- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
The cyclin-dependent kinase (CDK) family is a group of 21 kinases involved in transcription and cell cycle regulation. The CDKs are unique among the human kinases in their requirement of a
cyclin partner protein to form an active enzyme complex. Several members of the family are established as oncology drug targets, with inhibitors of CDK4/6 on the market and inhibitors of
CDK1, 2, 5, 7 and 9 in clinical trials. Despite the medical importance of this branch of the kinome, only half of its members have been well-characterized to date. Bibliographic analysis
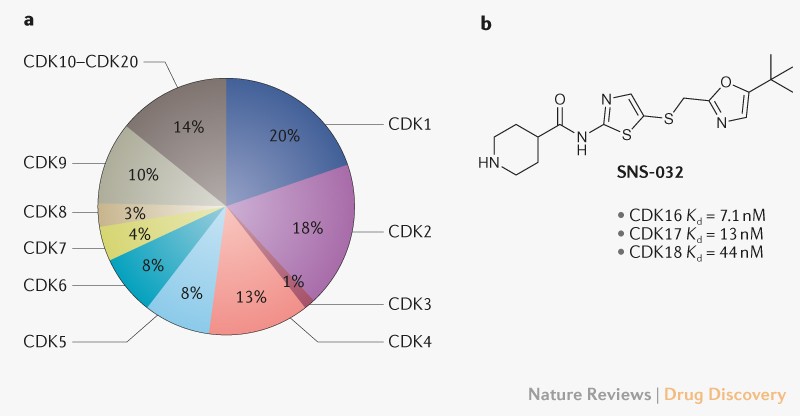
shows that the number of publications on CDK10–20 comprises less than 15% of the total CDK literature (Fig. 1a). To address this gap, the NIH Illuminating the Druggable Genome (IDG)
programme has included these eleven CDK family members on its list of understudied kinases for development of selective small-molecule tools and cellular assays to aid in the understanding
of their function. These CDK family members can be further stratified into groups by sequence and structural similarity. The PCTAIRE subfamily — comprising CDK16, CDK17 and CDK18 — is the
example that we focus on here. The subfamily was first cloned and characterized in 1992 (_EMBO J._ 11, 2909–2917; 1992), but a cyclin partner (cyclin Y) was not identified until 2011 (_Mol.
Cell. Biol._ 32, 868–879; 2012), which contributed to this subfamily being understudied. BIOLOGICAL FUNCTIONS _CDK16_. CDK16 has been shown to be widely expressed in various cell types as
well as a broad range of tissues (for a review, see _Cell Cycle_ 11, 3758–3768; 2012). The highest levels of CDK16 expression are in the brain and testis, and studies using mouse knockout
models have demonstrated that CDK16 is essential in spermatogenesis and has roles in neurite outgrowth and regulation of intracellular vesicles. Importantly, for its relevance as a potential
drug target, recent evidence indicates that increased CDK16 expression is linked to lower overall survival in several types of cancer (_Pharmacol. Ther. __173__, 83–105; 2017_). The
pro-tumorigenic activity of CKD16 may be mediated by downregulation of the tumour suppressor p27 (_Cell Cycle_ 14, 463–464; 2015). _CDK17 AND CDK18_. Compared with CDK16, the disease
relevance of CDK17 and CDK18 is poorly annotated (_EMBO J._ 11, 2909–2917; 1992). CDK17 shows high expression in the brain and the lungs, while CDK17 downregulation has been associated with
poorer prognosis in glioma. Very little is known about the expression and function of CDK18. CHEMICAL TOOLS The development of cell-active selective small-molecule inhibitors of CDK16 would
aid the study of its biological function. CDK16 has been characterized as chemically tractable in Pharos (see Related links) with eight small molecules reported to be potent inhibitors.
These compounds range from the non-selective kinase inhibitor R547, with a CDK16 dissociation constant (_K_d) value of 0.5 nM, to the CDK family-selective inhibitors AT-7519 and SNS-032 with
_K_d values of 1.1 nM and 7.1 nM, respectively. SNS-032, originally developed as a CDK2 inhibitor, has been shown to be a pan-CDK inhibitor with activity across the PCTAIRE subfamily in the
KINOMEscan panel (Fig. 1b). Dixon-Clarke and colleagues recently performed thermal melt experiments to identify several potent CDK16 inhibitors (_Biochem. J. __474__, 699–713; 2017)._
Co-crystal structures were also obtained with rebastinib (Protein Data Bank identifier: 5G6V) and indirubin E804 (PDB ID: 3MTL) that revealed CDK16 is able to bind both type I and type II
kinase inhibitors. These co-crystal structures provide chemical starting points and key structural information to enable the design of selective and potent CDK16 inhibitors.







