- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Lens epithelium-derived growth factor (LEDGF) maintains survival pathways by augmenting the transcription of stress-response genes such as small heat-shock protein 27. Recently,
aberrant expression of LEDGF was found in prostate cancer (PC). Herein, we showed that LEDGF overexpression upregulated Hsp27 in PC cells, DU145, PC-3 and LNCaP and promoted antiapoptotic
pathways in PCs. We found that these cells had higher abundance of Hsp27, which was correlated with the levels of LEDGF expression. Transactivation assay in DU145 cells revealed that
transactivation of Hsp27 was related to the magnitude of LEDGF expression. Silencing of LEDGF in DU145 cells abrogated Hsp27 expression and inhibited stimulated cell proliferation,
invasiveness and migration. These cells were arrested in S and G2 phase, and failed to accumulate cyclin B1, and showed increased apoptosis. Furthermore, LEDGF-depleted DU145 cells displayed
elevated Bax and cleaved caspase 9 expression and reduced levels of Bcl2, Bcl-XL. The activated survival pathway(s), ERK1/2 and Akt, were selectively decreased in these cells, which
characteristically have lower tumorigenicity. Conversely, the depleted cells, when re-overexpressed with LEDGF or Hsp27, regained tumorigenic properties. Collectively, results reveal the
involvement of LEDGF-mediated elevated expression of Hsp27-dependent survival pathway(s) in PC. Our findings suggest new lines of investigation aimed at developing therapies by targeting
LEDGF or its aberrant expression-associated stimulated antiapoptotic pathway(s). SIMILAR CONTENT BEING VIEWED BY OTHERS E2F TRANSCRIPTION FACTOR 2-ACTIVATED DLEU2 CONTRIBUTES TO PROSTATE
TUMORIGENESIS BY UPREGULATING SERUM AND GLUCOCORTICOID-INDUCED PROTEIN KINASE 1 Article Open access 24 January 2022 _HJURP_ PROMOTES PROLIFERATION IN PROSTATE CANCER CELLS THROUGH INCREASING
_CDKN1A_ DEGRADATION VIA THE _GSK3Β/JNK_ SIGNALING PATHWAY Article Open access 07 June 2021 CIRC_0001671 REGULATES PROSTATE CANCER PROGRESSION THROUGH MIR-27B-3P/BLM AXIS Article Open
access 28 May 2024 MAIN A multi-domain flexible nuclear protein, lens epithelium-derived growth factor (LEDGF), exercises a variety of functions involving cellular abnormalities by
interacting with DNA/protein.1, 2, 3, 4, 5 LEDGF was originally identified as a transcriptional protein that binds to chromatin/DNA1, 6 mapped to chromosome 9p22.3 genetic locus.2, 7
Recently, LEDGF has been shown to interact with multiple proteins such as Myc-interacting protein JPO28 and mixed-lineage leukemia/menin complex,9 a domesticated transposase pogo
transposable element-derived protein with zinc finger10 and Cdc7-activator of S-phase kinase (ASK).11 Furthermore, its helix-turn-helix-like motifs (aa, 421–442 and aa, 471–492) bind to
heat-shock element (nGAAn), and N-terminal LEDGF interacts with stress-response elements (nA/TGGGGA/Tn), thereby regulating transcription of small heat-shock proteins (Hsps) such as
_α_B-crystallin and Hsp271, 12, 13 and enhancing cell survival.1, 14, 15 Recently, aberrant expression of LEDGF was found to be involved in development of various types of cancers including
subcutaneous angiogenesis and lymphangiogenesis of ovarian carcinoma tumors.16, 17 More recently, elevated expression of LEDGF was reported in 61% of prostate tumors.18 Moreover,
stress-inducible LEDGF provides cell survival by upregulating Hsp27.1, 19 Hsp27 belongs to the family of ‘survival proteins’ and its involvement has been found in a number of cell death
pathways induced by cellular and environmental stresses.20 We posit that LEDGF-mediated overexpression of Hsp27 protein may alter the crucial balance between cell proliferation and cell
death, which in turn may lead to cell transformation. Hsp27 can modulate cell proliferation by interacting with the Akt pathway, which has a pivotal role in the survival of many types of
cancer cells.21, 22, 23, 24, 25 As LEDGF is an enhancer of Hsp27, and enhanced expression of Hsp27 is known to be a causative factor in prostate cancer (PC) progression and invasiveness,26
we hypothesize that LEDGF–induced tumorigenicity may be selectively associated with upregulation of Hsp27-mediated antiapoptotic signaling in PC cells.27, 28 During carcinogenesis, a large
majority of signaling molecules are affected, and their downstream effectors are generally transcription factors. The smaller numbers of these transcription factors may be an ideal target
for treating/postponing cancer formation.29 Thus, we think that LEDGF should be a potential and promising target for development of transcription-based therapeutics.30 The RNA interference
technology has been shown to be a successful and effective way of inhibiting the synthesis of specific protein.31 In the current study, we found that expression of LEDGF dramatically
elevated in PC cells, DU145, PC-3 and LNCaP. Additionally, we showed that if LEDGF was overexpressed in normal prostate cells, PWR-1E led to transformation, and these cells showed
characteristics of cancer cell, DU145. Notably, DU145 cells aberrantly expressing LEDGF or overexpressed with LEDGF displayed enhanced and aggressive characteristic features of
tumorigenicity (Figures 8 and 9). In this work, we provide evidence that knocking down of LEDGF in DU145 by LEDGF-targeted small interfering RNA (siRNA) reduced tumorigenicity, and reduced
tumorigenicity was related to reduced expression of Hsp27 and attenuation of Hsp27-mediated survival pathways(s). As a whole, our findings not only offer new perspectives in the modulation
of cellular events by LEDGF but also suggest a novel therapeutic target for cancer treatment. RESULTS ABERRANT EXPRESSION OF LEDGF IN HUMAN PROSTATE CARCINOMA CELLS DU145, PC-3 AND LNCAP WAS
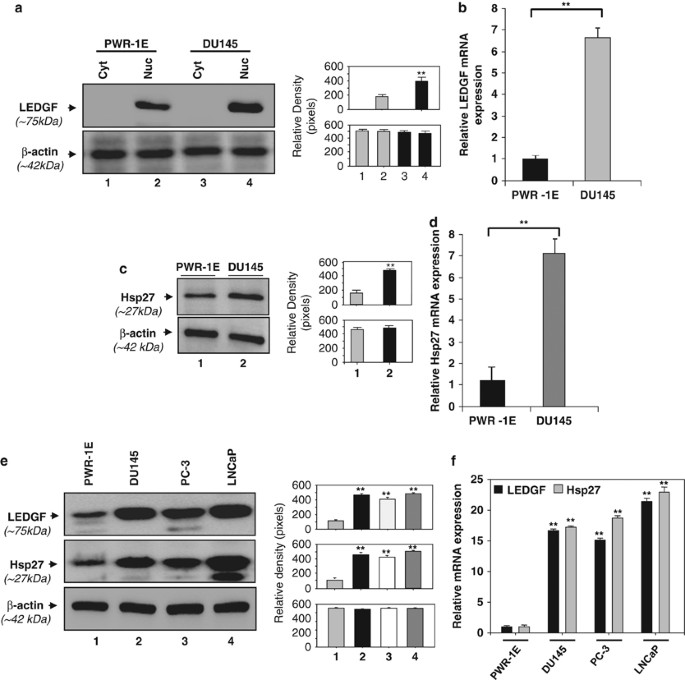
ASSOCIATED WITH INCREASED EXPRESSION OF ITS DOWNSTREAM TARGET HSP27 Using an androgen-independent PC cell line DU145 we studied whether increased expression of LEDGF was associated with
increased expression of Hsp27 and compared with normal prostate epithelial cell line PWR-1E. Analysis of LEDGF expression by real-time PCR and western blot analyses showed that the DU145
cells harbored significantly elevated expression of LEDGF protein in nucleus (Figure 1a, lane 2 _versus_ 4) and mRNA (Figure 1b, gray bar) compared with normal cells (PWR-1E). Interestingly,
mRNA expression of LEDGF in DU145 cells was approximately seven-fold higher than in normal cells (Figure 1b). Next, we tested whether levels of Hsp27 protein and mRNA were also increased in
DU145 cells, as LEDGF is a regulator of Hsp27. Figure 1c (lane 2) and Figure 1d (gray bar) demonstrate that indeed, the levels of Hsp27 were elevated in DU145 cells. Next, we examined
whether the expression of LEDGF was elevated in other androgen-dependent human PC cell lines, PC-3 and LNCaP. As shown in the Figure 1e, these cell lines also harbored elevated LEDGF protein
(Figure 1e, upper panel; lanes 2, 3 and 4). Observed increased levels of Hsp27 in these cell lines (Figure 1e, middle panel; lane 1 _versus_ 2, 3 and 4) indicating a correlation between
LEDGF expression and Hsp27. Furthermore, the expression levels of LEDGF and Hsp27 mRNA were also significantly higher in the PC cell lines (Figure 1f) as evidenced by real-time PCR. In the
current study, we have chosen to perform experiments using normal cells PWR-1E and DU145. LEDGF OVEREXPRESSION ENHANCED THE TRANSACTIVATION OF HSP27 PROMOTER AND INCREASED THE MIGRATION AND
INVASIVENESS OF DU145 CELLS On the basis of our earlier work,1 we hypothesized that the increased expression of LEDGF may be one factor in the increased Hsp27 expression in DU145 cells. To
test this, we first confirmed localization pattern and integrity of ectopically expressed pEGFP-LEDGF in DU145. Figure 2A showed that the ectopically expressed pEGFP-LEDGF protein localized
in nucleus. Next, we wished to examine DU145 containing elevated expression of LEDGF, whether these cells overexpressed with LEDGF display further increase in the expression of Hsp27 mRNA.
DU145 cells were overexpressed with different concentrations (250, 500 ng, 1 and 2 _μ_g) of pEGFP-LEDGF. Real-time PCR analysis revealed LEDGF concentration-dependent increased expression of
LEDGF mRNA (Figure 2B, black bars) and Hsp 27 (Figure 2B, gray bars). The increases in Hsp27 mRNA appeared to be associated with the abundance of LEDGF, further supports LEDGF as a
regulator of Hsp27. To examine whether LEDGF transactivated Hsp27 promoter in DU145, cells were co-transfected with varying concentrations of pEGFP-LEDGF or pEGFP-vector and Hsp27-CAT
(co-transfection and promoter activity) promoter. CAT values increased significantly with increasing concentration of the LEDGF constructs (Figure 2C), indicating that Hsp27 was
transactivated by LEDGF. To determine whether LEDGF overexpression in these cells was functionally involved in enhancing tumorigenicity, cells overexpressing LEDGF were subjected to
migration and invasion assays. In the migration assay, confluent and quiescent monolayers of cells were wounded. Photographs were taken 24 h after wounding to observe changes in migration
(Figure 2D), and the distance of cell migration to the wound area was measured (Figure 2D right panel). Cells overexpressing LEDGF were found to have greater migration potential (Figure 2D).
Also, we observed aggressive invasive behavior in cells overexpressing LEDGF (Figures 8C and Db). Data analysis revealed that cells ectopically expressed with LEDGF showed ∼45% increase in
invasive behavior in comparison with vector-transfected cells. KNOCKDOWN OF LEDGF IN DU145 EFFECTIVELY DOWNREGULATED ITS DOWNSTREAM TARGET HSP27 To examine the direct involvement of LEDGF in
tumorigenesis, DU145 cells were transfected with siRNA specific to LEDGF (Si-LEDGF) or scrambled siRNA vectors (Si-Control). Stably selected transfectants were examined for LEDGF mRNA
(Figure 3a) and protein (Figure 3b) by real-time PCR and western blot. The results indicated that cells stably transfected with siRNA LEDGF had significantly reduced expression of LEDGF.
Next, we tested whether these cells displayed lower expression of Hsp27 by performing western analysis using anti-Hsp27 antibody. Data revealed that reduced expression of Hsp27 was
associated with LEDGF expression level (Figure 3c). To rule out the down regulation of Hsp27 expression is specific to LEDGF depletion, and not due to nonspecific silencing effects of siRNA
used against LEDGF, we reprobed the membrane with specific antibodies for other proteins such as Sp1, Prdx6 and _β_-actin (Figure 3c). Densitometry of immunoblots disclosed no change in the
expression pattern of Sp1 or Prdx6 or _β_-actin, suggesting that Si-LEDGF specifically depleted LEDGF. To examine further whether Si-LEDGF reduced promoter activity of Hsp27, we performed
transactivation assay. We found that Si-LEDGF cells displayed reduced Hsp27 promoter activity compared with Si-Control cells (Figure 3d, open bar _versus_ gray bar). Furthermore, it is known
that activated STAT3 upregulates Hsp27 and facilitate phosphorylation of Hsp27 at serine residue 78 and aberrant STAT3 signaling induces cell malignancies. We, therefore, next examined the
level of STAT3 expression in LEDGF-depleted cells. We could observe a reduced level of total (Figure 3e, upper panel) and phosphorylated (Figure 3e, middle panel) forms of STAT3 protein.
KNOCKDOWN OF LEDGF IN DU145 CELLS SUPPRESSED PROLIFERATION AND SURVIVAL To determine whether Si-LEDGF attenuates the tumorigenic character of DU145 cells, we did proliferation/cell viability
of stably transfected Si-LEDGF-DU145. On day 0 (baseline), Si-LEDGF cells or Si-Control cells were seeded at equal density, and cell proliferation and survival were measured by MTS (A) and
_Brd_U incorporation (B) assays. At 24, 48 and 72 h, the increase in proliferation of tumor cells was assessed. At each time point, the mean fold increase in proliferation and survival of
Si-LEDGF cells was statistically less than that in scrambled siRNA-transfected cells (Figures 4A and B). Our next step was determining whether LEDGF knockdown cells would take a relatively
longer time to attain monolayer confluency compared with normal PWR-1E or scrambled Si-Control cells. Cells were cultured in 6-well plates, and chase culture was carried out up to 96 h and
photomicrographed (Figure 4C). Comparisons between PWR-1E or Si-Control and Si-LEDGF at different time points demonstrated that Si-LEDGF cells displayed decreased migration/proliferation
(Figures 4Ci) compared with PWR-1E (Figures 4Ca–d) or Si-Control (Figures 4Ce–h). KNOCKDOWN OF LEDGF INDUCED S-PHASE AND G2-M ARREST THROUGH DOWNREGULATION OF CYCLIN B1 IN DU145 CELLS To
characterize the process of inhibition of proliferation of Si-LEDGF-DU145 cells, we analyzed the cell cycle progression by FACS analysis as described elsewhere.32 As shown in Figure 5a,
transfection of scrambled or control siRNA did not affect the cell cycle profile, whereas Si-LEDGF-transfected cells showed an obvious decrease in the percentage of cells with 2N DNA
(∼36.21%) content (Figure 5a). Interestingly, LEDGF knockdown caused an enrichment of cells in the S-phase (51.31%) and G2 phase (∼12.48%). However, a corresponding decrease of cells in the
G1 phase (∼36.21%) was observed compared with Si-control cells (∼62.19%), indicating that LEDGF-deficient cells having an impaired cell cycle (Figure 5a). As before mitotic centrosome
activation at the G2–M transition, cell cycle progression through G2 to mitosis is accompanied by an accumulation of cyclin B1, it was appropriate to examine whether LEDGF knockdown cells
contained reduced levels of cyclins. Data revealed a significant reduction in the expression of G2-regulatory kinase cyclin B1 (Figure 5b, lane 2) in Si-LEDGF cells. LEDGF-DEPLETED DU145
CELLS DISPLAYED INCREASED EXPRESSION OF BAX AND UNDERWENT APOPTOSIS As expected, analysis with TdT-mediated dUTP-biotin nick end labeling (TUNEL) staining revealed more apoptotic nuclei in
Si-LEDGF-DU145 cells, with approximately 37% of the cell population showing characteristic apoptotic nuclei (Figures 6Aa). As shown in Figure 6C, LEDGF knockdown cells revealed a significant
increase in the expression of Bax protein (Figure 6Ca, lane 2). Next, we examined the expression pattern of Bcl2, Bcl-XL and procaspase or caspases 9. Data revealed a significant reduction
in expression of survival molecules Bcl2 (Figure 6Cb, lane 2) and Bcl-xL (Figure 6Cc, lane 2) and caspase 9 was activated; two apparent bands of molecular weight ∼47 kDa (procaspase 9) and
∼35 kDa (cleaved caspase-9) could be observed, and the level of cleaved band (active form) was found to be increased in Si-LEDGF-deficient cells (Figure 6Cd, lane 2). LEDGF DEPLETION
ATTENUATED CELL INVASION AND MIGRATION AND STABILIZED ACTIVATION OF ERK/AKT To address the role of LEDGF in PC invasion and metastasis, siRNA-LEDGF-and scrambled Si-Control-DU145 cells were
allowed to invade through polycarbonate membrane inserts. As shown in Figures 7A and B, LEDGF knockdown showed reduced invasive characteristics for cells (25% cell invasion) compared with
Si-Control cells (80% cell invasion). To further examine the involvement of LEDGF in invasive behavior, we performed an _in vitro_ wound-healing assay using LEDGF-depleted DU145 cells.
Confluent and quiescent monolayers of scrambled Si-Control or Si-LEDGF stable DU145 cells were wounded. At 24 h later, cell migration or the distance of cell migration to the wound area was
measured (Figure 7C, compare Ca _versus_ Cb; Cc _versus_ Cd). The wound-healing ability of LEDGF-depleted cells was much slower than that of scrambled Si-Control (Figures 7C and D). Next we
tested whether Hsp27-depedent ERK/Akt pathway is activated in DU145 cells, and whether that pathway could be inhibited by knocking down LEDGF. Western analysis revealed the deactivation of
ERK1/2 and Akt proteins in Si-LEDGF-transfected cells (Figure 7E, lane 2), suggesting that LEDGF stimulation of cancer cell progression may be associated with the ERK/Akt pathway. LEDGF
OVEREXPRESSION REVERSED THE STABILIZED VIABILITY, PROLIFERATION AND INVASIVE BEHAVIOR OF LEDGF-DEPLETED DU145 CELLS To determine whether re-overexpression of LEDGF would reverse the
stabilization of the viability, proliferation and invasiveness of Si-LEDGF cells, we overexpressed the Si-Control and Si-LEDGF cells with pEGFP-vector or pEGFP-LEDGF. Cell proliferation and
viability were assessed after 24, 48 and 72 h as stated in Materials and Methods. At all time points, the viability (Figure 8A) and proliferation (Figure 8B) rate was significantly increased
in Si-LEDGF cells overexpressed with LEDGF. Interestingly, Invasive assay using scrambled Si-Control and Si-LEDGF cells overexpressed with pEGFP-vector or pEGFP-LEDGF (Figures 8C and Dd),
revealed an increase in invasive potential of cells re-overexpressed with LEDGF. LEDGF KNOCK DOWN CELLS OVEREXPRESSING HSP27, REACTIVATED ERK1/2 AND AKT PHOSPHORYLATION, REGAINED ITS
VIABILITY, PROLIFERATION AND MIGRATORY BEHAVIOR To examine whether overexpression of Hsp27, the downstream target of LEDGF, in LEDGF-depleted cells could reverse the process displaying
tumorigenic properties, we ectopically expressed Hsp27 by transfecting pCMV-Hsp27 (1 and 2 _μ_g) into Si-LEDGF (LEDGF-depleted) and Si-Control cells. First, we confirmed the LEDGF silencing
(Figure 9Aa) and its dose-responsive effect on Hsp27 expression (Figure 9Ab). The same membrane was reprobed with ERK and Akt antibodies specific to phosphorylated or non-phosphorylated
forms. Interestingly, we could observe reactivation of both ERK1/2 (Figure 9Ac, right panel; lanes 2 and 3) and Akt (Figure 9Ae, right panel; lanes 2 and 3) proteins, indicating involvement
of LEDGF in PC progression is through Hsp27-ERK/Akt signaling. Next, we became interested to know whether above-observed events, reactivation of ERK1/2 and Akt may also reverse its
viability, proliferation and migration behaviors. We found that indeed, the overexpression of Hsp27 in LEDGF-depleted cells, significantly reversed its viability (Figure 9B), proliferation
(Figure 9C) and migratory behavior (Figure 9Dh). Conversely, Si-Control cells overexpressed with Hsp27 further enhanced its proliferation (Figures 9A and B) and migratory behavior (Figures
9Db). ECTOPIC EXPRESSION OF LEDGF IN NORMAL PROSTATE EPITHELIAL CELLS, PWR-1E, TRANSFORMED THEM INTO INVASIVE CELLS To determine whether normal prostate cells overexpressing LEDGF initiate
tumorigenesis, we transfected PWR-1E cells with 1 _μ_g of pEGFP-LEDGF (Figure 10A). The ectopic expression of LEDGF enhanced the survival (Figure 10B) and proliferation (Figure 10C) of these
cells as evidenced by viability (MTS) and BrdU assays. Furthermore, in a parallel experiment, these cells were also used for invasion assay. Data showed an induction of invasiveness in
these cells (Figures 10D and Eb). DISCUSSION Our present work showed that androgen-independent DU145 and androgen-dependent PC-3 and LNCaP PC cell lines tested using expression analysis
displayed aberrant expression of LEDGF and Hsp27. Importantly, the expression level of Hsp27 was well correlated with abundance of LEDGF protein. We also found that normal cells, PWR-1E when
overexpressed with LEDGF led to expression of tumorigenic property, arguing that LEDGF overexpression causes initiation of tumorigenesis (Figures 10D and E). Furthermore, DU145 having
aberrant expression of LEDGF when overexpressed with LEDGF revealed more aggressive tumorigenic properties (Figure 2). In the above scenario we concluded that transcriptional protein LEDGF
is a crucial factor required for progression and initiation of tumorigenesis. LEDGF achieved this by upregulating Hsp27, and thereby stimulating the survival signaling Akt/ERK pathway(s).
Effective knockdown of LEDGF in DU145 cells, abolished its tumorogenic properties through downregulation of Hsp27 and deactivation of ERK/Akt proteins. Interestingly, LEDGF-depleted DU145
cells when overexpressed with LEDGF or Hsp27, a reversal of tumorigenic properties occurred in these cells through the reactivation of ERK1/2/Akt proteins (Figure 9), supporting the
hypothesis that LEDGF has its role in cancer progression through the upregulation of Hsp27. Hsp27 has been reported to interact with STAT3, a crucial transcription factor implicated in the
maintenance and antiapoptotic status of cancerous cells.33, 34 We found that LEDGF-depleted cells exhibited reduced level of STAT3 and phosphorylated forms (Figure 3e). Furthermore, LEDGF
has recently attracted attention owing to its frequent aberrant expression in tumor cells, and to its cytoprotective and chemoresistance properties.30 Our data showed that it is possible to
markedly reduce the expression of LEDGF in DU145 cancer cells and its tumorigenicity/metastasis, by the use of Si-LEDGF (Figure 7E). Furthermore, the increased LEDGF expression in prostate
tumors and BPH compared with normal prostate tissue suggests that this protein might be upregulated during prostate carcinogenesis or development of inflammatory conditions of the
prostate.18, 30 Its relatively high expression in prostate tumors and their adjacent ‘normal’ tissue is likely induced by inflammation and oxidative stress, key factors underlying prostate
carcinogenesis.35 Inflammation and oxidative stress are associated with development of BPH, which may explain our observation that LEDGF is also strongly expressed in human prostate
carcinomas, and this enhanced expression in cancer cells such as DU145 may be a major event in progression of cancer (Figures 7B–D). Moreover, Hsp27 is a stress-induced molecular chaperone
that is highly expressed in castrate-resistant PC and other cancers, and is often associated with metastasis and poor prognosis.36 In the present study, we observed that LEDGF was aberrantly
expressed, and the transactivation of LEDGF promoter was much higher in human prostate carcinoma cells, DU145. Interestingly, the effective depletion of LEDGF resulted in a decreased level
of Hsp27 and promoter activity in these cells. However, the effective knockdown of LEDGF in DU145 cells led to a significant decrease in their proliferation rate as evidenced by BrdU assay
(Figure 3) and delayed monolayer confluency. Overexpression/re-overexpression of LEDGF significantly increased the proliferative property and viability in these cells as examined by BrdU and
MTS assays, respectively, emphasizing the indispensable role of LEDGF in controlling cell proliferation. Also, the knockdown of LEDGF reversed the apoptotic signaling in cancer cells, as
shown by an increase in Bax and cleaved caspase 9 expression and downregulation of procaspase 9, Bcl2 and Bcl-xL proteins (Figure 6D). Initiation of mitosis is known to be regulated by the
activation of cdc2–cyclin B complex.37 Although we could not show that cyclin B1 is the direct target of LEDGF, we did observe that silencing of LEDGF induced activation of the apoptotic
signaling and S-phase and G2-arrest of the cells significantly (Figure 5a). However, we can expect more remarkable and noticeable results following synchronization of cells. However, in
present study, we followed the methods of Long _et al._,32 to produce natural and _bona-fide_ outcomes. We think that synchronization of cells is not feasible or cumbersome to occur in
body’s natural cellular microenvironment. However, in a previously published study, LEDGF was found to interact with the S-phase kinase (Cdc7:ASK) during cell cycle progression. It is well
known that in higher eukaryotic cells, cyclin B1 is excluded from the nucleus by its NES-dependent transport mechanism during interphase, and that NES-mediated export of proteins from the
nucleus, including cyclin B1, has a role in the DNA damage-induced G2 checkpoint. Our results in this study suggest that the failure to detect cyclin B1 in the nuclear fraction of
siRNA-transfected cells may be due to its DNA damage-induced G2 checkpoint. Hsp27 is also involved in the regulation of Akt, inhibiting Bax activation to enhance cell survival. We found that
silencing of the _LEDGF_ gene deactivated these two important signaling molecules. However, in this work we could be able to demonstrate the efficiency of Si-LEDGF in blocking or reducing
growth and proliferation of PC cells _in vitro_ and inhibiting progression of tumorigenic properties of PC cells. Our results from cell viability (MTS assay) and proliferation (BrdU assay)
coupled with migration and invasion assays suggest that LEDGF may have role in cancer proliferation. Targeting LEDGF knockdown strategy may be useful tool to suppress growth of PC cells.
Furthermore, BrdU incorporation and cell cycle assays revealed that Si-LEDGF delivery to DU145 cells induces cell arrest at G2 level or their progression, and that in turn leads to apoptotic
cell death. In conclusion, we demonstrated, for the first time, that the underlying mechanism involved in LEDGF deficiency blocks carcinogenesis by suppressing Hsp 27-ERK1/2 and Akt
pathways in human PC cells DU145. Certainly, more work is needed for a full understanding of the role of LEDGF in PC. However, we were able to show that LEDGF knockdown should be an
effective strategy in developing more effective and curative anticancer therapeutics in combination with known cancer drugs, potentially leading to new treatments for metastatic PC.
MATERIALS AND METHODS CELL LINES Human prostate adenocarcinoma cell lines DU145, PC-3 and LNCaP were used in the present study. Observations were compared with normal prostate epithelium
cell line PWR-1E wherever necessary. All the cell lines were obtained from the American Tissue Culture Collection (Bethesda, MD, USA). The DU145 cells were cultured at 37 °C in a 5% CO2
atmosphere in minimum essential medium (MEM) (Gibco-BRL, Bethesda, MD, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS). The MEM also contained 1 mM L-glutamine, 25 mM
Hepes, 100 unit/ml penicillin and 100 mg/ml streptomycin. The PWR-1E cells were cultured in Keratinocyte serum-free medium supplemented with growth factors (Gibco-BRL). PC-3 and LNCaP cells
were maintained in RPMI medium supplemented with 10% heat-inactivated FCS, 1 mM L-glutamine, 25 mM Hepes, 100 unit/ml penicillin and 100 mg/ml streptomycin. REAL-TIME PCR Total RNA was
extracted from cells using TriZol Reagent (Invitrogen, Carlsbad, CA, USA), and was used for first-strand DNA synthesis using SuperScript II (Invitrogen). PCR was performed with the following
primer sequences: human LEDGF (Forward primer: 5′-CAGCAACAGCATCTGTTAATCTAAA-3′ and reverse primer: 5′-GGGCTGTTTTACCATCATTTTGG-3′); human Hsp27 (forward primer: 5′-TCCCTGGATGTCAACCACTT-3′
and reverse primer: 5′-GATGTAGCCATGCTCGTCCT-3′) and human _β_-actin (forward primer: 5′-CCAACCGCGAGAAGATGA-3′ and reverse primer: 5′-CCAGAGGCGTACAGGGATAG-3′). All PCR experiments are
performed in Light Cycler 480 with SYBR Green fluorescence detection (Roche Diagnostics GmbH, Indianapolis, IL, USA). WESTERN BLOT ANALYSIS Whole-cell extracts or nuclear extracts were
prepared as described earlier.38 Equal amounts of protein samples were resolved onto an SDS-PAGE. Following immunoblotting with primary antibodies; LEDGF monoclonal (BD Biosciences, San
Jose, CA, USA; Cat. no. 611714), Hsp27 polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA, USA; Cat. no. sc-1048), cyclin B1 (Cell Signaling Technology, Danvers, MA, USA; Cat. no. 4135),
Bax (Santa Cruz Biotechnology; Cat. no. sc-7480), Bcl2 (Cell Signaling Technology; Cat. no. 2876), Bcl-XL (Santa Cruz Biotechnology; Cat. no. sc-7195), caspase-9 (Cell Signaling Technology;
Cat. no. 9508), pERK1/2 (Cell Signaling Technology; Cat. no. 9106), ERK1/2 (Cell Signaling Technology; Cat. no. 9107), Akt (Cell Signaling Technology; Cat. no. 9272) and pAkt (Cell Signaling
Technology; Cat. no. 4051), Prdx6 (Santa Cruz Biotechnology; Cat. no. sc-271368), Sp1 (Santa Cruz Biotechnology; Cat. no.sc-17824), STAT3 (Santa Cruz Biotechnology; Cat. no.sc-482),
membrane was visualized following standard protocol and recorded with FUJIFILM-LAS-4000 luminescent image analyzer (FUJIFILM Medical system Inc., Stamford, CT, USA). CONSTRUCTION OF
HSP27-CAT AND EGFP-LEDGF CONSTRUCTS Hsp27-CAT construct was engineered as described previously.13 Briefly, a forward primer containing a _Sac_I site (5′-GCGTCGAGCTCTCGAATTCATTTGCTT-3′) and
reverse primer with a _Xho_I site (5′-GCTCTCGAGGTCTGCTCAGAAAAGTGC-3′) were used to generate the fragment, which was cloned between the _EcoR_I sites of the TA vector (Invitrogen). The
Hsp27-TA construct was digested with _Sac_I and _Xho_I and promoter fragments was ligated to pCAT-Basic vector (Promega, Madison, WI, USA). A construct containing a green fluorescent protein
(GFP) and LEDGF cDNA was generated with the ‘living color system’ (Clontech, Palo Alto, CA, USA) using the plasmid vector pEGFP-C1 (Clontech) for LEDGF protein overexpression studies.
Expression construct, pCMV-Hsp27 and empty vector, pCMV-V, were purchased from Addgene (Cambridge, MA, USA). CAT ASSAY The CAT assay was performed using a CAT-ELISA kit (Roche Diagnostics
GmbH). Cells were transfected/co-transfected with reporter construct (Hsp27-CAT) and/or EGFP-LEDGF/Si-LEDGF expression vectors. After 48 h of incubation, cells were harvested, and extracts
were prepared and protein was normalized. CAT-ELISA was performed to monitor CAT activity, following the manufacturer’s protocol. Absorbance was measured at 405 nm using a microtiter plate
ELISA reader. Transactivation activities were adjusted for transfection efficiencies using GFP or SEAP values. The transfection experiments were carried out either with Superfactamine
Reagent (Invitrogen) or using Neon Transfection system (Invitrogen). SIRNA ASSAY The LEDGF-specific siRNA expression plasmid was designed according to the method described earlier.39 The
sequence was selected from location 1340–1360 (5′-AAAGACAGCATGAGGAAGCGA-3′). The sense and antisense oligonucleotides with the internal loop were synthesized by Invitrogen. These were
annealed and ligated into the _Bam_HI and _Hind_III sites of pSilencer 4.1-CMV hygro (Ambion, Carlsbad, CA, USA). pSilencer 4.1-pCMVhygro expressing a scrambled siRNA (Ambion) was used as a
control. siRNA constructs were transfected into DU145 cell with Superfactamine Reagent (Invitrogen). At 24 h after transfection, stable transfected cells were selected using 400 _μ_g/ml of
hygromycin (Invitrogen) over a period of 9 days. Silencing was confirmed by LEDGF mRNA and protein expression. BRDU INCORPORATION AND MTS ASSAY Cell proliferation assay was performed with
_Brd_U incorporation assay kit according to manufacturer’s protocol (Roche Diagnostics GmbH). Briefly, equal number of cells was seeded in 96-well plates at a density of 5000 cells/well.
Following incubation cells were labeled with _Brd_U for 2 h, the OD was measured at 450 nm. MTS assay performed to measure the viability of the cells at different time points. In brief, 5000
cells/well were seeded into 96-well plates. Following incubation with MTS dye, absorbance was measured at 490 nm. FLOW CYTOMETRY FOR CELL CYCLE AND APOPTOSIS Scrambled Si-Control- and
Si-LEDGF-transfected cells were grown in T75 culture flask. After 24 h, cells were harvested, fixed with 5 ml of ice-cold 70% ethanol (4 °C). Cell pellets were collected by centrifugation
and resuspended in 400 _μ_l of PBS, 50 _μ_l of propidium iodide (PI) solution (0.6 mM) and 50 _μ_l of RNaseA (1 mg/ml). After 30 min cells were analyzed for DNA content using a FACS flow
cytometer. Fluorescence from the PI–DNA complex was estimated on a minimum of 20 000 cells per sample and analyzed with CellQuest Pro software. TUNEL ASSAY Si-LEDGF- and scrambled
Si-Control-transfected cells were seeded onto coverslips and incubated for 24 h. TUNEL staining (_in situ_ cell death detection kit, Roche Diagnostics GmbH) and staining with
4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Dojindo Laboratories, Kumamoto, Japan) were performed. The percentage of TUNEL-positive cells per total number of cells was counted from
the number of DAPI-stained nuclei in six different fields of each slide for each group using the image analyzing software. WOUND-HEALING ASSAY Si-LEDGF-transfected and
Si-Control-transfected cells or pEGFP-V or pEGFP-LEDGF-transfected cells were seeded into 60-mm dishes at 1.5 × 106 cells. The confluent cell monolayer was scraped with a sterile pipette tip
with a fixed diameter. For each dish, three–five wounds were made, and three sites of regular wounds were selected and marked. Wounded monolayers were then washed three times with PBS to
remove cell debris. Cells were permitted to migrate into the area of clearing for 24 h. Immediately after wounding and at the end of the experiment (after 24 h), wounds were photographed and
semiquantitative measurements of wounds were taken. A mean width was determined, and the average width of wound closure was calculated as described previously. CELL INVASION ASSAY Invasion
assay was performed with Cytoselect TM 96-well cell invasion assay (Cell Biolabs, INC., San Diego, CA, USA) according to manufacturer’s instructions. Briefly, around 2 × 106 cells per well
of both Si-Control and Si-LEDGF or pEGFP-V- or pEGFP-LEDGF-transfected cells were resuspended in serum-free MEM medium, and were seeded into the extracellular matrix layer, which had been
previously rehydrated at room temperature for 1–2 h. MEM media of 150 _μ_l containing 10% fetal bovine serum was added to the lower chamber as chemo attractant. Cells were incubated for 24 h
at 37 °C in a CO2 incubator (5% CO2). Invaded cells on the bottom of the insert membrane were dissociated from the membrane by incubation with cell detachment buffer and subsequently lysed
and detected by CyQuant GR dye. The fluorescence was quantified with a fluorescence plate reader using a 480/535-nm filter set. Percent invasion index was calculated by normalizing the
fluorescence reading of invaded cells with total cell fluorescence reading. Invaded cells on the membrane were photomicrographed under fluorescent microscope from each set of experiments, by
fixing the membrane in methanol and then stained with CyQuant GR dye. STATISTICAL ANALYSIS All the statistical analyses were done in Graphpad Prism software (La Jolla, CA, USA). Comparison
between two groups were done with student _t_-test. Multiple comparisons were done by ANOVA. A _P_-value of <0.01 and <0.001 was defined as indicating a statistically significant
difference. ABBREVIATIONS * LEDGF: lens epithelium-derived growth factor * PC: prostate cancer REFERENCES * Singh DP, Fatma N, Kimura A, Chylack LT, Shinohara T . LEDGF binds to heat shock
and stress-related element to activate the expression of stress-related genes. _Biochemi Biophys Res Commun_ 2001; 283: 943–955. Article CAS Google Scholar * Singh DP, Kimura A, Chylack
LT, Shinohara T . Lens epithelium-derived growth factor (LEDGF/p75) and p52 are derived from a single gene by alternative splicing. _Gene_ 2000; 242: 265–273. Article CAS Google Scholar *
Sutherland HG, Newton K, Brownstein DG, Holmes MC, Kress C, Semple CA _et al_. Disruption of Ledgf/Psip1 Results in Perinatal Mortality and Homeotic Skeletal Transformations. _Mol Cell
Biol_ 2006; 26: 7201–7210. Article CAS Google Scholar * Maurer-Stroh S, Dickens NJ, Hughes-Davies L, Kouzarides T, Eisenhaber F, Ponting CP . The Tudor domain ‘Royal Family’: Tudor, plant
Agenet, Chromo, PWWP and MBT domains. _Trends Biochem Sci_ 2003; 28: 69–74. Article CAS Google Scholar * Stec I, Nagl SB, van Ommen GJ, den Dunnen JT . The PWWP domain: a potential
protein-protein interaction domain in nuclear proteins influencing differentiation? _FEBS Lett_ 2000; 473: 1–5. Article CAS Google Scholar * Ge H, Si Y, Roeder RG . Isolation of cDNAs
encoding novel transcription coactivators p52 and p75 reveals an alternate regulatory mechanism of transcriptional activation. _EMBO J_ 1998; 17: 6723–6729. Article CAS Google Scholar *
Singh DP, Ohguro N, Chylack LT, Shinohara T . Lens epithelium-derived growth factor: increased resistance to thermal and oxidative stresses. _Invest Ophthalmol Vis Sci_ 1999; 40: 1444–1451.
CAS PubMed Google Scholar * Maertens GN, Cherepanov P, Engelman A . Transcriptional co-activator p75 binds and tethers the Myc-interacting protein JPO2 to chromatin. _J Cell Sci_ 2006;
119: 2563–2571. Article CAS Google Scholar * Yokoyama A, Cleary ML . Menin critically links MLL proteins with LEDGF on cancer-associated target genes. _Cancer Cell_ 2008; 14: 36–46.
Article CAS Google Scholar * Bartholomeeusen K, Christ F, Hendrix J, Rain JC, Emiliani S, Benarous R _et al_. Lens epithelium-derived growth factor/p75 interacts with the
transposase-derived DDE domain of PogZ. _J Biol Chem_ 2009; 284: 11467–11477. Article CAS Google Scholar * Hughes S, Jenkins V, Dar MJ, Engelman A, Cherepanov P . Transcriptional
co-activator LEDGF interacts with Cdc7-activator of S-phase kinase (ASK) and stimulates its enzymatic activity. _J Biol Chem_ 2010; 285: 541–554. Article CAS Google Scholar * Fatma N,
Singh DP, Shinohara T, Chylack LT . Transcriptional regulation of the antioxidant protein 2 gene, a thiol-specific antioxidant, by lens epithelium-derived growth factor to protect cells from
oxidative stress. _J Biol Chem_ 2001; 276: 48899–48907. Article CAS Google Scholar * Sharma P, Fatma N, Kubo E, Shinohara T, Chylack LT, Singh DP . Lens epithelium-derived growth factor
relieves transforming growth factor-beta1-induced transcription repression of heat shock proteins in human lens epithelial cells. _J Biol Chem_ 2003; 278: 20037–20046. Article CAS Google
Scholar * Matsui H, Lin L-R, Singh DP, Shinohara T, Reddy VN . Lens epithelium-derived growth factor: increased survival and decreased DNA breakage of human RPE cells induced by oxidative
stress. _Invest Ophthalmol Vis Sci_ 2001; 42: 2935–2941. CAS PubMed Google Scholar * Machida S, Chaudhry P, Shinohara T, Singh DP, Reddy VN, Chylack LT _et al_. Lens epithelium-derived
growth factor promotes photoreceptor survival in light-damaged and RCS rats. _Invest Ophthalmol Vis Sci_ 2001; 42: 1087–1095. CAS PubMed Google Scholar * Cohen B, Addadi Y, Sapoznik S,
Meir G, Kalchenko V, Harmelin A _et al_. Transcriptional regulation of vascular endothelial growth factor C by oxidative and thermal stress is mediated by lens epithelium-derived growth
factor/p75. _Neoplasia_ 2009; 11: 921–933. Article CAS Google Scholar * Sapoznik S, Cohen B, Tzuman Y, Meir G, Ben-Dor S, Harmelin A _et al_. Gonadotropin-regulated lymphangiogenesis in
ovarian cancer is mediated by LEDGF-induced expression of VEGF-C. _Cancer Res_ 2009; 69: 9306–9314. Article CAS Google Scholar * Daniels T, Zhang J, Gutierrez I, Elliot ML, Yamada B, Heeb
MJ _et al_. Antinuclear autoantibodies in prostate cancer: immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. _Prostate_ 2005; 62:
14–26. Article CAS Google Scholar * Sharma P, Singh DP, Fatma N, Chylack LT, Shinohara T . Activation of LEDGF gene by thermal- and oxidative-stresses. _Biochem Biophys Res Commun_ 2000;
276: 1320–1324. Article CAS Google Scholar * Mehlen P, Schulze-Osthoff K, Arrigo AP . Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and
staurosporine-induced cell death. _J Biol Chem_ 1996; 271: 16510–16514. Article CAS Google Scholar * Crowell JA, Steele VE, Fay JR . Targeting the AKT protein kinase for cancer
chemoprevention. _Mol Cancer Ther_ 2007; 6: 2139–2148. Article CAS Google Scholar * Hansen RK, Parra I, Lemieux P, Oesterreich S, Hilsenbeck SG, Fuqua SA . Hsp27 overexpression inhibits
doxorubicin-induced apoptosis in human breast cancer cells. _Breast Cancer Res Treat_ 1999; 56: 187–196. Article CAS Google Scholar * Kim EH, Lee HJ, Lee DH, Bae S, Soh JW, Jeoung D _et
al_. Inhibition of heat shock protein 27-mediated resistance to DNA damaging agents by a novel PKC delta-V5 heptapeptide. _Cancer Res_ 2007; 67: 6333–6341. Article CAS Google Scholar *
Aloy MT, Hadchity E, Bionda C, Diaz-Latoud C, Claude L, Rousson R _et al_. Protective role of Hsp27 protein against gamma radiation-induced apoptosis and radiosensitization effects of Hsp27
gene silencing in different human tumor cells. _Int J Radiat Oncol Biol Phys_ 2008; 70: 543–553. Article CAS Google Scholar * Matsui Y, Hadaschik BA, Fazli L, Andersen RJ, Gleave ME, So
AI . Intravesical combination treatment with antisense oligonucleotides targeting heat shock protein-27 and HTI-286 as a novel strategy for high-grade bladder cancer. _Mol Cancer Ther_ 2009;
8: 2402–2411. Article CAS Google Scholar * Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M _et al_. Heat shock protein expression independently predicts clinical
outcome in prostate cancer. _Cancer Res_ 2000; 60: 7099–7105. CAS PubMed Google Scholar * Eisenmann KM, VanBrocklin MW, Staffend NA, Kitchen SM, Koo HM . Mitogen-activated protein kinase
pathway-dependent tumor-specific survival signaling in melanoma cells through inactivation of the proapoptotic protein bad. _Cancer Res_ 2003; 63: 8330–8337. CAS PubMed Google Scholar *
Miller H, Poon S, Hibbert B, Rayner K, Chen YX, O’Brien ER . Modulation of estrogen signaling by the novel interaction of heat shock protein 27, a biomarker for atherosclerosis, and estrogen
receptor beta: mechanistic insight into the vascular effects of estrogens. _Arterioscler Thromb Vasc Biol_ 2005; 25: e10–e14. Article CAS Google Scholar * Darnell JE . Transcription
factors as targets for cancer therapy. _Nat Rev Cancer_ 2002; 2: 740–749. Article CAS Google Scholar * Huang T-s, Myklebust L, Kjarland E, Gjertsen B, Pendino F, Bruserud O _et al_.
LEDGF/p75 has increased expression in blasts from chemotherapy-resistant human acute myelogenic leukemia patients and protects leukemia cells from apoptosis _in vitro_. _Mol Cancer_ 2007; 6:
31. Article CAS Google Scholar * Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC . Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans.
_Nature_ 1998; 391: 806–811. Article CAS Google Scholar * Long XE, Gong ZH, Pan L, Zhong ZW, Le YP, Liu Q _et al_. Suppression of CDK2 expression by siRNA induces cell cycle arrest and
cell proliferation inhibition in human cancer cells. _BMB Rep_ 2010; 43: 291–296. Article CAS Google Scholar * Song H, Ethier SP, Dziubinski ML, Lin J . Stat3 modulates heat shock 27 kDa
protein expression in breast epithelial cells. _Biochem Biophys Res Commun_ 2004; 314: 143–150. Article CAS Google Scholar * Gibertzzz B, Eckelzzz B, Fasquellezzz L, Moulinzzz M,
Bouhallierzzz F, Goninzzz V _et al_. Knock down of heat shock protein 27 (HspB1) induces degradation of several putative client proteins. _PLoS ONE_ 2012; 7: e29719. Article Google Scholar
* Pinthus JH, Bryskin I, Trachtenberg J, Lu JP, Singh G, Fridman E _et al_. Androgen induces adaptation to oxidative stress in prostate cancer: implications for treatment with radiation
therapy. _Neoplasia_ 2007; 9: 68–80. Article CAS Google Scholar * Zoubeidi A, Zardan A, Wiedmann RM, Locke J, Beraldi E, Fazli L _et al_. Hsp27 promotes insulin-like growth factor-I
survival signaling in prostate cancer via p90Rsk-dependent phosphorylation and inactivation of BAD. _Cancer Res_ 2010; 70: 2307–2317. Article CAS Google Scholar * Tyson JJ . Modeling the
cell division cycle: cdc2 and cyclin interactions. _Proc Nat Acad Sci_ 1991; 88: 7328–7332. Article CAS Google Scholar * Fatma N, Singh P, Chhunchha B, Kubo E, Shinohara T, Bhargavan B
_et al_. Deficiency of Prdx6 in lens epithelial cells induces ER stress-response-mediated impaired homeostasis and apoptosis. _Am J Physiol Cell Physiol_ 2011; 301: C954–C967. Article CAS
Google Scholar * Takamura Y, Fatma N, Kubo E, Singh DP . Regulation of heavy subunit chain of gamma-glutamylcysteine synthetase by tumor necrosis factor-alpha in lens epithelial cells: role
of LEDGF/p75. _Am J Physiol Cell Physiol_ 2006; 290: C554–C566. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS Grants provided by the National Eye Institute (NIH)
(EY013394 and EY017613) (to DPS) and Research for Preventing Blindness (RPB) are gratefully acknowledged. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Ophthalmology and Visual
Sciences, University of Nebraska Medical Center, Omaha, 68198-5840, NE, USA B Bhargavan, N Fatma, B Chhunchha, V Singh & D P Singh * Department of Ophthalmology, University of Kanazawa,
Ishikawa, Japan E Kubo Authors * B Bhargavan View author publications You can also search for this author inPubMed Google Scholar * N Fatma View author publications You can also search for
this author inPubMed Google Scholar * B Chhunchha View author publications You can also search for this author inPubMed Google Scholar * V Singh View author publications You can also search
for this author inPubMed Google Scholar * E Kubo View author publications You can also search for this author inPubMed Google Scholar * D P Singh View author publications You can also search
for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to D P Singh. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL
INFORMATION Edited by A Stephanou RIGHTS AND PERMISSIONS This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy
of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bhargavan, B., Fatma, N., Chhunchha, B. _et al._
_LEDGF_ gene silencing impairs the tumorigenicity of prostate cancer DU145 cells by abating the expression of Hsp27 and activation of the Akt/ERK signaling pathway. _Cell Death Dis_ 3, e316
(2012). https://doi.org/10.1038/cddis.2012.57 Download citation * Received: 15 February 2012 * Revised: 16 April 2012 * Accepted: 23 April 2012 * Published: 31 May 2012 * Issue Date: May
2012 * DOI: https://doi.org/10.1038/cddis.2012.57 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * LEDGF * transcription * Hsp27 * DU145 * PWR-1E *
apoptosis





