- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Although mutations in mitochondrial tRNAs constitute the most common mtDNA defect, the presence of pathological variants in mitochondrial tRNAAsn is extremely rare. We were able to
identify a novel mtDNA _tRNA__Asn_ gene pathogenic mutation associated with a myopathic phenotype and a previously unreported respiratory impairment. Our proband is an adult woman with
ophthalmoparesis and respiratory impairment. Her muscle biopsy presented several cytochrome _c_ oxidase-negative (COX−) fibres and signs of mitochondrial proliferation (ragged red fibres).
Sequence analysis of the muscle-derived mtDNA revealed an m.5709T>C substitution, affecting mitochondrial _tRNA__Asn_ gene. Restriction-fragment length polymorphism analysis of the
mutation in isolated muscle fibres showed that a threshold of at least 91.9% mutated mtDNA results in the COX deficiency phenotype. The new phenotype further increases the clinical spectrum
of mitochondrial diseases caused by mutations in the _tRNA__Asn_ gene. SIMILAR CONTENT BEING VIEWED BY OTHERS IDENTIFICATION OF TWO NOVEL _RRM2B_ VARIANTS ASSOCIATED WITH AUTOSOMAL RECESSIVE
PROGRESSIVE EXTERNAL OPHTHALMOPLEGIA IN A FAMILY WITH PSEUDODOMINANT INHERITANCE PATTERN Article 23 March 2023 CHARACTERISATION OF A NOVEL _OPA1_ SPLICE VARIANT RESULTING IN CRYPTIC SPLICE
SITE ACTIVATION AND MITOCHONDRIAL DYSFUNCTION Article Open access 09 May 2022 PATHOGENIC DEEP INTRONIC _MTM1_ VARIANT ACTIVATES A PSEUDO-EXON ENCODING A NONSENSE CODON RESULTING IN SEVERE
X-LINKED MYOTUBULAR MYOPATHY Article 29 August 2020 INTRODUCTION Many mitochondrial disorders are associated with mutations in mitochondrial tRNAs. To date, over 200 point mutations
affecting mitochondrial _tRNA_ genes have been described.1 Nevertheless, mutations in the _tRNA__Asn_ gene are very rare; until now, only five pathogenic variants have been associated with
clinical phenotypes ranging from chronic external ophthalmoplegia (cPEO), with or without mitochondrial myopathy, to lethal early-onset encephalomyopathy.2 Here we present clinical and
molecular features of a patient with ophthalmoparesis and respiratory impairment associated with a novel heteroplasmic mutation (m.5709T>C) disclosed in the mitochondrial _tRNA__Asn_
gene. CASE REPORT The proband is a 51-year-old woman with a several year history of progressive external ophthalmoparesis (PEO) and bilateral eyelid ptosis, which was surgically corrected at
age 29, but which gradually worsened again in the following years. At age 47 years, she was admitted to hospital for subacute onset of a severe respiratory insufficiency and she was
diagnosed with a restrictive syndrome with indication for non-invasive ventilation (B-PAP) during the night. Her clinical history is complicated by hypothyroidism (she is taking replacement
therapy) and anxious–depressive syndrome. Neurological examination showed bilateral eyelid ptosis and bilateral, both vertical and horizontal, ophthalmoparesis without diplopia. She had mild
axial and proximal upper limb weakness (bilateral sternocleidomastoid and deltoid muscles) with brisk tendon reflexes and no sensitive alterations. Neither cerebellar nor gait dysfunction
were observed. Blood tests were normal, except for mildly elevated CK (between 290 and 450 U/L, nv <185 U/L), LDH (872 U/L with nv 125–243 U/L) and basal lactate (3.9 mmol/l venous, with
nv 0.90–1.70 mmol/l and 3.1 mmol/l arterial with nv 0.36–1.25 mmol/l). EMG examination disclosed mild non-specific abnormalities in both deltoid and quadriceps muscles. Cardiological
evaluation and tests were normal. Respiratory functional tests confirmed a chronic respiratory insufficiency with restrictive syndrome. Her younger sister, aged 42 years, has mild mental
retardation and unspecified psychiatric disorders, but no eyelid ptosis or ophthalmoparesis. Her serum CK levels are normal. Both the mother and the maternal grandmother are reported
affected with sarcoidosis and ophthalmoparesis (no clinical reports available). The father is healthy and neither the proband nor the sister has children. At age 49 years, the patient
underwent left deltoid muscle biopsy, which was consistent with a mitochondrial disorder. MATERIALS AND METHODS Histological and histochemical analysis of the muscle biopsy, Southern blot
analysis of muscle mtDNA and PCR assay for multiple deletions were performed as described.3, 4, 5 Fragments encompassing the 22 _tRNA_ genes were PCR-amplified, using a set of primers
contained in the MitoSEQ Resequencing System (Applied Biosystem, Foster City, CA, USA) and sequenced on an Applied Biosystem 3100 Genetic Analyzer. A 750-bp fragment encompassing
m.5709T>C mutation was PCR-amplified. The mutated nucleotide C introduces a BstXI-restriction site. BstXI cuts the mutated molecules into two fragments of 500 and 250 bp, whereas the
wild-type PCR fragment remains uncut. Single fibres were microdissected from 30-μm thick muscle sections using the Leica Laser Microdissection Microscope ASLMD (Leica Microsystems, Wetzlar,
Germany) and were processed as described.6 Mutational load assessment in single fibres, muscle biopsy and blood was performed by last-cycle hot PCR7 followed by restriction-fragment length
polymorphism (RFLP) analysis. Aliquots (20 _μ_l) of PCR products were digested and electrophoresed in 5% non-denaturing acrylamide gel. The proportion of mutant mtDNA was evaluated by
densitometry using the NIH ImageJ software (http://rsbweb.nih.gov/ij/). RESULTS MORPHOLOGICAL AND HISTOCHEMICAL INVESTIGATIONS Left deltoid muscle biopsy showed well-preserved muscle
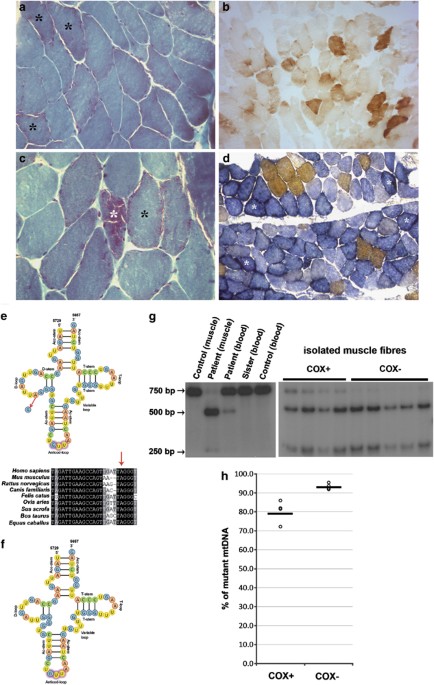
architecture with evidence of ragged red fibres (RRFs; Figures 1a and c). Histochemical analysis revealed several cytochrome _c_ oxidase (COX)-negative fibres (Figure 1b), many of them with
increased SDH activity (Figure 1d) indicating mitochondrial proliferation. MOLECULAR GENETIC ANALYSIS Multiple deletions of mtDNA were ruled out by Southern blot and PCR analyses. Direct
sequencing of PCR-amplified fragments encompassing the 22 _tRNA_ genes of muscle mtDNA revealed a T-to-C transition at nucleotide position 5709. This substitution results in the replacement
of a well-conserved A with a G in the cloverleaf secondary structure of the _tRNA__Asn_ gene, whose transcription proceeds on the opposite strand (Figure 1e). The A-to-G transition seems to
result in a shift in the nucleotides involved in the D-stem formation, leading to an impaired D-stem and loop structure (Figure 1f). The mutation was not found in 100 Caucasian controls.
PCR–RFLP analysis showed that the mutation was heteroplasmic in skeletal muscle and in white blood cells, percentage of mutated genomes being 89.0% and 21.7%, respectively. The mutation was
not detected in blood from the proband's sister (Figure 1g). Single fibre analysis showed a higher degree of heteroplasmy in COX-deficient fibres (93.1±1.5%, _n_=5) compared with
COX-positive muscle fibres (78.6±5.5%, _n_=4, Figures 1g and h). The difference between COX-positive and COX-negative fibres was statistically significant (_P_<0.01). DISCUSSION More than
half of the pathogenic mtDNA mutations are concentrated in _tRNA_ genes.2 So far, five pathogenic point mutations within _tRNA__Asn_ gene have been described in seven probands: m.5703G>A
associated with bilateral ptosis, cPEO and mitochondrial myopathy,8, 9 m.5692T>C associated with cPEO,10 m.5698G>A causing an isolated myopathy with cPEO in two sporadic patients,11,
12 m.5693T>C associated with a lethal early-onset encephalomyopathy13 and m.5728T>C leading to severe multiorgan failure.14 Here we report the novel substitution m.5709T>C in
muscle-derived mtDNA from a woman affected with ophthalmoparesis and respiratory impairment. Our study meets most of the consensus criteria used to assign pathogenicity to mitochondrial tRNA
variants:15 (i) m.5709T>C was found in heteroplasmic state; (ii) the proportion of mutated mtDNA was higher in the affected tissue (skeletal muscle) than in an unaffected tissue with
rapid turnover (blood); (iii) the mutation occurs at an evolutionary conserved site in the mitochondrial genome and it seems to cause a significant structural impairment in the tRNAAsn
D-stem and loop; (iv) m.5709T>C was absent in a cohort of ethnically matched healthy controls; (v) mutation load was significantly higher in isolated COX-negative fibres than in
COX-positive single muscle fibres. We could not prove the matrilineal transmission, suggested by both the kind of mutation and the family history, as maternal mtDNA was unavailable.
Muscle-derived mtDNA from the sister was not available to ascertain the presence of the mutation and, however, she does not have a myopathic phenotype. Because we did not detect mutated
molecules in her leukocytes, we are currently unable to say whether the sister's clinical condition, mainly affecting the central nervous system, is another possible manifestation of
the mutation-related clinical picture. The small number of tRNAAsn mutations reported so far makes a genotype–phenotype correlation a hazardous task. Heterogeneous clinical presentations can
be divided into two groups: an early onset syndromic disease leading to lethal mitochondrial encephalomyopathy13 or multi-organ failure,14 and an adult onset phenotype characterized by
cPEO, myopathy and a neurological syndrome resembling MERRF (myoclonus epilepsy and RRF).8, 9, 10, 11, 12 As expected, mutations resulting in severe phenotypes or fatal outcome were present
at almost homoplasmic levels in blood and skeletal muscle (>97%), whereas mutation load appeared lower in adult-onset patients, ranging from 46 to 80%. In our proband, the level of
heteroplasmy in leukocytes was moderately low, whereas the mutation appeared largely heteroplasmic in skeletal muscle tissue. A narrow, although significant, mutational threshold could be
still identified analysing isolated muscle fibres. Our patient shared the myopathic phenotype and developed cPEO, followed by a previously unreported respiratory impairment. Considering her
sister's clinical features, an involvement of the central nervous system by the same mutation cannot be excluded. Ventilatory failure is frequently described in both acute and
chronic-progressive neuromuscular disorders.16 In the latter, it usually appears late in the course of the disease, but occasional presentation with respiratory failure has been reported,
including restrictive syndrome in mitochondrial disorders.17 Two pathophysiological mechanisms leading to respiratory failure in patients with mitochondrial disorders have been proposed: (i)
abnormal respiratory drive caused by dysfunction in the central nervous system (brainstem) respiratory centers; (ii) weakness and/or fatigue of the inspiratory muscles. In our patient, the
first mechanism is unlikely to be involved, because other clinical signs of brainstem dysfunction were absent. We therefore hypothesise an inspiratory muscle dysfunction due to both the
fatigue from inappropriate energetic supply and from primary respiratory muscle weakness. Pulmonary function tests in our patient (single parameters not available) confirmed respiratory
muscle weakness with evidence of a restrictive syndrome. In conclusion, our data provide strong elements supporting the pathogenic role of the m.5709T>C mutation, and help define its
threshold level. The new phenotype further increases the clinical spectrum of mitochondrial diseases caused by mutations in the t_RNA__Asn_ gene. REFERENCES * Scaglia F, Wong LJ : Human
mitochondrial transfer RNAs: role of pathogenic mutation in disease. _Muscle Nerve_ 2008; 37: 150–171. Article CAS Google Scholar * Zifa E, Giannouli S, Theotokis P, Stamatis C, Mamuris
Z, Stathopoulos C : Mitochondrial tRNA mutations: clinical and functional perturbations. _RNA Biol_ 2007; 4: 38–66. Article CAS Google Scholar * Sciacco M, Prelle A, Comi GP _et al_:
Retrospective study of a large population of patients affected with mitochondrial disorders: clinical, morphological and molecular genetic evaluation. _J Neurol_ 2001; 248: 778–788. Article
CAS Google Scholar * Zeviani M, Gellera C, Pannacci M _et al_: Tissue distribution and transmission of mitochondrial DNA deletions in mitochondrial myopathies. _Ann Neurol_ 1990; 28:
94–97. Article CAS Google Scholar * Moraes CT, Atencio DP, Oca-Cossio J, Diaz F : Techniques and pitfalls in the detection of pathogenic mitochondrial DNA mutations. _J Mol Diagn_ 2003;
5: 197–208. Article CAS Google Scholar * Sciacco M, Bonilla E, Schon EA, Di Mauro S, Moraes CT : Distribution of wild-type and common deletion form of mtDNA on normal and respiration
deficient musclefibers from patients with mitochondrial myopathy. _Hum Mol Genet_ 1994; 3: 13–19. Article CAS Google Scholar * Moraes CT, Ricci E, Bonilla E, DiMauro S, Schon EA : The
mitochondrial tRNA(Leu(UUR)) mutation in mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS): genetic, biochemical, and morphological correlations in skeletal
muscle. _Am J Hum Genet_ 1992; 50: 934–949. CAS PubMed PubMed Central Google Scholar * Moraes CT, Ciacci F, Bonilla E _et al_: Two novel pathogenic mitochondrial DNA mutations affecting
organelle number and protein synthesis. Is the tRNA(Leu(UUR)) gene an etiologic hot spot? _J Clin Invest_ 1993; 92: 2906–2915. Article CAS Google Scholar * Vives-Bauza C, Del Toro M,
Solano A, Montoya J, Andreu AL, Roig M : Genotype-phenotype correlation in the 5703G>A mutation in the tRNA(ASN) gene of mitochondrial DNA. _J Inherit Metab Dis_ 2003; 26: 507–508.
Article CAS Google Scholar * Seibel P, Lauber J, Klopstock T, Marsac C, Kadenbach B, Reichmann H : Chronic progressive external ophthalmoplegia is associated with a novel mutation in the
mitochondrial tRNA(Asn) gene. _Biochem Biophys Res Commun_ 1994; 204: 482–489. Article CAS Google Scholar * Sternberg D, Chatzoglou E, Laforêt P _et al_: Mitochondrial DNA transfer RNA
gene sequence variations in patients with mitochondrial disorders. _Brain_ 2001; 124: 984–994. Article CAS Google Scholar * Spinazzola A, Carrara F, Mora M, Zeviani M : Mitochondrial
myopathy and ophthalmoplegia in a sporadic patient with the 5698G-->A mitochondrial DNA mutation. _Neuromuscul Disord_ 2004; 14: 815–817. Article Google Scholar * Coulbault L,
Herlicoviez D, Chapon F _et al_: A novel mutation in the mitochondrial tRNA Asn gene associated with a lethal disease. _Biochem Biophys Res Commun_ 2005; 329: 1152–1154. Article CAS Google
Scholar * Meulemans A, Seneca S, Lagae L _et al_: A novel mitochondrial transfer RNA(Asn) mutation causing multiorgan failure. _Arch Neurol_ 2006; 63: 1194–1198. Article Google Scholar *
McFarland R, Elson JL, Taylor RW, Howell N, Turnbull DM : Assigning pathogenicity to mitochondrial tRNA mutations: when ‘definitely maybe’ is not good enough. _Trends Genet_ 2004; 20:
591–596. Article CAS Google Scholar * Hutchinson D, Whyte K : Neuromuscular disease and respiratory failure. _Pract Neurol_ 2008; 8: 229–237. Article Google Scholar * Cros D, Palliyath
S, DiMauro S, Ramirez C, Shamsnia M, Wizer B : Respiratory failure revealing mitochondrial myopathy in adults. _Chest_ 1992; 101: 824–828. Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS Gratitude has to be expressed to the patient for participating in this research. We wish to thank especially the ‘Associazione Amici del Centro Dino Ferrari’ for their
support. The financial support of the following research grant is gratefully acknowledged: Telethon – UILDM Project GUP09004 ‘Construction of a database for a nation-wide Italian
collaborative network of mitochondrial diseases’, Associazione Amici del Centro Dino Ferrari, University of Milan, the Telethon project GTB07001, the Eurobiobank project QLTR-2001-02769 and
RF 02.187 Criobanca Automatizzata di Materiale Biologico. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Neurological Sciences, Dino Ferrari Centre, University of Milan, IRCCS
Foundation Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy Dario Ronchi, Monica Sciacco, Andreina Bordoni, Michela Ripolone, Elisa Fassone, Alessio Di Fonzo, Mafalda Rizzuti, Patrizia
Ciscato, Alessandra Cosi, Maura Servida, Maurizio Moggio, Stefania Corti, Nereo Bresolin & Giacomo P Comi * Department of Neurology, Neurocenter of Southern Switzerland, Lugano,
Switzerland Monika Raimondi * Centre of Excellence on Neurodegenerative Diseases, University of Milan, Milan, Italy Stefania Corti, Nereo Bresolin & Giacomo P Comi * IRCCS Eugenio Medea,
Bosisio Parini, Lecco, Italy Nereo Bresolin Authors * Dario Ronchi View author publications You can also search for this author inPubMed Google Scholar * Monica Sciacco View author
publications You can also search for this author inPubMed Google Scholar * Andreina Bordoni View author publications You can also search for this author inPubMed Google Scholar * Monika
Raimondi View author publications You can also search for this author inPubMed Google Scholar * Michela Ripolone View author publications You can also search for this author inPubMed Google
Scholar * Elisa Fassone View author publications You can also search for this author inPubMed Google Scholar * Alessio Di Fonzo View author publications You can also search for this author
inPubMed Google Scholar * Mafalda Rizzuti View author publications You can also search for this author inPubMed Google Scholar * Patrizia Ciscato View author publications You can also search
for this author inPubMed Google Scholar * Alessandra Cosi View author publications You can also search for this author inPubMed Google Scholar * Maura Servida View author publications You
can also search for this author inPubMed Google Scholar * Maurizio Moggio View author publications You can also search for this author inPubMed Google Scholar * Stefania Corti View author
publications You can also search for this author inPubMed Google Scholar * Nereo Bresolin View author publications You can also search for this author inPubMed Google Scholar * Giacomo P
Comi View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Giacomo P Comi. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ronchi, D., Sciacco, M., Bordoni, A. _et al._ The novel
mitochondrial _tRNA__Asn_ gene mutation m.5709T>C produces ophthalmoparesis and respiratory impairment. _Eur J Hum Genet_ 20, 357–360 (2012). https://doi.org/10.1038/ejhg.2011.238
Download citation * Received: 29 April 2011 * Revised: 28 October 2011 * Accepted: 11 November 2011 * Published: 21 December 2011 * Issue Date: March 2012 * DOI:
https://doi.org/10.1038/ejhg.2011.238 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * progressive external ophthalmoplegia * tRNA(Asn) *
mitochondrial myopathy