- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Multidrug resistance (MDR) is a major problem in cancer chemotherapy. One of the best known mechanisms of MDR is the elevated expression of ATP-binding cassette (ABC) transporters.
While some members of human ABC transporters have been shown to cause drug resistance with elevated expression, it is not yet known whether the over-expression of other members could also
contribute to drug resistance in many model cancer cell lines and clinics. The recent development of microarrays and quantitative PCR arrays for expression profiling analysis of ABC
transporters has helped address these issues. In this article, various arrays with limited or full list of ABC transporter genes and their use in identifying ABC transporter genes in drug
resistance and chemo-sensitivity prediction will be reviewed. SIMILAR CONTENT BEING VIEWED BY OTHERS SYSTEMATIC PREDICTION OF DRUG RESISTANCE CAUSED BY TRANSPORTER GENES IN CANCER CELLS
Article Open access 01 April 2021 A MEMBRANE TRANSPORTER DETERMINES THE SPECTRUM OF ACTIVITY OF A POTENT PLATINUM–ACRIDINE HYBRID ANTICANCER AGENT Article Open access 16 September 2020 A
14-GENE GEMCITABINE RESISTANCE GENE SIGNATURE IS SIGNIFICANTLY ASSOCIATED WITH THE PROGNOSIS OF PANCREATIC CANCER PATIENTS Article Open access 17 March 2021 INTRODUCTION Multidrug resistance
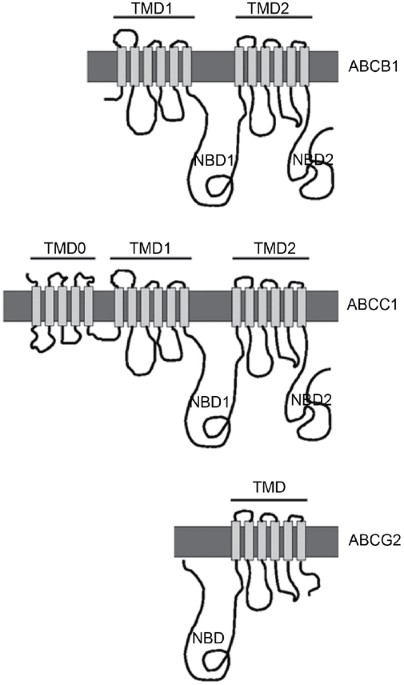
(MDR) is a major problem in cancer chemotherapy. One of the best known mechanisms of MDR is the elevated expression of ATP-binding cassette (ABC) transporters such as ABCB1 (MDR1, Pgp) 1,
2, 3, ABCC1 (MRP1) 4, and ABCG2 (BCRP, MXR, ABCP) 5 (Figure. 1). ABC transporters comprise a superfamily of more than 1000 members from bacteria to human. Human alone has 49 members of the
superfamily which are divided into 7 subfamilies, ABCA through ABCG (Table 1), and they transport a wide variety of substrates including drugs, lipids, metabolites, and ions. These human
transporters are expressed in various tissues and some of them are known to function as drug efflux pumps to cause drug resistance by actively extruding multiple anticancer drugs with
expenses of ATP. However, two of these human proteins (ABCE1 and ABCF1) do not contain any known putative transmembrane domain and, therefore, may not function as transporters by themselves
(see http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=mono_001). While some members of human ABC transporters have been shown to cause drug resistance with elevated expression, it is not yet
known whether over-expression of other members could also contribute to drug resistance. Many model cancer cell lines selected by anticancer drugs are also available for investigating the
mechanisms of drug resistance. However, dissecting which ABC transporter is activated in a particular case is not an easy task. Knowledge of expression profiles of ABC transporters and other
genes involved in MDR will likely help therapeutic optimization for cancer patients in clinics. The recent development of genomic approaches such as microarrays and quantitative PCR arrays
(Table 2) for expression profiling analyses has helped address these issues. In this paper, I will review recent progress in the application of these genomic tools to identify ABC
transporter genes likely responsible for MDR in model cancer cell lines and their potential use as predictors of chemo-sensitivity. However, it should also be noted that in some cases, the
findings from these profiling studies await further validation for the expression at the protein level, and the functional role of the implicated transporters in drug resistance remains to
be verified. GENERATION OF DRUG SENSITIVITY PREDICTORS USING HIGH-DENSITY MICROARRAYS Many high density microarrays have been used to profile gene expression patterns of a large number of
cell lines with known responses to multiple drugs and compounds. Correlation between the expression profiles and the drug sensitivity data of these cell lines helped generate a number of
predictors for specific drugs or compounds. Some of the 49 human ABC transporters found in these predictors are highlighted in Table 2. However, further testing is needed to determine if
these predictors are useful in clinics. The earliest studies of drug sensitivity involved cDNA microarray analysis of the NCI-60 cell lines 6, 7. In these studies, a microarray containing
approximately 8 000 cDNA clones (Table 2) that represent unique human genes including a limited number of ABC transporters such as ABCA3, ABCB1, ABCB2, ABCB7, ABCC1, ABCC6, and ABCF1, was
used. Analysis of gene expression profiles of the NCI-60 cell lines and clustering the cell lines on the basis of their responses to 1400 compounds or drugs revealed many gene-drug response
relationships. It appears that the cell lines with elevated expression of ABCB1 clustered on the same branch for drug responses. This is also true for ABCC1, suggesting that the expression
of these ABC transporter genes likely contribute to the poor drug sensitivity of these cancer cell lines (Table 3). Both ABCB1 and ABCC1 are well known genes involved in resistance to
multiple anticancer drugs. In addition to these ABC transporter genes, this study also revealed correlative gene-drug relationships for other 1376 genes. One of these genes,
dihydropyrimidine dehydrogenase, was found to have a significant negative correlation between its expression and 5-FU potency in the 60 cell lines, suggesting that its over-expression may
cause resistance to 5-FU. A similar study of NCI-60 cell lines was also conducted, but instead used the Affymetrix oligonucleotide microarray Hu6800 (Table 2) containing 6 817 human genes
including ABC transporters such as ABCA3, ABCA4, ABCB1, ABCB2, and ABCB3 8. The correlation of drug sensitivity to gene expression was generated from a training set of data and this was then
used to predict the sensitivity of cell lines to any particular drug using data in a testing set. Expression-based classifiers containing multiple genes for each of the 232 compounds were
generated to predict drug sensitivity. Eighty-eight such classifiers performed accurately to predict drug sensitivity. Unfortunately, no particular analyses on ABC transporters and their
roles in drug sensitivity prediction were reported in this study. Dan _et al._ 9 performed a similar study using a cDNA microarray containing 9216 human genes (Table 2), but with only 39
cell lines. Of these 39 cell lines, 9 were not included in the original set of NCI-60 collection used in previous studies 6, 7, 8. The drug database was also smaller with only 55 anticancer
drugs. Fifty genes were found to have a significant positive (e.g., aldose reductase) or negative (e.g., LIM domain kinase 2) correlation between their expression and sensitivity to ≥10 of
the 55 drugs tested. Interestingly, ABCB6, the only ABC transporter gene among the 50 genes, was found to have a significant positive correlation with sensitivity to 20 anticancer drugs
including Adriamycin, camptothecin, and mitoxantrone, suggesting that over-expression of ABCB6 may contribute to cellular sensitivity to these drugs (Table 3). ABCB6 is a mitochondrial ABC
transporter known for its role in mitochondrial porphyrin uptake and possibly in controlling haem biosynthesis 11. It should be noted, however, that in the study by Yasui _et al._, it was
found that the ABCB6 gene was amplified in the camptothecin-resistant A549 cells 10. Currently, there is no direct evidence regarding whether over-expression of ABCB6 causes resistance or
sensitivity to anticancer drugs. Clearly, further studies are needed to more directly test the role of ABCB6 in drug responses. Most recently, Gyorffy _et al._ 12 analyzed 30 cancer cell
lines for their responses to 11 common anticancer agents and their gene expression profiles using the Affymetrix HG-U133 microarray containing all 49 human ABC transporter genes (Table 2).
They found that the elevated expression of ABCA1 correlates with mitoxantrone resistance, ABCC1 with etopside resistance, ABCC2 with 5-FU resistance, and ABCD3 with topotecan resistance
(Table 3). In addition, another 67 genes were found by these authors to have correlation with responses to multiple anticancer drugs. One of the notable genes is tripartite motif-containing
2 of which the expression correlates with cellular resistance to 8 different anticancer drugs. A 70-mer oligonucleotide array containing probes for 640 transporter genes (transportome)
including 40 ABC transporters (Table 2) has also been engineered to investigate the potential contribution of membrane transporters to drug responses of cancer cell lines 13. Using this
array, many genes that encode ABC transporters and transporters of nucleosides, amino acids, glucose, folate, channels for calcium and water, Ca++-ATPase and Na+/K+-ATPase were found to
significantly correlate with either drug resistance or sensitivity of the NCI-60 cell lines. Highly statistically significant negative correlations with drug sensitivity, indicative of a
possible role in chemoresistance, were found for ABCB1, ABCB5, ABCC1, and ABCC3 (Table 3). The differential expression of these genes, except for ABCC1, was also confirmed using real time
RT-PCR. While ABCB1, ABCC1, and ABCC3 were known to cause drug resistance, the expression of ABCB5, which was not previously known to affect drug responses, was found to negatively correlate
with camptothecin and 1,2,4,5-Tetramethylbenzene sensitivity. The follow-up study using siRNA to down-regulate ABCB5 expression in melanoma SK-MEL-28 cells caused sensitization to several
anticancer drugs including camptothecin, 10-OH camptothecin, and 5-FU. These findings suggest that ABCB5 over-expression likely also causes MDR and may serve as a predictor along with ABCB1,
ABCC1, and ABCC3 for poor drug sensitivity. In addition to ABC transporters, other transporters have also been found to have significant correlations with drug responses by using the
transportome array. While some of them such as the folate carrier are known to affect sensitivity to drugs such as methotrexate, others such as amino acid transporters have not been
implicated in such a process. However, the sufficient structural similarity between amino acids and their analogue drugs may warrant the involvement of these transporters in drug responses
of these cancer cells. This speculation awaits verification from future studies. IDENTIFICATION OF ABC TRANSPORTER GENES IN DRUG-SELECTED CELL LINES USING HIGH DENSITY MICROARRAYS
Comparative gene expression profiling has been conducted by using the Clontech Atlas Rat cDNA array (Table 2) containing 588 genes, including 4 ABC transporters (ABCB1, ABCB4, ABCC1, and
ABCC9), to investigate genes potentially responsible for arsenic tolerance in chronically exposed rat liver epithelial cell line TRL1215 14. Compared with the parental arsenic sensitive
TRL1215 cells, the arsenic resistant TRL1215 has ∼80 genes with altered expressions. Of these genes, ABCB1, ABCB4, and ABCC1 were among the genes with increased expression (Table 4). In a
follow-up study, Liu _et al._ verified the altered expression of these three ABC transporters by using RT-PCR and western blot analyses 15. They further demonstrated that inhibition of ABCB1
and ABCC1 function using their respective inhibitors could significantly increase arsenic sensitivity of the arsenic tolerant TRL1215 cell line, suggesting that over-expression of ABCB1 and
ABCC1 may contribute to arsenic tolerance. However, it is not yet known if arsenic or its metabolites are directly effluxed by these ABC transporters. Furthermore, because the rat array
contains only a limited number of ABC transporter genes, its usefulness is likely limited as it can not identify the potential roles of other ABC transporters in arsenic tolerance. The
anticancer drug NB-506, a Topo I inhibitor, has been shown not to be a substrate of the known drug transporter ABCB1 or ABCC1 16, although NB-506 resistant cell lines showed decreased
intracellular accumulation of NB-506 17. To discover genes that could be involved in NB-506 resistance and elimination, Komatani _et al._ performed comparative genomic analyses using
Affymetrix arrays (Mu11K and Mu19K, Table 2) containing over 34 000 mouse genes 17. They found that ABCG2, in addition to 11 other genes, had the most prominent elevation in expression in
NB-506 resistant mouse fibroblast cell line LY/NR2 compared with the parental cell line LY (Table 4). The role of ABCG2 in cellular resistance to NB-506 was validated subsequently by ectopic
over-expression of ABCG2 in the lung cancer cell line PC-13 17. In a similar study to discover resistance mechanisms of Adriamycin-selected acute myeloid leukemia cell line K562/A02 using
the Clontech Human 1.2 II Atlas array (Table 2) containing only 1 ABC transporter gene (ABCD1), Tan _et al._ found 12 differentially expressed genes between K562/A02 and its parental
sensitive line K562 18. Because of the limited ABC transporter genes on the array, no ABC transporter gene would have been found here despite the fact that ABCB1 is known to be strongly
over-expressed in the K562/A02 cell line. Thus, the usefulness of this array for ABC transporter analysis is very limited. A regular Affymetrix HG-U95Av2 array (Table 2) containing 9 600
known human genes including 14 ABC transporters was used to identify molecular signatures that are possibly responsible for paclitaxel resistance in 3 stepwise selected SKOV3 ovarian cancer
cell lines 19. Of the 14 ABC transporter genes collected on this array, only ABCB1 was found to have increased expression correlative with the sequential increase in drug resistance of the 3
stepwise selected cell lines. Interestingly, the expression of ABCC3 and ABCC4 was decreased in the drug resistant cell lines. In a similar study of ovarian cancer cell line KF28TX
resistant to paclitaxel using an IntelliGene microarray (Table 2) containing 557 human cancer-related cDNAs including ABCB1 and ABCC3, ABCB1 was also found to be over-expressed and likely
contribute to paclitaxel resistance 20. Similar to the Clontech Rat cDNA array (see above), the usefulness of these arrays in ABC transporter profiling, however, is also limited. In the
study using Affymetrix HG-U95Av2 array 19, some other genes in addition to ABC transporters, were also found to have increased expression either in the early cell line with low paclitaxel
resistance level or in the later cell line with intermediate or high level of resistance; and they were clustered into 3 categories based on their emerged increase in the 3 stepwise selected
cell lines. The early respondent genes are primarily inflammatory response genes such as IL-8. The intermediate genes include carrier and primary active transporter genes. The late
respondent genes include tumor and cell surface antigens and signal transducers. Still many other genes were also found to have altered expression in the cell line either with low or higher
level of resistance. However, the expression of these genes did not follow the evolution trend with increased expression correlating with the increased drug resistance in the 3 stepwise
selected cell lines. Therefore, their contributions to paclitaxel resistance in ovarian cancer cells may be limited. Using the same transportome array developed by Huang _et al._ 13 (see
above), Xiao _et al._ analyzed the underlying mechanism of acquired resistance of cancer cell lines HCT-15, IGROV1, MCF7, and K562 to the histone deacetylase inhibitor FK228 21. These
authors found that ABCB1 expression is dramatically up-regulated in all four FK228-resistant derivative cell lines (Table 4). It was also found that ABCB1 expression was induced by FK228
likely via alterations in histone acetylation at the ABCB1 promoter. However, ABCB1-mediated efflux of FK228 and its direct involvement in FK228 resistance have not yet been established.
ET-743, a new anticancer agent derived from marine products, is currently under clinical trial; and variation in responses to this drug has been observed, suggesting that clinical resistance
to ET-743 may develop. Using the Human 1 cDNA microarray containing 16,281 cDNA probes with 45 ABC transporter genes from Agilent Technologies (Table 2), Manara _et al._ profiled the
differential gene expression between two ET-743 resistant Ewing's sarcoma cell lines, TC/ET 6 nM and TC/ET 12 nM, and their parental sensitive cell line TC-71 22. The expressions of
sixty-five genes were found commonly changed in the two resistant sublines compared with the sensitive cells. Among these genes, ABCB1 and ABCB4 were up-regulated most prominently in the
resistant cells (Table 4). Using comparative genomic hybridization, ABCB1, but not ABCB4, was also found to have been amplified in the resistant cells. Since ABCB1 is the gene with most
prominent increases in expression, it is likely very important for ET-743 resistance. Indeed, inhibition of ABCB1 activity by PSC-833 dramatically reduced ET-743 resistance level 22.
However, the potential contribution of the remaining 64 genes including ABCB4 to ET-743 resistance has not yet been rigorously examined. The mechanism of Adriamycin resistance in five pairs
of hepatocarcinoma cell lines has also been examined using a 19 K EST microarray 23 (Table 2). The expression of over 60 genes including ABCB2 and ABCB9 was found to have commonly increased
in the 5 resistant sublines compared with their sensitive parental hepatocellular carcinoma cells. While ABCB2 participates in peptide transport in ER for antigen presentation, the function
of ABCB9 was not yet known. Neither of these two genes has been shown previously to cause drug resistance. However, in the profiling study by Gillet _et al._ using their low density DualChip
human ABC microarray (see below), ABCB2 and ABCB9 were also found to be over-expressed in Adriamycin-resistant HL-60 and CCRF-CEM cell lines, respectively 24 (see below). It is currently
unknown if the over-expression of ABCB2 and ABCB9 causes Adriamycin resistance. The high density Affymetrix arrays have also been used for focused analysis of ABC transporter gene expression
despite the fact that they contain many more genes. One of these studies was performed using Affymetrix HG-U133 Plus 2.0 GeneChip which contains all 49 human ABC transporter genes (Table
2). ABC transporter expression profiles were generated using this array from breast cancer patients who received neoadjuvant chemotherapy including 5-FU and paclitaxel 25. Comparison between
patients who had complete response and those who had residual disease revealed that the expression of ABCA1, ABCA12, ABCB6, ABCC5, ABCC11, ABCC13 was elevated in the residual disease group,
suggesting that their expression may contribute to the incomplete response of these patients to neoadjuvant therapy. Of these transporters, ABCC5 was found to be the elevated gene with the
highest significance and highest expression level (Table 4). ABCC5 has been shown previously to cause resistance to several drugs including 5-FU 26, 27. Thus, its high expression level in
breast cancers may contribute to clinical resistance to drugs such as 5-FU. Gillet _et al._ also performed an analysis of 16 breast cancer samples using their low density DualChip human ABC
microarray (see below) and found 23 ABC transporters including ABCC1 and ABCC5 were expressed in the majority (≥10 of 16) of the breast cancer samples tested 28. Unlike the study by Park _et
al._ 25, however, the Gillet study 28 did not have the correlation analysis between patient response to chemotherapy and their ABC transporter expression level. Thus, the implication of ABC
transporter expression in this study is limited. The Affymetrix MuU74v2 GeneChip with 33 000 mouse genetic elements contains annotated oligonucleotide probes for 43 of the 51 mouse ABC
transporter genes (Table 2). Using this array, Mutch _et al._ 29 conducted a focused analysis of ABC transporter expression profiles along the intestinal tract of mice. While most ABC
transporters do not show differential expressions in the duodenum, jejunum, and colon, 8 ABC transporter genes (ABCB2, ABCB3, ABCB9, ABCC3, ABCC6, ABCD1, ABCG5, and ABCG8) show significant
alterations in expression along the intestinal tract. The alterations in expression of these 8 genes have also been validated using real-time PCR. These findings suggest that the Affymetrix
MuU74v2 GeneChip may also be used for a focused analysis to investigate differential ABC transporter gene expression in drug resistant mouse cell lines compared with their parental sensitive
lines. Gottesman's laboratory has developed a high density ABC-ToxChip for microarray analysis of toxicological response genes 30. On this microarray, in addition to 36 human ABC
transporter genes, a comprehensive set of detoxifying genes were also included. By comparing the KB-3-1 cell line with its corresponding resistant derivative cell line selected using
colchicine (KB-8-5), they showed that ABCB1 had dramatic over-expression in the KB-8-5 cell line (Table 4), the known mechanism of resistance in this cell, thus validating the usefulness of
the array. Using this array to analyze DU-145 cell line and its corresponding resistant derivative cell line (RCO.1) selected using 9-nitro-camptothecin revealed that RCO.1 over-expresses
ABCC2 (Table 4), suggesting that ABCC2 may contribute to 9-nitro-camptothecin resistance. However, the notion that over-expression of ABCC2 causes 9-nitro-camptothecin resistance has not yet
been validated. Furthermore, RCO.1 also over-expresses many other genes of the 20 000 genes tested such as the EGF-like repeats and discoidin I-like domain protein (U70312), which may also
contribute to 9-nitro-camptothecin resistance. IDENTIFICATION OF ABC TRANSPORTER GENES IN DRUG-SELECTED CELL LINES USING LOW DENSITY MICROARRAYS Use of high-density arrays to profile only
ABC transporter expression is apparently not cost-effective. Recently, low density arrays containing only ABC transporter genes have been developed which can be used more cost-effectively
for focused studies of ABC transporters in drug resistant versus sensitive cells (Table 2). The first of such arrays contains 38 human ABC transporter genes (DualChip human ABC low-density
microarray) and it has been validated using three MDR cell lines, CEM/ADR5000, HL60/AR, and MCF7/AdVp1000 in comparison with their respective parental CEM, HL60, and MCF7 cells 24. Using
this array it was found that CEM/ADR5000, HL60/AR, and MCF7/AdVp1000 over-expressed ABCB1, ABCC1, and ABCG2, respectively, as previously demonstrated 5, 31, 32, thus validating the
usefulness of this low density array. In this study, several other ABC transporters were also found to be over-expressed in the drug resistant cell lines (Table 4), suggesting that they may
also contribute to the resistance selected in these cells. However, none of these additional ABC transporter genes was validated for their functional role in drug resistance. The same array
was also used to profile ABC transporter expression and its correlation to clinical responses of 42 childhood acute myeloid leukemia (AML) patients 33. It was found that, of all 38 human ABC
transporters examined, only ABCA3 had significant correlation of over-expression to poor response (Table 4), suggesting that ABCA3 may contribute to clinical drug resistance in AML.
Knocking down ABCA3 expression in the osteosarcoma cell line 143B with siRNA caused a statistically significant increase in sensitivity to Adriamycin. Nevertheless, this increase in
sensitivity is very small (<20%) 33. Because ABCA3 is normally located at intracellular membranes 34, it seems unlikely to mediate drug resistance by directly effluxing drugs as do the
traditional ABC drug transporters such as ABCB1 and ABCC1. In another study, the same research group analyzed 21 childhood T cell acute lymphoblastic leukemia (ALL) samples using the same
DualChip human ABC low-density microarray and found consistent over-expression of ABCA2 and ABCA3, similar to the findings in childhood myeloid leukemia 35. However, correlation analysis
between ABC transporter expression and the clinical outcome did not generate any statistically significant differences in the ALL. Although ABCA3 knock-down in the osteosarcoma cell line
143B by siRNA appeared to generate a significant increase in cellular sensitivity to Adriamycin 33, 35, it did not result in any survival effect in response to vinblastine and methotrexate.
Knock-down of ABCA2 alone in 143B cells did not appear to have any effect on survival in response to Adriamycin, vinblastine, or methotrexate. However, the combined knock down of ABCA2 and
ABCA3 resulted in a significant increase in cell death in response to all three anticancer drugs 35, suggesting a potential compensatory effect between the two ABC transporters. QUANTITATIVE
PCR ARRAYS TO IDENTIFY ABC TRANSPORTER GENES The same 60 NCI cell lines discussed above were also examined for the correlation of their drug sensitivities with the expression of 48 human
ABC transporters using a focused real-time RT-PCR approach 36 (Table 2). Correlation between the expression of ABC transporter genes and resistance to candidate drugs was analyzed. While
there were interesting positive correlations between ABC transporter expression and resistance to drugs, along with the discovery of some previously unknown substrates for certain ABC
transporters such as ABCB1, negative correlations between ABC transporter expression and drug resistance were also observed. One of the novel compounds that have positive correlations in
resistance with ABCB1 expression is NSC363997. MTT assay using KB-3-1 and the drug-selected KB-V1 cells, which over-express ABCB1, showed that NSC363997 is likely an ABCB1 substrate 36. In
contrast, NSC73306 is an example for compounds that have negative correlations in resistance with ABCB1 expression. Validation studies showed that cells over-expressing ABCB1 are less
resistant to NSC73306 36. More recently, Ludwig _et al._ followed up on the observation of NSC73306 and found that NSC73306 toxicity is actually mediated by ABCB1 over-expression 37.
Inhibiting the function of ABCB1 using inhibitors or decreasing its expression using siRNA both decreased cellular sensitivity to NSC73306. Interestingly, long term selection of several
cancer cell lines with NSC73306 also resulted in NSC73306-resistant derivative cells, which apparently have decreased expression of ABCB1. These findings are interesting because ABCB1
over-expression has previously been recognized only to cause resistance but not sensitivity to drugs. Nevertheless, the mechanism of ABC-transporter-mediated cytotoxicity is currently
unknown and further studies are needed to interpret this phenomenon and investigate the mechanism of action. The TaqMan real-time RT-PCR approach involving 47 ABC transporters has been
developed by another group 38 to profile ABC transporter expression in human tissues. The expression profiles of ABC transporters in human tissues appear to be consistent with their known
ubiquitous distribution. However, some ABC transporters show restricted expression patterns such as ABCG5 and ABCG8 in liver and intestine. The utility of this approach for analyzing drug
resistant cells has yet to be tested. While the above real-time PCR approach represents a focused assay on ABC transporters, performing this assay routinely on all ABC transporter genes is
time-consuming. Recognizing this problem, Langmann _et al._ 39 recently improved their TagMan real-time RT-PCR approach by developing a low-density array of 47 ABC transporters based on an
Applied Biosystems 7900HT Micro Fluidic Card, which allows simultaneous PCR analysis of 47 ABC transporters (Table 2). Although this array has not been tested for analyzing drug resistant
cells, it was used to analyze the expression profiles of ABC transporters in primary human monocytes, differentiated macrophages, and macrophages treated with LXR and RXR agonist T0901317
and retinoic acid. It was found that 30 ABC transporters were expressed in human primary monocytes. Following differentiation into macrophages, the number of expressed ABC transporters
decreased to 26. Although the expression level of most of these ABC transporters is lower than that in monocytes, ABCB1, ABCB11, and ABCG2 are the new genes detected while the expression of
ABCB9 and ABCC5 is increased in macrophages. Treatment of macrophages with LXR and RXR agonist T0901317 and retinoic acid further decreased the number of expressed ABC transporter genes to
12. It may be too premature to conclude that ABCB1, ABCB9, ABCB11, ABCC5, and ABCG2 expression plays important roles in differentiation. However, future detailed studies should help answer
the question regarding the relationship between these ABC transporters and differentiation. Another simpler and inexpensive AmpArray approach (Figure 2, Table 2) has also been designed
recently and tested for investigating the expression profiles of 47 ABC transporters using RT-PCR on a 96-well plate 40. This comparative assay is quick, convenient, and economical, as 47
ABC transporters are assayed at the same time on a regular thermal cycler that houses a 96-well plate format. This approach was tested using parental cell line MCF7 and its drug resistant
derivative subline MCF7/AdVp3000. It was found that ABCG2 expression was dramatically increased in the resistant MCF7/AdVp3000 cells, which are consistent with previous findings that
increased ABCG2 expression is a major cause of resistance in MCF7/AdVp3000 cells 5. Interestingly, several other ABC transporters were also found to show altered expression levels.
Particularly, the expression of ABCA4 and ABCC3 was drastically increased in the drug resistant subline and then reversed completely along with ABCG2 in the revertant MCF7/AdVpRev cells as
determined by real-time PCR. The altered expression and possible contribution of ABCC3 to Adriamycin and mitoxantrone resistance were also validated by using western blot and SRB assays,
respectively. However, the altered expression of ABCA4 could not be confirmed at the protein level. These findings suggest that more than one ABC transporters could be up-regulated to cause
resistance in drug selected cell lines and that the altered expression at the mRNA level as determined by arrays or PCR does not necessarily correlate with the level of the corresponding
protein. Compared to the real-time PCR low-density arrays, which require the use of specialized instrumentation and reagents, the AmpArray analysis is less expensive and simpler to perform.
However, AmpArray suffers from its semi quantitative nature. COMPARATIVE GENOMIC APPROACHES TO INVESTIGATE ABC TRANSPORTER GENE AMPLIFICATIONS AND THEIR CONTRIBUTION TO DRUG RESISTANCE In
addition to microarrays and real time quantitative PCRs to investigate the potential involvement of ABC transporters in drug resistance, other approaches have recently been developed. One of
these approaches is subtractive comparative genomic hybridization of metaphase DNA on slides 41. By using this approach to profile ABC transporter gene amplifications in 23 drug resistant
cancer cell lines, Yasui _et al._ recently found that ABCA3, ABCB1, ABCB6, ABCB8, ABCB10, ABCB11, ABCC1, ABCC4, ABCC9, ABCD3, ABCD4, ABCE1, and ABCF2 were amplified in various drug resistant
cancer cell lines relative to their parental sensitive ones (Table 4). The amplified genes of ABCA3, ABCC1, and ABCC9 were also shown to have increased expression in the drug resistant
cells at the RNA level using northern blot or RT-PCR analyses. However, it is currently unknown if amplifications of other ABC transporter genes also cause their over-expression in the drug
resistant cells. Such a potential problem was better addressed in another similar study by Boonstra _et al._ who also used the similar subtractive comparative genomic hybridization to
analyze a mitoxantrone resistant small cell lung cancer cell line GLC4-MITO 42. Of the 7 amplified ABC transporter genes (ABCA2, ABCB2, ABCB3, ABCB6, ABCC4, ABCC10 and ABCF1) detected using
comparative genomic hybridization, only ABCA2 was found to show corresponding over-expression at RNA and protein levels, and its over-expression likely causes mitoxantrone resistance (Table
4). This observation suggests that caution should be taken when the comparative genomic hybridization approach is used and validations using other approaches to determine the expression
level should be performed. CONCLUDING REMARKS AND PERSPECTIVES Both high and low density microarrays and quantitative PCR approaches with limited or full list of ABC transporter genes have
been developed and tested. These arrays clearly can be used for a variety of studies to profile ABC transporter gene expression in drug resistant cancer cell lines or clinical tissues, which
could not have been accomplished in the past. Predictors for drug sensitivity can also be built from the results of ABC transporter expression profiling which may assist the design of
individualized therapies. It is clear from the studies reviewed here that any given drug resistant cancer cell line likely has multiple ABC transporters selected for contribution to
resistance (Table 4). Although the most frequently selected ABC transporters in various drug resistant cell lines appeared to be ABCB1 and ABCC1, consistent with their known roles in MDR,
new roles in drug resistance previously unknown for some ABC transporters were also found using these profiling analyses. For example, ABCC3 and ABCC5 were both found up-regulated in the
Adriamycin-selected MCF7/AdVp1000 24 and MCF7/AdVp3000 40 cell lines using two different arrays, strongly suggesting that these transporters may contribute to Adriamycin resistance. Although
these transporters may be selected by verapamil that was present during the selection of these cell lines, the role of ABCC3 in Adriamycin resistance has been validated functionally by the
siRNA knock down strategy 40. Clearly, further studies are still needed to more definitively demonstrate that the over-expression of these transporters causes Adriamycin resistance. The
findings that drug resistant cancer cells may over-express multiple ABC transporters simultaneously impose a challenge for successful clinical cancer chemotherapy. Sensitization of the drug
resistance phenotype to optimize cancer chemotherapy may require considerations of inhibiting multiple drug efflux ABC transporters. It is noteworthy that in some studies members of ABC
transporters were found to have decreased expression in drug resistant cells. It is currently unknown whether the decreased expression of these ABC transporters contributes to drug
resistance. In other studies, it has been found that the over-expression of ABC transporter mediates cytotoxicity of some compounds. Again, the mechanism of action is unknown. Clearly, more
studies are needed to investigate if decreased expression of some ABC transporters causes drug resistance and how certain ABC transporter over-expression mediates cytotoxicity of some drugs.
Based on the studies reviewed here, it is clear that while mass information could be produced from the array profiling studies for potential drug resistance contributors, most of the
studies are correlative and lack functional validation of the implicated genes that may be responsible for the drug resistance/sensitivity observed. Of course, simultaneous analyses of cell
lines with intermediate drug resistance generated during stepwise selections and revertant cell lines that have lost resistance as demonstrated in the studies by Lamendola _et al._ 19 and
Liu _et al._ 40 would help identify genes that likely contribute to the resistance of the cells. Further functional validation of these genes in drug resistance (Table 3) using
siRNA-mediated silencing, ectopic over-expression, and known inhibitors would be very fruitful and could help verify the new drug resistance functions of these ABC transporters. Another
problem associated with ABC transporter gene profiling using microarrays or quantitative PCRs is that these approaches only detect the changes in expression at the mRNA level. The protein
level of the corresponding transporter may or may not be changed. For example, the expression of ABCA4 in the AmpArray study by Liu _et al._ 40 was increased at the mRNA level in the drug
resistant MCF7/AdVp3000 cells, as detected by the array profiling and confirmed by real time RT-PCR. However, the change of ABCA4 protein was not detected using several antibodies. Rather
than validating the gene expression using real time PCR, as shown in most of the studies discussed above, western blot analyses would be preferred if antibodies are available. Furthermore,
immunohistochemistry using tissue microarrays has been developed to examine the expression of ABC transporters, which would also facilitate the study using clinical human tissue samples and
eliminate the potential problems from detection of mRNAs as mentioned above 43, 44. Proteomic approaches have also been developed to investigate drug resistance mechanisms and may help solve
the aforementioned problem of gene profiling studies. However, the proteomic approach can be problematic and limited in separating and identifying polytopic membrane proteins including ABC
transporters (Zhang and Liu, manuscript submitted). Because ABC transporters share high homology, there is a potential problem of cross hybridization associated with microarrays, although
the oligonucleotide arrays contain multiple probes targeting different regions of each target. As suggested by Lee _et al._ 45, performing similar studies using both cDNA and oligonucleotide
microarrays, which are subjected to different artifactual influences during hybridization, may help minimize this potential problem. For quantitative PCR approaches, the use of certain
reference genes such as GAPDH can also be problematic. It has been found that GAPDH expression was increased in the Adriamycin-resistant MCF7/Adr cells 46, 47. In a metabolic marker-focused
proteomic profiling analysis of 101 breast cancer tissues, Isidoro _et al._ 48 also found that the expression of GAPDH was significantly increased in patients with poor survival. These
studies suggest that GAPDH may not be an ideal internal reference gene for the profiling studies using PCR. Most recently, Calcagno showed that plasma membrane calcium-ATPase 4 (PMCA4) is a
better reference gene for real time PCR quantification of ABC transporter genes 49. PMCA4 is a ubiquitously expressed house keeper plasma membrane protein and its expression in several
cancer cell lines appeared to be unaffected by treatment with various agents including anticancer drugs. Some arrays are limited in the number of ABC transporter genes (Table 1), and thus,
their usefulness in profiling ABC transporter expression is likely also limited. The past studies using these arrays would likely have missed some ABC transporter genes that have _bona fide_
roles in drug resistance. Furthermore, using the low density arrays and quantitative PCRs also likely would miss information about other potential mechanisms of drug resistance when
profiling drug resistant and sensitive cancer cells, although the low density arrays are more cost effective. Taken together, it is clear that comprehensiveness, cost effectiveness,
simplicity of operation, and fidelity should all be considered when choosing any of these comparative genomic profiling approaches. At least two independent approaches are recommended to
validate the findings. Furthermore, it is known that ABC transporter polymorphism such as that in ABCG2 50 could affect substrate specificity and, thus, resistance to certain anticancer
drugs. These polymorphisms of ABC transporters have not yet been considered in the ABC transporter arrays developed thus far. Future studies to develop ABC transporter arrays with
consideration of polymorphisms would be important for studies of MDR and drug responses. REFERENCES * Gerlach JH, Endicott JA, Juranka PF, _et al_. Homology between P-glycoprotein and a
bacterial haemolysin transport protein suggests a model for multidrug resistance. _Nature_ 1986; 324: 485–489. Article CAS PubMed Google Scholar * Chen CJ, Chin JE, Ueda K, _et al_.
Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. _Cell_ 1986; 47:381–389. Article CAS PubMed
Google Scholar * Gros P, Croop J, Housman D . Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. _Cell_ 1986; 47:371–380.
Article CAS PubMed Google Scholar * Cole SP, Bhardwaj G, Gerlach JH, _et al_. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line [see comments].
_Science_ 1992; 258:1650–1654. Article CAS PubMed Google Scholar * Doyle LA, Yang W, Abruzzo LV, _et al_. A multidrug resistance transporter from human MCF-7 breast cancer cells. _Proc
Natl Acad Sci USA_ 1998; 95:15665–15670. Article CAS PubMed PubMed Central Google Scholar * Scherf U, Ross DT, Waltham M, _et al_. A gene expression database for the molecular
pharmacology of cancer. _Nat Genet_ 2000; 24:236–244. Article CAS PubMed Google Scholar * Ross DT, Scherf U, Eisen MB, _et al_. Systematic variation in gene expression patterns in human
cancer cell lines. _Nat Genet_ 2000; 24:227–235. Article CAS PubMed Google Scholar * Staunton JE, Slonim DK, Coller HA, Tamayo P, Angelo MJ, Park J, _et al_. Chemosensitivity prediction
by transcriptional profiling. _Proc Natl Acad Sci USA_ 2001; 98:10787–10792. Article CAS PubMed PubMed Central Google Scholar * Dan S, Tsunoda T, Kitahara O, _et al_. An integrated
database of chemosensitivity to 55 anticancer drugs and gene expression profiles of 39 human cancer cell lines. _Cancer Res_ 2002; 62:1139–1147. CAS PubMed Google Scholar * Yasui K,
Mihara S, Zhao C, _et al_. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. _Cancer Res_ 2004; 64:1403–1410. Article CAS PubMed Google Scholar *
Krishnamurthy PC, Du G, Fukuda Y, _et al_. Identification of a mammalian mitochondrial porphyrin transporter. _Nature_ 2006; 443:586–589. Article CAS PubMed Google Scholar * Gyorffy B,
Surowiak P, Kiesslich O, _et al_. Gene expression profiling of 30 cancer cell lines predicts resistance towards 11 anticancer drugs at clinically achieved concentrations. _Int J Cancer_
2006; 118:1699–1712. Article PubMed Google Scholar * Huang Y, Anderle P, Bussey KJ, _et al_. Membrane transporters and channels: role of the transportome in cancer chemosensitivity and
chemoresistance. _Cancer Res_ 2004; 64:4294–4301. Article CAS PubMed Google Scholar * Chen H, Liu J, Merrick BA, Waalkes MP . Genetic events associated with arsenic-induced malignant
transformation: applications of cDNA microarray technology. _Mol Carcinog_ 2001; 30:79–87. Article PubMed Google Scholar * Liu J, Chen H, Miller DS, _et al_. Overexpression of glutathione
S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. _Mol Pharmacol_ 2001; 60:302–309. Article CAS PubMed Google
Scholar * Voigt W, Vanhoefer U, Yin MB, Minderman H, Schmoll HJ, Rustum YM . Evaluation of topoisomerase I catalytic activity as determinant of drug response in human cancer cell lines.
_Anticancer Res_ 1997; 17:3707–3711. CAS PubMed Google Scholar * Komatani H, Kotani H, Hara Y, Nakagawa R, Matsumoto M, Arakawa H, _et al_. Identification of breast cancer resistant
protein/mitoxantrone resistance/placenta-specific, ATP-binding cassette transporter as a transporter of NB-506 and J-107088, topoisomerase I inhibitors with an indolocarbazole structure.
_Cancer Res_ 2001; 61:2827–2832. CAS PubMed Google Scholar * Tan Y, Li G, Zhao C, _et al_. Expression of sorcin predicts poor outcome in acute myeloid leukemia. _Leuk Res_ 2003;
27:125–131. Article CAS PubMed Google Scholar * Lamendola DE, Duan Z, Yusuf RZ, Seiden MV . Molecular description of evolving paclitaxel resistance in the SKOV-3 human ovarian carcinoma
cell line. _Cancer Res_ 2003; 63:2200–2205. CAS PubMed Google Scholar * Goto T, Takano M, Sakamoto M, _et al_. Gene expression profiles with cDNA microarray reveal RhoGDI as a predictive
marker for paclitaxel resistance in ovarian cancers. _Oncol Rep_ 2006; 15:1265–1271. CAS PubMed Google Scholar * Xiao JJ, Huang Y, Dai Z, _et al_. Chemoresistance to depsipeptide FK228
[(E)-(1S,4S,10S,21R)-7-[(Z)-ethylidene]-4,21-diisopropyl-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo8, 7, 6-tricos-16-ene-3,6,9,22-pentanone] is mediated by reversible MDR1 induction in
human cancer cell lines. _J Pharmacol Exp Ther_ 2005; 314:467–475. Article CAS PubMed Google Scholar * Manara MC, Perdichizzi S, Serra M, _et al_. The molecular mechanisms responsible
for resistance to ET-743 (Trabectidin; Yondelis) in the Ewing's sarcoma cell line, TC-71. _Int J Oncol_ 2005; 27:1605–1616. CAS PubMed Google Scholar * Pang E, Hu Y, Chan KY, _et
al_. Karyotypic imbalances and differential gene expressions in the acquired doxorubicin resistance of hepatocellular carcinoma cells. _Lab Invest_ 2005; 85:664–674. Article CAS PubMed
Google Scholar * Gillet JP, Efferth T, Steinbach D, _et al_. Microarray-based detection of multidrug resistance in human tumor cells by expression profiling of ATP-binding cassette
transporter genes. _Cancer Res_ 2004; 64:8987–8993. Article CAS PubMed Google Scholar * Park S, Shimizu C, Shimoyama T, _et al_. Gene expression profiling of ATP-binding cassette (ABC)
transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. _Breast Cancer Res Treat_ 2006; 99:9–17. Article CAS PubMed Google Scholar *
Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W 3rd, Dantzig AH . The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its
monophosphorylated metabolites. _Mol Cancer Ther_ 2005; 4:855–863. Article CAS PubMed Google Scholar * Wielinga P, Hooijberg JH, Gunnarsdottir S, _et al_. The human multidrug resistance
protein MRP5 transports folates and can mediate cellular resistance against antifolates. _Cancer Res_ 2005; 65:4425–4430. Article CAS PubMed Google Scholar * Gillet JP, Schneider J,
Bertholet V, De Longueville F, Remacle J, Efferth T . Microarray expression profiling of ABC transporters in human breast cancer. _Cancer Genomics Proteomics_ 2006; 3:97–106. CAS PubMed
Google Scholar * Mutch DM, Anderle P, Fiaux M, _et al_. Regional variations in ABC transporter expression along the mouse intestinal tract. _Physiol Genomics_ 2004; 17:11–20. Article CAS
PubMed Google Scholar * Annereau JP, Szakacs G, Tucker CJ, _et al_. Analysis of ATP-Binding Cassette Transporter Expression in Drug-Selected Cell Lines by a Microarray Dedicated to
Multidrug Resistance. _Mol Pharmacol_ 2004; 66:1397–1405. Article CAS PubMed Google Scholar * Kimmig A, Gekeler V, Neumann M, _et al_. Susceptibility of multidrug-resistant human
leukemia cell lines to human interleukin 2-activated killer cells. _Cancer Res_ 1990; 50:6793–6799. CAS PubMed Google Scholar * Brugger D, Herbart H, Gekeler V, _et al_. Functional
analysis of P-glycoprotein and multidrug resistance associated protein related multidrug resistance in AML-blasts. _Leuk Res_ 1999; 23:467–475. Article CAS PubMed Google Scholar *
Steinbach D, Gillet JP, Sauerbrey A, _et al_. ABCA3 as a possible cause of drug resistance in childhood acute myeloid leukemia. _Clin Cancer Res_ 2006; 12:4357–4363. Article CAS PubMed
Google Scholar * Yamano G, Funahashi H, Kawanami O, _et al_. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. _FEBS Lett_ 2001; 508:221–225. Article CAS
PubMed Google Scholar * Efferth T, Gillet JP, Sauerbrey A, _et al_. Expression profiling of ATP-binding cassette transporters in childhood T-cell acute lymphoblastic leukemia. _Mol Cancer
Ther_ 2006; 5:1986–1994. Article CAS PubMed Google Scholar * Szakacs G, Annereau JP, Lababidi S, _et al_. Predicting drug sensitivity and resistance: profiling ABC transporter genes in
cancer cells. _Cancer Cell_ 2004; 6:129–137. Article CAS PubMed Google Scholar * Ludwig JA, Szakacs G, Martin SE, _et al_. Selective toxicity of NSC73306 in MDR1-positive cells as a new
strategy to circumvent multidrug resistance in cancer. _Cancer Res_ 2006; 66:4808–4815. Article CAS PubMed PubMed Central Google Scholar * Langmann T, Mauerer R, Zahn A, _et al_.
Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. _Clin Chem_ 2003; 49:230–238. Article CAS
PubMed Google Scholar * Langmann T, Mauerer R, Schmitz G . Human ATP-binding cassette transporter TaqMan low-density array: analysis of macrophage differentiation and foam cell formation.
_Clin Chem_ 2006; 52:310–313. Article CAS PubMed Google Scholar * Liu Y, Peng H, Zhang JT . Expression Profiling of ABC Transporters in a Drug-Resistant Breast Cancer Cell Line Using
AmpArray. _Mol Pharmacol_ 2005; 68:430–438. CAS PubMed Google Scholar * Kallioniemi A, Kallioniemi OP, Sudar D, _et al_. Comparative genomic hybridization for molecular cytogenetic
analysis of solid tumors. _Science_ 1992; 258:818–821. Article CAS PubMed Google Scholar * Boonstra R, Timmer-Bosscha H, van Echten-Arends J, _et al_. Mitoxantrone resistance in a small
cell lung cancer cell line is associated with ABCA2 upregulation. _Br J Cancer_ 2004; 90:2411–2417. Article CAS PubMed PubMed Central Google Scholar * Wilson MW, Fraga CH, Fuller CE,
_et al_. Immunohistochemical detection of multidrug-resistant protein expression in retinoblastoma treated by primary enucleation. _Invest Ophthalmol Vis Sci_ 2006; 47:1269–1273. Article
PubMed Google Scholar * Zhang W, Shannon WD, Duncan J, Scheffer GL, Scheper RJ, McLeod HL . Expression of drug pathway proteins is independent of tumour type. _J Pathol_ 2006; 209:213–219.
Article CAS PubMed Google Scholar * Lee JK, Bussey KJ, Gwadry FG, _et al_. Comparing cDNA and oligonucleotide array data: concordance of gene expression across platforms for the NCI-60
cancer cells. _Genome Biol_ 2003; 4:R82. Article PubMed PubMed Central Google Scholar * Brown KJ, Fenselau C . Investigation of doxorubicin resistance in MCF-7 breast cancer cells using
shot-gun comparative proteomics with proteolytic 18O labeling. _J Proteome Res_ 2004; 3:455–462. Article CAS PubMed Google Scholar * Gehrmann ML, Hathout Y, Fenselau C . Evaluation of
metabolic labeling for comparative proteomics in breast cancer cells. _J Proteome Res_ 2004; 3:1063–1068. Article CAS PubMed Google Scholar * Isidoro A, Casado E, Redondo A, _et al_.
Breast carcinomas fulfill the Warburg hypothesis and provide metabolic markers of cancer prognosis. _Carcinogenesis_ 2005; 26:2095–2104. Article CAS PubMed Google Scholar * Calcagno AM,
Chewning KJ, Wu CP, Ambudkar SV . Plasma membrane calcium ATPase (PMCA4): a housekeeper for RT-PCR relative quantification of polytopic membrane proteins. _BMC Mol Biol_ 2006; 7:29. Article
PubMed PubMed Central Google Scholar * Han B, Zhang JT . Multidrug resistance in cancer chemotherapy and xenobiotic protection mediated by the half ATP-binding cassette transporter
ABCG2. _Curr Med Chem Anti-Canc Agents_ 2004; 4:31–42. Article CAS Google Scholar * Zhang JT, Ling V . Study of membrane orientation and glycosylated extracellular loops of mouse
P-glycoprotein by _in vitro_ translation. _J Biol Chem_ 1991; 266:18224–18232. CAS PubMed Google Scholar * Zhang JT, Duthie M, Ling V . Membrane topology of the N-terminal half of the
hamster P-glycoprotein molecule. _J Biol Chem_ 1993; 268:15101–15110. CAS PubMed Google Scholar * Skach WR, Calayag MC, Lingappa VR . Evidence for an alternate model of human
P-glycoprotein structure and biogenesis. _J Biol Chem_ 1993; 268:6903–6908. CAS PubMed Google Scholar * Chen Q, Yang Y, Liu Y, Han B, Zhang JT . Cytoplasmic retraction of the amino
terminus of human multidrug resistance protein 1. _Biochemistry_ 2002; 41:9052–9062. Article CAS PubMed Google Scholar * Chen Q, Yang Y, Li L, Zhang JT . The Amino Terminus of the Human
Multidrug Resistance Transporter ABCC1 Has a U-shaped Folding with a Gating Function. _J Biol Chem_ 2006; 281:31152–31163. Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported in part by the National Institutes of Health Grants CA94961 and CA120221. Editorial proof reading by Jeff Russ is appreciated. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Department of Pharmacology and Toxicology, Walther Oncology Center/Walther Cancer Institute and IU Cancer Center, Indiana University School of Medicine, 1044 W.
Walnut Street, R4-166, Indianapolis, IN, 46202, USA Jian-Ting Zhang Authors * Jian-Ting Zhang View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to Jian-Ting Zhang. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, JT. Use of arrays to investigate the
contribution of ATP-binding cassette transporters to drug resistance in cancer chemotherapy and prediction of chemosensitivity. _Cell Res_ 17, 311–323 (2007).
https://doi.org/10.1038/cr.2007.15 Download citation * Published: 03 April 2007 * Issue Date: April 2007 * DOI: https://doi.org/10.1038/cr.2007.15 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * genomics * MDR * drug resistance * ABC transporter * microarray * real time quantitative PCR










