- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Glioblastoma multiforme (GBM) are resistant to TNF_α_-induced apoptosis and blockade of TNF_α_-induced NF-_κ_B activation sensitizes glioma cells to apoptosis. As Casein kinase-2
(CK2) induces aberrant NF-_κ_B activation and as we observed elevated CK2 levels in GBM tumors, we investigated the potential of CK2 inhibitors (CK2-Is) - DRB and Apigenin in sensitizing
glioma cells to TNF_α_-induced apoptosis. CK2-Is and CK2 small interfering RNA (siRNA) reduced glioma cell viability, inhibited TNF_α_-mediated NF-_κ_B activation, and sensitized cell to
TNF_α_-induced apoptosis. Importantly, CK2-Is activated p53 function in wild-type but not in p53 mutant cells. Activation of p53 function involved its increased transcriptional activation,
DNA-binding ability, increased expression of p53 target genes associated with cell cycle progression and apoptosis. Moreover, CK2-Is decreased telomerase activity and increased senescence in
a p53-dependent manner. Apoptotic gene profiling indicated that CK2-Is differentially affect p53 and TNF_α_ targets in p53 wild-type and mutant glioma cells. CK2-I decreased MDM2-p53
association and p53 ubiquitination to enhance p53 levels. Interestingly, CK2-Is downregulated SIRT1 activity and over-expression of SIRT1 decreased p53 transcriptional activity and rescued
cells from CK2-I-induced apoptosis. This ability of CK2-Is to sensitize glioma to TNF_α_-induced death via multiple mechanisms involving abrogation of NF-_κ_B activation, reactivation of
wild-type p53 function and SIRT1 inhibition warrants investigation. SIMILAR CONTENT BEING VIEWED BY OTHERS CARD16 RESTORES TUMORIGENESIS AND RESTRAINTS APOPTOSIS IN GLIOMA CELLS VIA
FOXO1/TRAIL AXIS Article Open access 08 November 2024 PLK1 INHIBITION PROMOTES APOPTOSIS AND DNA DAMAGE IN GLIOMA STEM CELLS BY REGULATING THE NUCLEAR TRANSLOCATION OF YBX1 Article Open
access 17 February 2023 HYPOXIA-INDUCED CIRCADAMTS6 IN A TDP43-DEPENDENT MANNER ACCELERATES GLIOBLASTOMA PROGRESSION VIA ANXA2/ NF-ΚB PATHWAY Article 17 November 2022 MAIN Despite its
ability to induce apoptosis, several tumors are resistant to TNF_α_-mediated apoptosis.1 This resistance has been attributed to TNF_α_-mediated NF-_κ_B activation as blockade of NF-_κ_B
sensitizes cells to TNF_α_-induced cell death.2, 3 Some anti-cancer chemotherapeutic drugs in combination with TNF_α_ can kill resistant tumor cells.4 Glioblastoma multiforme (GBM) – the
most aggressive malignant brain tumor, is largely resistant to current therapeutic regimens. We have reported that organoselenium Ebselen sensitizes glioma cells to TNF_α_-induced apoptosis
by abrogating NF-_κ_B activation.5 Casein kinase-2 (CK2)– a protein kinase important for cell survival and resistance to apoptosis,6 is over-expressed in several malignancies.7 CK2
negatively regulates caspase activity8 and CK2 inhibitors (CK2-Is) increase the susceptibility of cancer cells to chemotherapeutic agents or apoptotic stimuli.9 CK2 inhibitor, DRB,
sensitizes tumor cells to TRAIL (TNF_α_-related apoptosis inducing ligand)-induced apoptosis.10 CK2 inhibitor, Apigenin, inhibits NF-_κ_B activation in breast cancer cell lines11 and DRB
enhances Apo2L/TRAIL-induced glioma cell death.12 CK2-I blocks TNF_α_-induced p65 phosphorylation13 and reduces constitutive NF-_κ_B activity in breast cancer cells.14 TNF_α_-activated
NF-_κ_B represses p53 transcriptional activity and vice versa.15 Most GBMs harbor p53 mutations, which are considered critical in GBM development16 and p53 rescue compounds induce
p53-dependent growth arrest and sensitizes glioma cells to Apo2L/TRAIL-induced apoptosis.17 SIRT1 an NAD+ (nicotinamide adenine dinucleotide)-dependent deacetylase, known to decrease
transcriptional activity of p53,18 is a component of CK2-regulated anti-apoptotic network.19 Silencing of SIRT1 enhances sensitivity of glioblastoma to radiotherapy.20 Accordingly, we
investigated whether CK2-Is could sensitize glioma cells to TNF_α_-induced apoptosis through regulation of molecules associated with cell survival and resistance to apoptosis such as
NF-_κ_B, p53 and SIRT1. RESULTS ELEVATED CK2 LEVELS IN GLIOBLASTOMA BIOPSY SAMPLES Though the ability of CK2 inhibitor to regulate DNA damage repair response and induce antitumor activity in
a mouse xenograft model of human glioblastoma has been reported,21 nothing is known about the status of CK2 in human glioma samples. We therefore investigated the status of CK2 in GBM
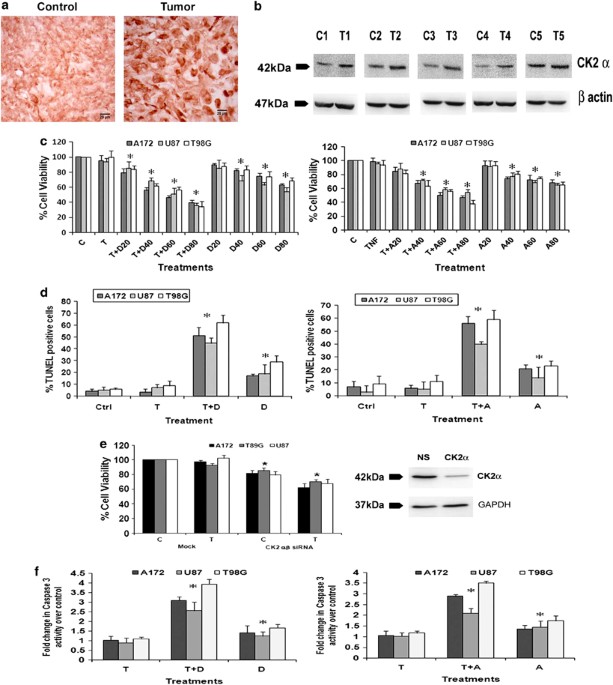
tumors. Immunohistochemistry (IHC) revealed an elevated expression of CK2_α_ in GBM tumors as compared with adjacent normal tissues (Figure 1a). Western blot analysis also confirmed an
increased CK2_α_ expression in glioma tissue as compared with the surrounding non-neoplastic tissue (Figure 1b). CK2 REGULATES GLIOMA CELL VIABILITY AND CONFERS RESISTANCE TO TNF_Α_–INDUCED
APOPTOSIS As CK2 levels were elevated in GBM samples, we investigated its role in glioma cell survival and resistance to apoptosis. Although treatment with 20 _μ_M of DRB had no effect on
cell viability, ∼20–30% reduction in viability was observed upon treatment with 40, 60 and 80 _μ_M of DRB, respectively (Figure 1c). Although treatment with TNF_α_ alone had no effect on
viability of glioma cells, co-treatment with 20, 40, 60 and 80 _μ_M of DRB resulted in ∼30–65% decrease in viability, as compared with control (Figure 1c). Similarly, Apigenin also affected
cell viability (Figure 1c). Thus, both DRB and Apigenin sensitized glioma cells to TNF_α_-induced apoptosis. We chose 40 μM concentration of DRB and Apigenin for subsequent treatments. To
further confirm CK2-I-mediated apoptotic death, TUNEL assay was performed (Figure 1d). The 15–25% increase in TUNEL-positive cells observed upon DRB and Apigenin treatment was further
elevated to ∼ 50–60% when DRB or Apigenin was co-administered with TNF_α_ (Figure 1d). To further confirm the involvement of CK2 in glioma cell survival, viability of cells transfected with
small interfering RNAs (siRNAs) against _α_ and _β_ subunit of CK2, both in presence and absence of TNF_α_ was determined. The 20–25% decrease in cell viability observed upon siRNA-mediated
knock-down of CK2 _α_/_β_ subunits was further elevated to 30–40% in the presence of TNF_α_ (Figure 1e). The significant 1.3, 1.5 and 1.7-fold increase in caspase-3 activity observed in
U87MG, A172 and T98G cells upon treatment with DRB, respectively, was further elevated to 2.9, 3.2 and 4.0-fold in the presence of TNF_α_. Similar activation of caspase-3 was also observed
in cells treated with Apigenin either alone or in combination with TNF_α_ (Figure 1f). CK2-I-MEDIATED APOPTOSIS DOES NOT INVOLVE CASPASE-8 ACTIVATION Interaction of Fas with FADD recruits
procaspase-8 that induces subsequent caspase-8 and caspase-3 activation leading to apoptosis. Although CK2 inhibition is known to trigger apoptosis through FADD and caspase-8,22 CK2-Is had
no effect on FAS or FADD expression (Figure 2a) or caspase-8 activity (Figure 2b) in glioma cells, both in presence and absence of TNF_α_. To further confirm the role of caspase-8, the
viability of cells treated with different combinations of TNF_α_ and CK2-I in the presence and absence of caspase-8 inhibitor was determined. CK2-I-mediated decrease in cell viability
remained unaffected in the presence of caspase-8 inhibitor (Figure 2c). This ruled out the possible involvement of caspase-8 in CK2-I-induced apoptosis. CK2-IS ABROGATE TNF_Α_ INDUCED
NF-_Κ_B ACTIVITY IN GLIOMA CELLS CK2 is known to regulate NF-_κ_B through regulation of IKK.23 Treatment with CK2 inhibitors decreased TNF_α_-induced pI_κ_K_α_/_β_ level in glioma cells
(Figure 2d). This decrease was concomitant with increase in I_κ_B_α_ and decrease in NF-_κ_B levels (Figure 2d). As CK2-I prevents NF-_κ_B activation,11 we investigated whether CK2-Is
increase sensitivity to TNF_α_-induced apoptosis through inhibition of NF-_κ_B activity. Transfection of glioma cells with NF-_κ_B luciferase reporter followed by TNF_α_ treatment
demonstrated ∼9–11-fold increase in luciferase activity over the vector control (Figure 2e). Although treatment with DRB or Apigenin alone had no significant effect on NF-_κ_B activity,
CK2-Is reduced TNF-induced NF-_κ_B activity to levels comparable to control (Figure 2d). Similar decrease in TNF_α_-induced NF-_κ_B luciferase activity was observed in cells transfected with
CK2_α_/_β_ siRNAs (Figure 2f), confirming the involvement of CK2 in TNF_α_-induced aberrant NF-_κ_B activation. CK2-IS INCREASES P53 EXPRESSION IN GLIOMA CELLS As TNF_α_-activated NF-_κ_B
represses p53 transcriptional activity and vice versa,15 and as CK2-Is decreased TNF_α_-induced NF-_κ_B activation, we determined p53 status in CK2-I- and TNF_α_-treated cells. A dramatic
increase in p53 levels accompanied with an increase in phosphorylation (Ser-15) and acetylation (Lys373, 382) of p53 was observed upon treatment with CK2-Is either alone or in the presence
of TNF_α_ (Figure 3a). This increase was greater in p53 wild-type (U87MG and A172) cells as compared with the slight increase observed in p53 mutant (T98G) cells. Though A172 is known to be
a p53 mutant cell line,24 sequencing of DNA-binding region of p53 suggested it to be p53 wild type for DNA-binding domain. p53 status in the glioma cell lines was confirmed by sequencing
(Supplementary Table 1). CK2 INHIBITION INCREASES DNA-BINDING ABILITY AND TRANSCRIPTIONAL ACTIVITY OF WILD-TYPE P53 Further, we went on to investigate the effect of CK2-I-mediated increase
in p53 phosphorylation and acetylation on its functional activity. Although CK2-Is increased DNA-binding ability of p53 in U87MG and A172 by ∼ 2–4-fold either alone or in combination with
TNF_α_, it had no significant effect on the binding ability of mutant p53 in T98G cells (Figure 3b). This suggested that CK2 inhibition restores the function of wild-type p53 independent of
TNF_α_. Similarly, although a ∼2–3-fold increase in p53 transcriptional activity was observed in CK2-I-treated U87MG and A172 cells, its activity remained unaltered in T98G cells (Figure
3c). This CK2-I-induced increase in p53 DNA-binding ability and transcriptional activity in U87MG and A172 cells was significantly reduced in the presence of p53 inhibitor, Pifithrin-_α_
(Figures 3b and c). The relevance of CK2 in the activation of p53 function was further confirmed in U87MG and A172 cells, where siRNA-mediated knockdown of CK2_α_/_β_ increased p53
transcriptional activity (Figure 3d). CK2-I-MEDIATED INCREASED P53 ACTIVITY REDUCES GLIOMA CELLS VIABILITY To establish the functional significance of this increased p53 levels, we
determined the viability of CK2-I-treated glioma cells in the presence of TNF_α_, p53 inhibitor Pifithrin-_α_ or both. The 50% decrease in cell viability observed in U87MG and A172 upon
treatment with TNF_α_ and DRB was significantly reduced to ∼25% when TNF_α_ and CK2-I treatment was supplemented with Pifithrin-_α_ (Figure 3e). Similar results were obtained with Apigenin
also. This ability of Pifithrin-_α_ to reverse TNF_α_ and CK2-I-mediated decrease in cell viability indicated the involvement of p53 in CK2-I-induced cell death in p53 wild-type glioma
cells. However, ability of Pifithrin-_α_ to reverse TNF_α-_ and CK2-I-mediated cell death in p53 mutant T98G cells was not significant (Figure 3e). Similarly, siRNA-mediated p53 knockdown
was able to rescue CK2-I-induced cell death both in the presence and absence of TNF_α_ in p53 wild-type glioma cells only (Figure 3f). These results confirm the involvement of p53 in
CK2-I-mediated glioma cell death. P53 NULL CELLS ARE RESISTANT TO CK2-I-INDUCED APOPTOSIS To confirm the involvement of CK2-I-induced p53 activation in inducing apoptosis, the effect of
CK2-I on the viability of p53 null cells H1229 and SaOS2 was investigated. Both the p53 null cell lines were resistant to CK2-I-induced apoptosis, as treatment with DRB and Apigenin failed
to induce apoptosis in both these cells. However, CK2-I-mediated sensitization of p53 null cells to TNF_α_ could have resulted from downregulation of NF-_κ_B activity (Figure 3g). CK2-IS
INCREASE EXPRESSION OF P53 TARGET GENES ASSOCIATED WITH CELL CYCLE REGULATION AND APOPTOSIS We next investigated whether activation of p53 function induces genes that promote cell cycle
arrest (p21and GADD45_β_) and apoptosis (Noxa). Although TNF_α_ alone had no effect on p21, CK2-Is either alone or in combination with TNF_α_ elevated p21 expression (Figure 4a). CK2-Is
either alone or in combination with TNF_α_ increased mRNA levels of p53-induced pro-apoptotic molecules GADD45_β_ and Noxa (Figure 4a). This increase in p21, Noxa and GADD45_β_ level was
downregulated by Pifithrin-_α_ (Figures 4a and b). No change in Noxa and GADD45_β_ levels was observed in cells treated with TNF_α_ alone (Figure 4b). As changes in p21 expression indicated
alteration in cell cycle progression, we performed FACS analysis to determine the cell cycle profile of glioma cells treated with different combination of CK2-Is, TNF_α_ and Pifithrin_α_.
Although TNF_α_ had no significant effect on cell cycle progression, treatment with CK2-I induced G2/M arrest both in the presence and absence of TNF_α_ (Figure 4b). A 5.4, 5.8, 15.3 and
14.3%, cells at G2/M phase was observed in control, TNF_α_, Apigenin, TNF_α_ and Apigenin-treated A172 cells, respectively. Treatment with Pifithrin-_α_ reversed CK2-I-induced cell cycle
arrest (Figure 4c). Similar results were obtained in U87 cells also (data not shown). CK2-IS INDUCES TELOMERE SHORTENING AND SENESCENCE IN A P53-DEPENDENT MANNER Activation of p53 leads to
telomere shortening, and repression of human telomerase reverse transcriptase (hTERT) activity by p53 is mediated by p21.25 As p53 and p21 levels were elevated in CK2-I-treated p53 wild-type
cells, we determined hTERT activity in cells treated with CK2-Is or TNF_α_ or both. Although CK2-Is decreased hTERT activity, this decrease was greater in the presence of TNF_α_.
Importantly, CK2-I mediated reduction in hTERT activity was p53-dependent as the hTERT activity was reversed significantly in the presence of Pifithrin-_α_ (Figure 5a). As activation of
p53-p21 pathway also acts as a major mediator of cellular senescence induced by CK2 inhibition,26 we determined _β_-galactosidase activity in CK2-I-treated glioma cells. The increase in
senescence-positive cells observed in cells treated with CK2-I (Figure 5b) both in the presence and absence of TNF_α_ (Figure 5b), was abrogated in the presence of Pifithrin-_α_.
CK2-I-MEDIATED ABROGATION OF NF-ΚB AND INCREASE IN P53 ACTIVATION ARE INDEPENDENT EVENTS Given that TNF_α_-activated NF-_κ_B represses p53 transcriptional activity and vice versa,15 we
investigated whether elevated p53 levels could have resulted in decreased NF-_κ_B activation in cells treated with a combination of TNF_α_ and CK2-Is. However, the ability of CK2-Is to
downregulate TNF_α_-induced NF-_κ_B transcriptional activity was unaffected by Pifithrin-_α_ (Figure 5c). Thus, p53 activation and NF-_κ_B downregulation in CK2-I- and TNF_α_-treated cells
are two independent events. CK2-IS DIFFERENTIALLY REGULATES EXPRESSION OF GENES ASSOCIATED WITH APOPTOSIS IN TNF_Α_-TREATED P53 WILD-TYPE AND MUTANT GLIOMA CELLS To further establish which
apoptosis-related genes were altered upon treatment with CK2 inhibitor, we performed a quantitative real-time PCR (qRT-PCR)-based apoptotic gene array analysis. We observed distinct patterns
of gene expression in cells treated with either TNF_α_ or Apigenin or both in p53 wild-type (A172) and mutant (T98G) glioma cells. In p53 wild-type A172 glioma cells, among the 84 genes
tested, transcript levels of 20 genes were increased upon TNF_α_ treatment and their increased levels were abrogated significantly with CK2-Is (Table 1a). Most of these anti-apoptotic genes
are known to be regulated in TNF_α-_ and NF-_κ_B-dependent manner (_TNFRSF9, TNFSF10, BIRC3, CD40, TNFRSF11B, RIPK2, BCL2L11, BID, BIK, XIAP, BNIP3, NOD1, CARD8, TNFRSF1A, CD70, TRADD,
TRAF2, TRAF3, BCL2_ and _BCLAF1_). Moreover, 10 genes (_BCL2L1, BCL2L10, PYCARD, APAF1, GADD45A, CIDEA, TNFRSF10A, CASP10, CASP14_ and _CASP5_) were found to be upregulated by CK2-I
independent of TNF_α_ (Table 1a). Several of these genes were found to be p53-dependent pro-apoptotic factors. This indicated the importance of CK2-I-induced p53 in regulating the
pro-apoptotic response. On the other hand, in p53 mutant T98G cell lines, transcript levels of 10 TNF_α_ and NF-_κ_B target genes (_BIRC3, TNFRSF9, RIPK2, BCL2A1, PYCARD, CFLAR, TRADD,
TRAF2, TRAF3_ and _CARD6_) were increased upon TNF_α_ treatment and their increased levels were brought down significantly by CK2-I. Moreover, levels of four pro-apoptotic genes (_CASP10,
CASP14, CASP5_ and _CIDEA_) were upregulated upon CK2 inhibition. Similarly, transcript levels of four genes (_XIAP, BIRC6, NOL3_ and _TRAF4_) were downregulated significantly upon CK2-I
treatment either alone or in presence of TNF_α_. All of them are known apoptotic inhibitors (Table 1b). CK2-I INCREASES THE STABILITY OF P53 BY DECREASING ITS UBIQUITINATION As CK2-I induced
p53 expression without effecting its transcript level (Table 1), we investigated whether CK2-I-mediated increase in p53 level was the consequence of increased stability of the p53 protein.
The role of CK2 on p53 stability was determined by treating glioma cells with protein synthesis inhibitor, Cycloheximide (CHX), in the presence and absence of CK2-I. Although expression of
p53 decreased rapidly after incubation with CHX in untreated cells, treatment with Apigenin prevented such degradation (Figure 6a). This suggested increased stabilization of p53 upon CK2
inhibition. DECREASED MDM2–P53 ASSOCIATION UPON CK2 INHIBITION LEADS TO P53 STABILIZATION Ubiquitin ligase MDM2 negatively regulates p53 stability by promoting its ubiquitin-mediated
degradation27 and CK2 is known to regulate p53 turnover through MDM2.28 We therefore investigated whether CK2-mediated stabilization of p53 is MDM2-dependent. As no change in MDM2 levels was
observed upon treatment of glioma cells with CK2-I, both in the presence and absence of TNF_α_ (data not shown), we investigated whether CK2-Is alter the association of p53 with MDM2.
Association of p53 with MDM2 decreased upon CK2-I treatment (Figure 6b). It is possible that decreased MDM2–p53 association prevents rapid degradation of p53, leading to its accumulation in
CK2-I-treated glioma cells. This decreased MDM2–p53 interaction was concomitant with decrease in p53 ubiquitination, suggesting that CK2-I increases p53 levels by preventing
ubiquitin-mediated p53 degradation (Figure 6b). CK2 REGULATES SIRT1 ACTIVITY IN GLIOMA CELLS As CK2 regulate p53 activity through SIRT1-dependent deacetylation18 and as we have observed
increased p53 acetylation upon CK2-I treatment, we determined SIRT1 activity in cells treated with CK2-Is both in the presence and absence of TNF_α_. A ∼30–40% decrease in SIRT1 activity was
observed in CK2-I-treated cells independent of TNF_α_ (Figure 7a). ELEVATED SIRT1 LEVELS IN GLIOMA SAMPLES As SIRT1 is associated with tumorigenesis and resistant to radio- and
chemotherapy,29 we determined its expression levels in GBM tumor samples as compared with adjacent normal tissue. We report for the first time an elevated expression of SIRT1 in GBM tumor
samples as compared with the surrounding non-neoplastic tissue, as demonstrated by IHC and western blotting (Figure 7b). ROLE OF SIRT1 IN CK2-I-INDUCED P53-DEPENDENT GLIOMA CELL APOPTOSIS In
order to confirm the involvement of CK2-I-induced decrease in SIRT1 activity on glioma cell proliferation, we determined viability of cells over-expressing WT-SIRT1 in the presence and
absence of CK2-Is. Over-expression of SIRT1 significantly prevented CK2-I-induced apoptosis both in the presence and absence of TNF_α_ (Figure 7c). As SIRT1 regulates p53,18 we next
investigated the role of SIRT1 in CK2-I-induced p53 transcription activity in cells transfected with WT-SIRT1 in the presence and absence of CK2-Is and TNF_α_. Increase in p53
transcriptional activity observed upon treatment with CK2-I was abrogated upon SIRT1 over-expression (Figure 7d). These results clearly suggested the involvement of SIRT1 in the regulation
of CK2-I-induced p53 activation and subsequent decrease in glioma cells viability. DISCUSSION Casein kinase-2 is reported to have a role in overcoming resistance of glioma cells towards
radiation therapy.30 Moreover, CK2-Is target NF-_κ_B signaling11 and as inactivation of NF-_κ_B sensitizes glioma cells to TNF_α_-induced apoptotic cell death.2, 3 These findings compelled
us to investigate whether inhibition of CK2 could sensitize glioma cells to TNF_α_-induced apoptosis. We report for the first time an elevated expression of CK2 in GBM tumors as compared
with the surrounding non-neoplastic tissue. Our studies indicate that CK2-Is DRB and Apigenin as well as CK2 siRNA sensitizes glioma cells to TNF_α_-induced cell death. Though CK2 inhibitor
trigger apoptosis through FADD and caspase-8,22 neither DRB nor Apigenin increased Fas or FADD expression or utilized caspase-8-mediated signaling to induce glioma cell death. CK2 is known
to regulate TNF_α_-dependent NF-_κ_B activity,31 and similarly in glioma cells, treatment with both DRB and Apigenin abrogated TNF_α_-induced NF-_κ_B activation. CK2 inhibition in glioma
cells triggered activity of wild-type p53 as evidenced by increased p53 levels, its phosphorylation and acetylation, DNA-binding ability and transcriptional activation. However, CK2-I failed
to affect the functionality of mutant p53. It is known that receptor-interacting protein 1, which is a critical component of NF-_κ_B signaling, inhibits p53 function through NF-_κ_B
activation in glioblastoma.32 It is possible that although CK2-I-induced p53 activation triggered cell death, it was the ability of CK2-Is to inhibit TNF_α_-induced NF-_κ_B activation that
lead to synergistically enhanced cell death in the presence of TNF_α_. Restoration of p53 function by CK2-Is was accompanied by elevated expression of p53 target genes p21, NOXA and
GADD45_β_ that are associated with apoptosis and cell cycle arrest. The results of gene expression profiling suggested differential responsiveness of p53 wild-type and mutant cells to CK2
inhibition, in terms of the expression of genes that are known p53 targets, as well as those relevant for the activation of caspases and induction of TNF_α_-mediated apoptosis. Gene
profiling array also confirmed our observation that CK2 inhibition does not involve caspase-8, as no change in its levels was observed in cells treated with TNF_α_ in the presence of
Apigenin. Though CK2-I had no effect on the expression of p53 mRNA levels as demonstrated by the PCR array, it increased the stability of p53 protein by decreasing its ubiquitination and
interaction with MDM2 to prevent subsequent degradation. The decrease in SIRT-1 activity in glioma cells upon CK2 inhibition was associated with p53 activation, as over-expression of SIRT1
prevented CK2-I-mediated increase in p53 transcriptional activation. This was not surprising as CK2 regulates p53 activity directly and indirectly through SIRT-1.19 Importantly, SIRT1
over-expression rescued glioma cells from CK2-I-induced apoptosis. An increase in senescence was also observed in CK2-I-treated cells. It is possible that decrease in SIRT1 activity lead to
senescence, as induction of senescence-like growth arrest in human breast and lung cancer cells by Sirtinol– a specific inhibitor for SIRT1 has been reported.33 Given that both CK2 and SIRT1
are elevated in GBM and their downregulation is concomitant with apoptosis and senescence, our study suggests that targeting CK2-SIRT1 nexus might shed light on how CK2 regulates glioma
progression. Our studies indicate that CK2 inhibitor (i) sensitizes glioma cells to TNF_α_-induced apoptosis in an caspase-8-independent manner (ii) decreases TNF_α_-induced NF-_κ_B
activation (iii) reactivates wild-type p53 function (iv) inhibits SIRT1 activity (v) inhibits telomerase activity and (vi) induces senescence (Figure 8). Taken together, CK2-I sensitizes
both p53 wild-type and mutant glioma cells to TNF_α_-induced apoptosis. Depending on the p53 status, this is achieved by CK2-Is either through abrogation of NF-_κ_B activation and/or rescue
of wild-type p53 function. Importantly, this ability of CK2-I to induce glioma cell death irrespective of p53 status may be of clinical significance for the treatment of glioma, where p53
mutation is considered critical for its progression.16 Although activation of p53 triggers apoptosis, stimulation of NF-_κ_B promotes resistance to apoptosis. Interestingly, CK2-Is
co-ordinate these two opposing events to overcome TNF_α_-induced resistance to apoptosis. As induction of both senescence and apoptosis in human cancer cells has been reported,34 the ability
of CK2-I to induce both these events to reduce cell viability warrants further investigation of CK2 as a potential anti-glioma target. MATERIALS AND METHODS PROCESSING OF TISSUE AND IHC IHC
was performed on tissue samples collected from patients with histologically confirmed GBM (_n_=19) to determine CK2 and SIRT1 expression as described.35 Non-neoplastic brain tissue (_n_=7)
obtained from margins of the tumors whenever possible was used as control. Samples were obtained as per the guidelines of Institutional Human Ethics Committee of NBRC. CELL CULTURE AND
TREATMENT Glioblastoma cell lines A172, U87MG and T98G were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in DMEM supplemented with 10% fetal bovine
serum. On attaining semi-confluence, cells were switched to serum-free media (SFM) and after 12 h, cells were treated with different concentration of DRB or Apigenin (in Dimethyl sulphoxide,
DMSO) or Pifithrin-_α_ in the presence or absence of TNF_α_ (50 ng/ml) in SFM for 24 h. DMSO-treated cells were used as controls. All reagents were purchased from Sigma unless otherwise
stated. The p53 null cell lines H1229 and SaOS2 were a kind gift from Dr. Sanjeev Das, National institute of Immunology, India. DETERMINATION OF CELL VIABILITY Viability of cells treated
with different combinations of TNF_α_, CK2-Is and Pifithrin-_α_ for 24 h in 96-well plates was assessed using the MTS assay (Promega, Madison, WI, USA) as described.36 Values were expressed
as a percentage relative to those obtained in controls. WESTERN BLOT AND IMMUNOPRECIPITATION Protein was isolated from whole-cell lysates and nuclear extracts from cells treated with
different combinations of TNF_α_, CK2-Is and Pifithrin-_α_ and western blot was performed as described.37 The following antibodies were used: p21 (BD Biosciences, San Diego, CA, USA), pp53
ser15, MDM2, SIRT1 (Abcam, Cambridge, UK), acetylated p53 lys373, lys382 (Millipore, Temecula, CA, USA), pIKK_α_/_β_ (Ser176/180), I_κ_B_α_, p53 (Cell Signaling, Danvers, MA, USA), Ub (Dako,
Glostrup, Denmark) CK2_α_, Fas and FADD, p53, p65, c23, GAPDH. Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) unless otherwise mentioned. Secondary antibodies
were purchased from Vector Laboratories Inc. (Burlingame, CA, USA). The blots were stripped and re-probed with anti-_β_-actin (Sigma, St. Louis, MO, USA) to determine equivalent loading as
described.37 Immunoprecipitation was performed with p53 antibody to determine the association of p53-MDM2 as described.38 ASSAY OF CASPASE-3 AND 8 ACTIVITIES The Colorimetric Assay kits for
caspases-3 and 8 (Sigma) were used to determine the enzymatic activity of caspases in cells treated with different combinations of TNF_α_ and CK2-Is as described.39 _LUCIFERASE_ REPORTER
GENE ASSAY Reporter assay in cells transfected with p53 or NF-_κ_B luciferase or WT SIRT1 constructs, and treated with different combinations of TNF_α_, CK2-Is and Pifithrin-_α_ was
performed as described.37 The p53 luciferase and SIRT1 constructs were obtained from Addgene (Cambridge, MA, USA) and NF-_κ_B luciferase construct obtained from Clontech (Madison, WI, USA).
SIRNA TRANSFECTION Before 18 h of transfection, 3 × 104 cells were seeded onto 24-well plates in medium without antibiotics and transfection with 50 nmol/l duplex CK2_α_, CK2_β_, p53 or
non-specific siRNA (Thermo Fischer Scientific, Lafayette, CO, USA) was carried using Lipofectamine RNAi Max reagent (Life Technologies-Invitrogen, Carlsbad, CA, USA) as described.40 P53
DNA-BINDING ASSAY DNA-binding activity of p53 was measured with the DuoSet IC (IntraCellular) Assay Development System (R&D Systems, Minneapolis, MN, USA) according to
manufacturer's instructions. TELOMERASE ACTIVITY Telomerase activity of cells treated with TNF_α_ in the presence and absence of CK2-Is and Pifithrin-_α_ was determined using the
_T_elo_TAGGG_ Telomerase PCR ELISA Plus kit (Roche Diagnostics, Mannheim, Germany) as described.39 FLOW CYTOMETRIC ANALYSIS OF DNA CONTENT FACS analysis was performed to determine the effect
of TNF_α_ or Apigenin or both in the presence and absence of Pifithrin _α_ on cell cycle progression of glioma cells, using Cell Quest program on FACS Calibur (Becton Dickinson, San Diego,
CA, USA) as described.5 Results were analyzed using Cell Quest pro software (Becton Dickinson). SENESCENCE-ASSOCIATED _Β_-GAL STAINING Cells treated with CK2-Is either alone or in presence
of TNF_α_ and/or Pifithrin were stained with the senescence-associated _β_-galactosidase (SA-_β_-gal) staining solution (Sigma) as per the manufacturer's instructions. The numbers of
blue-stained (SA-_β_-gal-positive) cells and total cells were counted microscopically and percentage of SA-_β_-gal-positive cells was calculated. SIRT1 ACTIVITY ASSAY Deacetylase activity of
SIRT1 was measured with SIRT1 activity assay kit from Calbiochem (Merck KGaA, Darmstadt, Germany) according to manufacturer's instructions. RT-PCR AND HUMAN APOPTOSIS QRT-PCR ARRAY
RT-PCR analyses for NOXA and GADD45_β_ expression in cells treated with different combinations of TNF_α_ and CK2-Is was performed as described.37 PCR primers for NOXA and GADD45_β_ are
listed in Supplementary Table 2. qRT-PCR was performed using an apoptosis PCR Array containing 84 apoptosis-related genes (SuperArray Biosciences, Frederick, MD, USA) as per
manufacturer's instruction. Briefly, total RNA samples from TNF_α_ and Apigenin-treated A172 and T98G glioma cells were isolated using RNeasy Mini Kit (Qiagen, Hilden, Germany). An
equal amount of RNA (2 _μ_g) was used for reverse transcription using RT2 First Strand Kit (SuperArray Biosciences). PCR reactions were done using the RT2 profiler PCR array PAHS-012 (Human
Apoptosis PCR Array) on the ABI 7500 using RT2 Real-time SYBR Green PCR master mix PA-012. The total volume of the PCR reaction was 25 _μ_l. The thermocycler parameters were 95°C for 10 min,
followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Five housekeeping genes were included on the array (_B2M, HPRT1, RPL13A, GAPDH,_ and _ACTB_) to normalize the transcript levels.
Results were analyzed as per user manual guidelines using integrated web-based software package for the PCR Array System (SuperArray Biosciences RT2 Profiler PCR Array Human Apoptosis
PAHS-012–A-12). STATISTICAL ANALYSIS All comparisons between groups were performed using two-tailed paired student's _t_-test. All _P_-values <0.05 were taken as significant.
ABBREVIATIONS * CK2-I: casein kinase-2 inhibitor * NF-_κ_B: nuclear factor kappa B * SIRT1: sirtuin 1 * TNF_α_: tumor necrosis factor alpha * GBM: glioblastoma multiforme * DRB:
5,6-dichlorobenzimidazole 1-_β_-D-ribofuranoside * CK2a/b siRNA: siRNA against a and b subunits of casein kinase-2 * FADD: Fas-associated protein with death domain * I_κ_K: inhibitor of
nuclear factor kappa B kinase * MDM2: murine double minute * NS siRNA: non-specific siRNA REFERENCES * Tsujimoto M, Yip YK, Vilcek J . Tumor necrosis factor: specific binding and
internalization in sensitive and resistant cells. _Proc Natl Acad Sci USA_ 1985; 82: 7626–7630. Article CAS Google Scholar * Beg AA, Baltimore D . An essential role for NF-kappaB in
preventing TNF-alpha-induced cell death. _Science_ 1996; 274: 782–784. Article CAS Google Scholar * Wang CY, Mayo MW, Baldwin Jr AS . TNF- and cancer therapy-induced apoptosis:
potentiation by inhibition of NF-kappaB. _Science_ 1996; 274: 784–787. Article CAS Google Scholar * Safrit JT, Bonavida B . Sensitivity of resistant human tumor cell lines to tumor
necrosis factor and adriamycin used in combination: correlation between down-regulation of tumor necrosis factor-messenger RNA induction and overcoming resistance. _Cancer Res_ 1992; 52:
6630–6637. CAS PubMed Google Scholar * Sharma V, Tewari R, Sk UH, Joseph C, Sen E . Ebselen sensitizes glioblastoma cells to Tumor Necrosis Factor (TNFalpha)-induced apoptosis through two
distinct pathways involving NF-kappaB downregulation and Fas-mediated formation of death inducing signaling complex. _Int J cancer_ 2008; 123: 2204–2212. Article CAS Google Scholar *
Litchfield DW . Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. _Biochem j_ 2003; 369 (Pt 1): 1–15. Article CAS Google Scholar * Munstermann U,
Fritz G, Seitz G, Lu YP, Schneider HR, Issinger OG . Casein kinase II is elevated in solid human tumours and rapidly proliferating non-neoplastic tissue. _Eur J Biochem_ 1990; 189: 251–257.
Article CAS Google Scholar * Yamane K, Kinsella TJ . CK2 inhibits apoptosis and changes its cellular localization following ionizing radiation. _Cancer Res_ 2005; 65: 4362–4367. Article
CAS Google Scholar * Ravi R, Bedi A . Sensitization of tumor cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of casein kinase II. _Cancer Res_ 2002; 62: 4180–4185. CAS PubMed
Google Scholar * Izeradjene K, Douglas L, Delaney A, Houghton JA . Influence of casein kinase II in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human
rhabdomyosarcoma cells. _Clin Cancer Res_ 2004; 10: 6650–6660. Article CAS Google Scholar * Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Traish AM, Mercurio F, Sonenshein GE . Roles of
IKK kinases and protein kinase CK2 in activation of nuclear factor-kappaB in breast cancer. _Cancer Res_ 2001; 61: 3810–3818. CAS PubMed Google Scholar * Rieger J, Frank B, Weller M,
Wick W . Mechanisms of resistance of human glioma cells to Apo2 ligand/TNF-related apoptosis-inducing ligand. _Cell Physiol Biochem_ 2007; 20: 23–34. Article CAS Google Scholar * Wang D,
Westerheide SD, Hanson JL, Baldwin AS Jr . Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. _J Biol Chem_ 2000; 275: 32592–32597.
Article CAS Google Scholar * Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE . Protein kinase CK2 promotes aberrant activation of nuclear factor-kappaB, transformed
phenotype, and survival of breast cancer cells. _Cancer Res_ 2002; 62: 6770–6778. CAS PubMed Google Scholar * Webster GA, Perkins ND . Transcriptional cross talk between NF-kappaB and
p53. _Mol Cell Biol_ 1999; 19: 3485–3495. Article CAS Google Scholar * Kato H, Kato S, Kumabe T, Sonoda Y, Yoshimoto T, Kato S _et al_. Functional evaluation of p53 and PTEN gene
mutations in gliomas. _Clin Cancer Res_ 2000; 6: 3937–3943. CAS PubMed Google Scholar * Weinmann L, Wischhusen J, Demma MJ, Naumann U, Roth P, Dasmahapatra B _et al_. A novel p53 rescue
compound induces p53-dependent growth arrest and sensitises glioma cells to Apo2L/TRAIL-induced apoptosis. _Cell Death Diff_ 2008; 15: 718–729. Article CAS Google Scholar * Vaziri H,
Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK _et al_. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. _Cell_ 2001; 107: 149–159. Article CAS Google Scholar * Kang H,
Jung JW, Kim MK, Chung JH . CK2 is the regulator of SIRT1 substrate-binding affinity, deacetylase activity and cellular response to DNA-damage. _PLOS One_ 2009; 4: e6611. Article Google
Scholar * Chang CJ, Hsu CC, Yung MC, Chen KY, Tzao C, Wu WF _et al_. Enhanced radiosensitivity and radiation-induced apoptosis in glioma CD133-positive cells by knockdown of SirT1
expression. _Biochem Biophys Res Commun_ 2009; 380: 236–242. Article CAS Google Scholar * Prudent R, Moucadel V, Nguyen CH, Barette C, Schmidt F, Florent JC _et al_. Antitumor activity of
pyridocarbazole and benzopyridoindole derivatives that inhibit protein kinase CK2. _Cancer Res_ 2010; 70: 9865–9874. Article CAS Google Scholar * Llobet D, Eritja N, Encinas M, Llecha N,
Yeramian A, Pallares J _et al_. CK2 controls TRAIL and Fas sensitivity by regulating FLIP levels in endometrial carcinoma cells. _Oncogene_ 2008; 27: 2513–2524. Article CAS Google Scholar
* Yu M, Yeh J, Van Waes C . Protein kinase casein kinase 2 mediates inhibitor-kappaB kinase and aberrant nuclear factor-kappaB activation by serum factor(s) in head and neck squamous
carcinoma cells. _Cancer Res_ 2006; 66: 6722–6731. Article CAS Google Scholar * Badie B, Goh CS, Klaver J, Herweijer H, Boothman DA . Combined radiation and p53 gene therapy of malignant
glioma cells. _Cancer Gene Ther_ 1999; 6: 155–162. Article CAS Google Scholar * Shats I, Milyavsky M, Tang X, Stambolsky P, Erez N, Brosh R _et al_. p53-dependent down-regulation of
telomerase is mediated by p21waf1. _J Biol Chem_ 2004; 279: 50976–50985. Article CAS Google Scholar * Kang JY, Kim JJ, Jang SY, Bae YS . The p53-p21(Cip1/WAF1) pathway is necessary for
cellular senescence induced by the inhibition of protein kinase CKII in human colon cancer cells. _Mol Cell_ 2009; 28: 489–494. Article CAS Google Scholar * Haupt Y, Maya R, Kazaz A, Oren
M . Mdm2 promotes the rapid degradation of p53. _Nature_ 1997; 387: 296–299. Article CAS Google Scholar * Allende-Vega N, Dias S, Milne D, Meek D . Phosphorylation of the acidic domain
of Mdm2 by protein kinase CK2. _Mol Cell Biochem_ 2005; 274: 85–90. Article CAS Google Scholar * Saunders LR, Verdin E . Sirtuins: critical regulators at the crossroads between cancer and
aging. _Oncogene_ 2007; 26: 5489–5504. Article CAS Google Scholar * Olsen BB, Issinger OG, Guerra B . Regulation of DNA-dependent protein kinase by protein kinase CK2 in human
glioblastoma cells. _Oncogene_ 2010; 29: 6016–6026. Article CAS Google Scholar * Piazza FA, Ruzzene M, Gurrieri C, Montini B, Bonanni L, Chioetto G _et al_. Multiple myeloma cell survival
relies on high activity of protein kinase CK2. _Blood_ 2006; 108: 1698–1707. Article CAS Google Scholar * Park S, Hatanpaa KJ, Xie Y, Mickey BE, Madden CJ, Raisanen JM _et al_. The
receptor interacting protein 1 inhibits p53 induction through NF-kappaB activation and confers a worse prognosis in glioblastoma. _Cancer Res_ 2009; 69: 2809–2816. Article CAS Google
Scholar * Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M _et al_. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer
cells. _Oncogene_ 2006; 25: 176–185. Article CAS Google Scholar * Chen CR, Wang W, Rogoff HA, Li X, Mang W, Li CJ . Dual induction of apoptosis and senescence in cancer cells by Chk2
activation: checkpoint activation as a strategy against cancer. _Cancer Res_ 2005; 65: 6017–6021. Article CAS Google Scholar * Tewari R, Choudhury SR, Ghosh S, Mehta VS, Sen E .
Involvement of TNFalpha-induced TLR4-NF-kappaB and TLR4-HIF-1alpha feed-forward loops in the regulation of inflammatory responses in glioma. _J Mol Med_ 2012; 90: 67–80. Article CAS Google
Scholar * Koul N, Sharma V, Dixit D, Ghosh S, Sen E . Bicyclic triterpenoid Iripallidal induces apoptosis and inhibits Akt/mTOR pathway in glioma cells. _BMC cancer_ 2010; 10: 328. Article
Google Scholar * Sharma V, Dixit D, Koul N, Mehta VS, Sen E . Ras regulates interleukin-1beta-induced HIF-1alpha transcriptional activity in glioblastoma. _J Mol Med_ 2011; 89: 123–136.
Article CAS Google Scholar * Tewari R, Sharma V, Koul N, Ghosh A, Joseph C, Hossain Sk U _et al_. Ebselen abrogates TNFalpha induced pro-inflammatory response in glioblastoma. _Mol Oncol_
2009; 3: 77–83. Article CAS Google Scholar * Sharma V, Koul N, Joseph C, Dixit D, Ghosh S, Sen E . HDAC inhibitor Scriptaid induces glioma cell apoptosis through JNK activation and
inhibits telomerase activity. _J Cell Mol Med_ 2009; 14: 2151–2161. Article Google Scholar * Sharma V, Dixit D, Koul N, Mehta VS, Sen E . Ras regulates interleukin-1beta-induced HIF-1alpha
transcriptional activity in glioblastoma. _J Mol Med_ 2011; 89: 123–136. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The work was supported by a research grant from
the Department of Biotechnology (DBT, Government of India #BT/PR/12924/Med/30/235/2009). DD is supported by a research fellowship from Council of Scientific and Industrial Research (CSIR,
Government of India). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * National Brain Research Centre, Manesar, Gurgaon, 122050, Haryana, India D Dixit, V Sharma, S Ghosh, V S Mehta & E Sen
* Paras Hospitals, Gurgaon, 122050, Haryana, India V S Mehta Authors * D Dixit View author publications You can also search for this author inPubMed Google Scholar * V Sharma View author
publications You can also search for this author inPubMed Google Scholar * S Ghosh View author publications You can also search for this author inPubMed Google Scholar * V S Mehta View
author publications You can also search for this author inPubMed Google Scholar * E Sen View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING
AUTHOR Correspondence to E Sen. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Edited by A Verkhratsky Supplementary Information
accompanies the paper on Cell Death and Disease website SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLE 1 (JPG 33 KB) SUPPLEMENTARY TABLE 2 (JPG 23 KB) RIGHTS AND PERMISSIONS This work is
licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Dixit, D., Sharma, V., Ghosh, S. _et al._ Inhibition of Casein kinase-2
induces p53-dependent cell cycle arrest and sensitizes glioblastoma cells to tumor necrosis factor (TNF_α_)-induced apoptosis through SIRT1 inhibition. _Cell Death Dis_ 3, e271 (2012).
https://doi.org/10.1038/cddis.2012.10 Download citation * Received: 09 November 2011 * Revised: 15 December 2011 * Accepted: 12 January 2012 * Published: 09 February 2012 * Issue Date:
February 2012 * DOI: https://doi.org/10.1038/cddis.2012.10 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * glioblastoma * casein kinase-2 *
TNF_α_ * NF-_κ_B * p53



)

