Abecasis GR _et al_: A map of human genome variation from population-scale sequencing. _Nature_ 2010;
467: 1061–1073. Article Google Scholar * Murphy DL, Moya PR, Wendland JR, Timpano KR : _Genetic Contributions to Obsessive-Compulsive Disorder (OCD) and OCD-Related Disorders_. Cambridge,
UK: Cambridge University Press, 2012. Book Google Scholar Download references ACKNOWLEDGEMENTS We are indebted to Diane Kazuba and Brenda Justement for conducting proband interviews, to
Teresa Tolliver and Su-Jan Huang for excellent technical assistance in DNA extraction and general lab assistance and to Theresa DeGuzman for development of the OCD phenotype database and
editing and graphical assistance with this manuscript. GA Heiman, RA King and JA Tischfield are supported by grants from the New Jersey Center for Tourette Syndrome and Associated Disorders
and NIMH (R01MH092293). Support for the experimental studies was from the NIMH Intramural Research Program and a grant from the Simons Foundation (LF Reichardt). AUTHOR INFORMATION Author
notes * Pablo R Moya and Nicholas H Dodman: Shared first authorship. * Edward I Ginns and Jens R Wendland: Shared senior authorship. AUTHORS AND AFFILIATIONS * Laboratory of Clinical
Science, NIMH-Intramural Research Program, Bethesda, MD, USA Pablo R Moya, Liza M Rubenstein, Zaker Rana & Ruby L Fried * Cummings School of Veterinary Medicine, Tufts University, North
Grafton, MA, USA Nicholas H Dodman * University of Miami, Coral Gables, FL, USA Kiara R Timpano * Department of Physiology, University of California, San Francisco, CA, USA Louis F Reichardt
* Human Genetics Institute of New Jersey and Department of Genetics, Rutgers University, Piscataway, NJ, USA Gary A Heiman & Jay A Tischfield * Child Study Center of Yale University,
New Haven, CT, USA Robert A King * Molecular Diagnostics Laboratory and Clinical Labs, University of Massachusetts Medical School/UMass Memorial Medical Center, Worcester, MA, USA Marzena
Galdzicka & Edward I Ginns * Pharma Research and Early Development, F Hoffman-La Roche Ltd., Basel, Switzerland Jens R Wendland Authors * Pablo R Moya View author publications You can
also search for this author inPubMed Google Scholar * Nicholas H Dodman View author publications You can also search for this author inPubMed Google Scholar * Kiara R Timpano View author
publications You can also search for this author inPubMed Google Scholar * Liza M Rubenstein View author publications You can also search for this author inPubMed Google Scholar * Zaker Rana
View author publications You can also search for this author inPubMed Google Scholar * Ruby L Fried View author publications You can also search for this author inPubMed Google Scholar *
Louis F Reichardt View author publications You can also search for this author inPubMed Google Scholar * Gary A Heiman View author publications You can also search for this author inPubMed
Google Scholar * Jay A Tischfield View author publications You can also search for this author inPubMed Google Scholar * Robert A King View author publications You can also search for this
author inPubMed Google Scholar * Marzena Galdzicka View author publications You can also search for this author inPubMed Google Scholar * Edward I Ginns View author publications You can also
search for this author inPubMed Google Scholar * Jens R Wendland View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to
Pablo R Moya. ETHICS DECLARATIONS COMPETING INTERESTS JRW is a Senior Principal Scientist, Pharma Research and Early Development at F Hoffmann-La Roche Ltd. None of the other authors has
anything to disclose. ADDITIONAL INFORMATION Supplementary Information accompanies the paper on European Journal of Human Genetics website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1
(PPT 152 KB) SUPPLEMENTARY FIGURE 2 (PPT 507 KB) SUPPLEMENTARY INFORMATION (DOC 55 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Moya, P., Dodman,
N., Timpano, K. _et al._ Rare missense neuronal cadherin gene (_CDH2_) variants in specific obsessive-compulsive disorder and Tourette disorder phenotypes. _Eur J Hum Genet_ 21, 850–854
(2013). https://doi.org/10.1038/ejhg.2012.245 Download citation * Received: 07 May 2012 * Revised: 01 October 2012 * Accepted: 11 October 2012 * Published: 16 January 2013 * Issue Date:
August 2013 * DOI: https://doi.org/10.1038/ejhg.2012.245 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * N-cadherin * _CDH2_ * rare gene variants *
canine compulsive disorder * obsessive-compulsive disorder * Tourette disorder