- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Hyperglycemia is detrimental to _β_-cell viability, playing a major role in the progression of _β_-cell loss in diabetes mellitus. The permeability transition pore (PTP) is a
mitochondrial channel involved in cell death. Recent evidence suggests that PTP inhibitors prevent hyperglycemia-induced cell death in human endothelial cells. In this work, we have examined
the involvement of PTP opening in INS-1 cell death induced by high levels of glucose or fructose. PTP regulation was studied by measuring the calcium retention capacity in permeabilized
INS-1 cells and by confocal microscopy in intact INS-1 cells. Cell death was analyzed by flow cytometry. We first reported that metformin and cyclosporin A (CsA) prevented Ca2+-induced PTP
opening in permeabilized and intact INS-1 cells. We then showed that incubation of INS-1 cells in the presence of 30 mM glucose or 2.5 mM fructose induced PTP opening and led to cell death.
As both metformin and CsA prevented glucose- and fructose- induced PTP opening, and hampered glucose- and fructose- induced cell death, we conclude that PTP opening is involved in high
glucose- and high fructose- induced INS-1 cell death. We therefore suggest that preventing PTP opening might be a new approach to preserve _β_-cell viability. SIMILAR CONTENT BEING VIEWED BY
OTHERS IMEGLIMIN MITIGATES THE ACCUMULATION OF DYSFUNCTIONAL MITOCHONDRIA TO RESTORE INSULIN SECRETION AND SUPPRESS APOPTOSIS OF PANCREATIC Β-CELLS FROM _DB/DB_ MICE Article Open access 14
March 2024 METFORMIN PREVENTS METHYLGLYOXAL-INDUCED APOPTOSIS BY SUPPRESSING OXIDATIVE STRESS IN VITRO AND IN VIVO Article Open access 10 January 2022 EPIGALLOCATECHIN GALLATE ATTENUATED
HIGH GLUCOSE-INDUCED PANCREATIC BETA CELL DYSFUNCTION BY MODULATING DRP1-MEDIATED MITOCHONDRIAL APOPTOSIS PATHWAYS Article Open access 22 July 2024 MAIN Under physiological condition,
glucose serum concentration fluctuates between 3.6 and 7 mM,1 whereas fructose serum concentration remains below the limit of detection by enzymatic methods.2 Under diabetic conditions,
fructose serum concentration increases, most probably because glucose is converted into fructose via the polyol pathway.3, 4 Moreover, the splanchnic territory is physiologically exposed to
high concentrations of glucose and fructose during the postprandial period.5 Therefore, _β_-cells can face hyperglycemia or hyperfructosemia in type 2 diabetes or when pancreatic islets are
transplanted into the splanchnic territory. The permeability transition pore (PTP) is a Ca2+-sensitive mitochondrial inner membrane channel.6, 7 Normally closed in order to allow ATP
synthesis, the PTP leads to mitochondrial depolarization and cell death after extended opening.8 Ca2+ is the single most important factor for PTP opening. The amount of Ca2+ required to open
the pore varies according to a number of factors. ‘PTP-inhibitors’ and so-called ‘PTP-inducers’ refer to factors that increase and decrease the amount of Ca2+ required to induce PTP
opening.9 Cyclosporin A (CsA) is the reference PTP inhibitor, whereas oxidative stress is well recognized as favoring PTP opening.6 In several cell types, direct or indirect inhibition of
respiratory chain complex 1 inhibits PTP opening.10, 11, 12 In such cells, the antidiabetic drug metformin inhibits PTP opening via a mild inhibition of complex 1.11 Chronic exposure to
elevated glucose or fructose concentrations impairs _β_-cells survival13, 14 by a mechanism that may involve oxidative stress.13, 15, 16 Hyperglycemia-induced oxidative stress has been shown
to induce mitochondrial permeability transition and subsequent cell death in human endothelial cells.11 Concerning pancreatic-derived cells, CsA has been shown to inhibit Ca2+-induced PTP
opening in permeabilized INS-1 and MIN-6 cells.17 It has been proposed that cytokine-induced apoptosis may be due to PTP opening in pancreatic RINm5F cells18 whereas CsA has been reported to
prevent PK11195-induced cell death in isolated human pancreatic islets.19 Finally, it has recently been shown that CsA protects MIN-6 cells against Pdx1 insufficiency-induced cell death,
although genetic ablation of the endogenous PTP-inducers cyclophilin D prevents diabetes in Pdx1+/− mice.20 Whether metformin regulates PTP opening in _β_-cells and whether hyperglycemia or
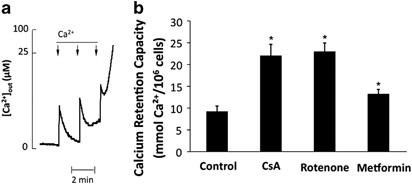
hyperfructosemia induces PTP opening in pancreatic-derived cells has not been studied yet. RESULTS CSA AND METFORMIN INHIBIT PTP OPENING IN PERMEABILIZED INS-1 CELLS The Ca2+ retention
capacity (CRC) represents the minimum Ca2+ load required to induce PTP opening in an entire population of mitochondria. Therefore, CRC measurement represents a suitable method to quantify
and compare the potency of different PTP regulators. CRC is measured by loading mitochondria with a train of Ca2+ pulses until a rapid Ca2+ release occurs as illustrated in Figure 1a. As
shown in Figure 1b, CsA increased CRC (i.e. inhibited PTP opening) in permeabilized INS-1 cells. Interestingly, complex 1 inhibitor rotenone also inhibited PTP opening in that cell line.
Accordingly, metformin also inhibited PTP opening but to a lower extent, which was expected because metformin is less potent than rotenone in complex 1 inhibition.21 CSA AND METFORMIN
PREVENT PTP OPENING IN INTACT INS-1 CELLS We next checked that CsA and metformin inhibited PTP opening in intact INS-1 cells. PTP status was assessed by double channel imaging of NAD(P)H
autofluorescence and mitochondrial electrical membrane potential (i.e. tetramethylrhodamine methyl ester (TMRM) fluorescence) as recently described,22 whereas Ca2+-induced PTP opening was
triggered off by exposing INS-1 cells to the Ca2+ ionophore A23187. As shown in Figure 2 (left panels), A23187-induced PTP opening was followed by an increase in NAD(P)H autofluorescence
both in terms of intensity and surface distribution. On the contrary, TMRM fluorescence decreased in term of intensity but not in term of surface distribution, resulting in an increase in
NAD(P)H/TMRM surface distribution ratio. No change in the NAD(P)H and TMRM fluorescence was observed in the absence of A23187 (data not shown). As shown in Figure 2 (middle and right
panels), A23187 did not affect NAD(P)H and TMRM signals when PTP opening was prevented by either CsA or metformin. These data indicate that PTP opening increases the NAD(P)H/TMRM surface
distribution ratio in INS-1 cells, as previously shown in human endothelial cells.22 HIGH GLUCOSE AND HIGH FRUCTOSE OPEN PTP IN INS-1 CELLS We next measured the NAD(P)H and TMRM fluorescence
of INS-1 cells incubated in control conditions or in the presence of 30 mM glucose or 2.5 mM fructose for 24 h. Note that comparisons were performed only between images acquired the same
day with exactly the same microscope settings and the same load of TMRM. As shown in Figure 3, the NAD(P)H/TMRM surface distribution ratio was dramatically increased when cells were
incubated in the presence of 30 mM glucose or 2.5 mM fructose, as compared with the control conditions. As expected, when PTP opening was prevented by either CsA or metformin, the
NAD(P)H/TMRM surface distribution ratio did not increase (Figure 3). These data indicate PTP was opened in INS-1 cells exposed to 30 mM glucose or 2.5 mM fructose for 24 h. HIGH GLUCOSE- AND
HIGH FRUCTOSE-INDUCED PTP OPENING LEADS TO CELL DEATH We finally studied the effect of 30 mM glucose or 2.5 mM fructose on the viability of INS-1 cells. As shown in Figure 4, ∼80% of the
cells were alive (i.e. Annexin V-/PI-) in control conditions. The viability of INS-1 cells was not affected by osmotic change (exposure to mannitol), whereas it was dramatically affected by
30 mM glucose or 2.5 mM fructose. Importantly, 30 mM glucose-induced toxicity was hampered by CsA or metformin, whereas 2.5 mM fructose-induced toxicity was totally prevented by CsA or
metformin, indicating that PTP opening was involved in high glucose- and high fructose-induced cell death. DISCUSSION In this work, we have reported that in INS-1 insulinoma cells (i) both
CsA and metformin inhibited PTP opening, (ii) high glucose and high fructose led to PTP opening, (iii) PTP inhibitors hampered high glucose- and high fructose-induced cell death. Note that
we have used a concentration of glucose that can be observed in clinical practice, while 30 mM glucose or 2.5 mM fructose are physiologically reached in the splanchnic territory during the
postprandial period.5 Glucotoxicity (i.e. high glucose induced cell death) on _β_-cell lines or islets is now well documented.13, 14 A very high concentration of fructose (i.e. >50 mM)
has been shown to induce cell death in hamster pancreatic _β_-cell-derived cell line HIT.13 Hyperglycemia or hyperfructosemia have been shown to increase ROS production.13, 15, 16 As
pancreatic _β_-cells express a low level of antioxidants,23, 24 this increased ROS production probably results in an oxidative stress that may affect the survival of _β_-cells. Supporting
this scenario, several antioxidants have been proved to prevent hyperglycemia-induced cell death.16 It is well acknowledged that oxidative stress triggers PTP opening.6 Whether or not high
glucose and high fructose led to PTP opening in INS-1 insulinoma cell because they increased ROS production is not demonstrated in this work, but this hypothesis is in agreement with the
literature. Alternatively, it has been shown that high glucose concentrations modulate the balance of proapoptotic and antiapoptotic Bcl proteins in cultured human pancreatic islets by
overexpressing Bad and Bid.25 Note, however, that among different effects, Bcl proteins are able to regulate PTP opening.26, 27, 28 Therefore, the observed overexpression of Bad and Bid,
which may favor PTP opening,27, 28 remains consistent with our results. High glucose concentration has also been shown to activate the hexosamine pathway, resulting in an impaired activation
of PI 3-kinase/Akt survival pathway.29 As the activation of the survival PI 3-kinase/Akt pathway has been shown to prevent PTP opening,30 the activation of the hexosamine pathway may
indirectly favor PTP opening. Therefore, high glucose concentration may (i) induce oxidative stress, (ii) modulate the Bcl proteins, and (iii) prevent the activation of the PI 3-kinase/Akt
pathway. These phenomena are known to favor PTP opening and might act synergistically. _In vitro_ studies have suggested that sulfonylurea may induce apoptosis of pancreatic _β_-cell via a
Ca2+-dependent process.31 Assuming that (i) sulfonylurea-induced apoptosis involves PTP opening and (ii) secondary failure to sulfonylurea is due to _β_-cell death, one may hypothesize that
metformin would prevent or delay the risk of secondary failure to sulfonylurea. To the best of our knowledge, such a study has not been performed yet. However, it has been shown that the
cumulative incidence of monotherapy failure is higher with sulfonylurea than with metformin.32 In a prospective human observational study measuring the risk of secondary failure to
sulfonylurea, the same percentage of patients receiving sulfonylurea plus metformin was found in the group well controlled with oral treatment and in the group with secondary failure.33 Note
however that metformin seems to have been added either after glucose rose over 300 mg/dl or after patients developed hyperglycemic symptoms,33 that is, after pancreatic _β_-cells apoptosis
occurred. By reducing the mitochondria-related toxicity of high glucose and high fructose level in _β_-cells, pharmacological inhibition of PTP opening may soon represents a new strategy to
prevent _β_-cell loss during diabetes mellitus in its various aspects. Islet transplantation could represent a credible application. Although substantial progress has occurred regarding
islet isolation and immunosuppression protocols,34 obstacles still compromise islet transplant success. Islets (50–70%) are estimated to be destroyed in the immediate post transplant
period,35 making _β_-cell apoptosis a crucial issue that prevents islet transplantation from spreading. Among several factors, this work suggests that islet exposure to high glucose and
fructose levels might have a relevant role in graft death. Therefore preventing PTP opening during the islet transplant procedure (either by engraftment outside the splanchnic territory or
by pharmacological inhibition of PTP opening) may enhance _β_ survival and improve islet transplant outcomes. Confirmatory studies with human islets are needed before proposing such a
strategy in clinical trials. MATERIALS AND METHODS CELL CULTURE Isolated insulinoma cell lines INS-1, a generous gift of Dr. F De Fraipont (CHU-Grenoble), were maintained in RPMI 1640 medium
supplemented with 10 mM HEPES, 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin, 100 _μ_g/ml streptomycin, 1 mM sodium pyruvate and 50 mM 2-mercaptoethanol.
Cells were incubated at 37°C in a humidified atmosphere (95% air, 5% CO2). CALCIUM RETENTION CAPACITY ASSESSMENT Cells were permeabilized immediately before the experiment by incubation for
2 min at 25°C in a buffer containing 10 mM MOPS (pH 7.35), 250 mM sucrose, 1 mM Pi–Tris, 5 mM succinate and 100 _μ_g/ml digitonin. The calcium retention capacity was measured
fluorimetrically using a PTI Quantamaster C61 spectrofluorimeter in the presence of 0.25 _μ_M Calcium Green (Molecular Probes, Illkirch, France) with excitation and emission wavelengths set
at 506 and 527 nm, respectively. IMAGING INS-1cells set on a Lab-Tek-Chamber Slide System (Nalge Nunc International, Rochester, NY, USA) were studied by time-lapse laser confocal microscopy
at 37°C in a humidified atmosphere (95% air, 5% CO2) using a microscope equipped with a perfusion chamber (POC chamber, LaCom, Erbach, Germany) and an incubation system (O2-CO2-°C, PeCom,
Erbach, Germany). Images were collected with a Leica TCS SP2 AOBS inverted laser scanning confocal microscope equipped with a Coherent 351–364 UV laser (Coherent Inc., Santa Clara, CA, USA)
laser using a 63 × oil immersion objective (HCX PL APO 63.0 X 1.40 W Corr). Laser excitation was 351–364 nm for NAD(P)H, and 543 nm for TMRM. Fluorescence emission adjusted with AOBS was
390–486 nm for NAD(P)H, and 565–645 nm for TMRM. In order to allow overlay of NAD(P)H and TMRM signals, image acquisition was set with the same pinhole aperture (Airy 2.03), necessarily
increased because of the low signal of NAD(P)H autofluorescence. Each experiment was performed on a randomly chosen field containing 15–25 cells. Background noise of NADH autofluorescence
was removed by fine filter (Kernel 3 × 3) using Volocity software. Image quantification was performed using the ImageJ (NIH images) and Volocity (Improvision, Cergy Saint Christophe, France)
softwares as described in.22 CELL DEATH INDUCTION AND DRUG TREATMENTS For glucose-induced cell death, cells were incubated for 72 h in complete RPMI 1640 medium supplemented with 19 mM
D-glucose (final concentration, 30 Mm D-Glucose). Osmotic control was performed supplementing RPMI 1640 medium with 19 mM mannitol. For fructose-induced cell death, cells were exposed for 72
h to 2.5 mM D-fructose. Before these treatments, INS-1 cells were incubated in the presence, or not, of 1 _μ_M CsA for 1 h or 100 _μ_M metformin for 24 h. QUANTIFICATION OF CELL DEATH BY
FLOW CYTOMETRY Apoptosis analyses were performed with a double-stain system using Annexin V (Interchim, Montluçon, France) combined with FluoProbes 488 and propidium iodide (PI) (Sigma
Aldrich, Saint Quentin Fallavier, France). INS-1 cells were detached by trypsination, washed by centrifugation, and incubated with 100 _μ_l of Annexin-V buffer 1 × (10 mM HEPES NaOH, pH 7.4,
150 mM NaCl, 5 mM KCl, 1 mM MgCl2 and 1.8 mM CaCl2). Cells were then incubated for 15 min at room temperature in the dark in the presence of 5 _μ_l of AnnexinV-FP488. Labeled cells were
transferred in a 5 ml propylene tube containing 900 _μ_l PBS. A volume of 10 _μ_l from a 1 mg/ml stock solution of PI were added to the suspension and immediately analyzed. Data acquisition
(∼5000 cells) was carried out using a FACSCAN flow cytometer (Becton Dickinson Biosciences, Le Pont de Claix, France) equipped with a 15-mW argon ion laser tuned at 488 nm, using the Cell
Quest Pro software (Becton Dickinson Biosciences). Data were plotted as a function of fluorescence intensity on FL-1 (530 nm/30 nm band–pass filter) (Annexin V) and FL-3 channels (PI)
(585–42 nm band pass filter). The Annexin V−/PI− population was regarded as normal healthy cells. STATISTICS Results are presented as means±S.E. The statistical significance of differences
was analyzed using the Student's _t_-test. Values were considered to be different from one another when _P_-values were lower than 0.05. ABBREVIATIONS * PTP: permeability transition
pore * CsA: cyclosporin A * CRC: Ca2+ retention capacity * TMRM: tetramethylrhodamine methyl ester REFERENCES * Service FJ, Nelson RL . Characteristics of glycemic stability. _Diabetes Care_
1980; 3: 58–62. Article CAS PubMed Google Scholar * Wahjudi PN, Patterson ME, Lim S, Yee JK, Mao CS, Lee WN . Measurement of glucose and fructose in clinical samples using gas
chromatography/mass spectrometry. _Clin Biochem_ 2010; 43: 198–207. Article CAS PubMed Google Scholar * Kashiwagi A, Obata T, Suzaki M, Takagi Y, Kida Y, Ogawa T _et al_. Increase in
cardiac muscle fructose content in streptozotocin-induced diabetic rats. _Metabolism_ 1992; 41: 1041–1046. Article CAS PubMed Google Scholar * Tilton RG, Chang K, Nyengaard JR, Van den
Enden M, Ido Y, Williamson JR . Inhibition of sorbitol dehydrogenase. Effects on vascular and neural dysfunction in streptozocin-induced diabetic rats. _Diabetes_ 1995; 44: 234–242. Article
CAS PubMed Google Scholar * Bizeau ME, Pagliassotti MJ . Hepatic adaptations to sucrose and fructose. _Metabolism_ 2005; 54: 1189–1201. Article CAS PubMed Google Scholar * Zoratti
M, Szabo I . The mitochondrial permeability transition. _Biochim Biophys Acta_ 1995; 1241: 139–176. Article PubMed Google Scholar * Bernardi P, Krauskopf A, Basso E, Petronilli V,
Blachly-Dyson E, Di Lisa F _et al_. The mitochondrial permeability transition from _in vitro_ artifact to disease target. _FEBS J_ 2006; 273: 2077–2099. Article CAS PubMed Google Scholar
* Desagher S, Martinou JC . Mitochondria as the central control point of apoptosis. _Trends Cell Biol_ 2000; 10: 369–377. Article CAS PubMed Google Scholar * Bernardi P . Mitochondrial
transport of cations: channels, exchangers, and permeability transition. _Physiol Rev_ 1999; 79: 1127–1155. Article CAS PubMed Google Scholar * Chauvin C, De Oliveira F, Ronot X,
Mousseau M, Leverve X, Fontaine E . Rotenone inhibits the mitochondrial permeability transition-induced cell death in U937 and KB cells. _J Biol Chem_ 2001; 276: 41394–41398. Article CAS
PubMed Google Scholar * Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N _et al_. Metformin prevents high-glucose-induced endothelial cell death through a
mitochondrial permeability transition-dependent process. _Diabetes_ 2005; 54: 2179–2187. Article CAS PubMed Google Scholar * Guigas B, Detaille D, Chauvin C, Batandier C, De Oliveira F,
Fontaine E _et al_. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological _in vitro_ study. _Biochem J_ 2004; 382 (Pt 3): 877–884. Article CAS PubMed
PubMed Central Google Scholar * Kaneto H, Fujii J, Myint T, Miyazawa N, Islam KN, Kawasaki Y _et al_. Reducing sugars trigger oxidative modification and apoptosis in pancreatic beta-cells
by provoking oxidative stress through the glycation reaction. _Biochem J_ 1996; 320 (Pt 3): 855–863. Article CAS PubMed PubMed Central Google Scholar * Kim WH, Lee JW, Suh YH, Hong SH,
Choi JS, Lim JH _et al_. Exposure to chronic high glucose induces beta-cell apoptosis through decreased interaction of glucokinase with mitochondria: downregulation of glucokinase in
pancreatic beta-cells. _Diabetes_ 2005; 54: 2602–2611. Article CAS PubMed Google Scholar * Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H . Glucose toxicity in beta-cells: type 2
diabetes, good radicals gone bad, and the glutathione connection. _Diabetes_ 2003; 52: 581–587. Article CAS PubMed Google Scholar * Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson
RP . Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. _Proc Natl Acad Sci USA_ 1999; 96: 10857–10862. Article CAS PubMed Google Scholar *
Koshkin V, Bikopoulos G, Chan CB, Wheeler MB . The characterization of mitochondrial permeability transition in clonal pancreatic beta-cells. Multiple modes and regulation. _J Biol Chem_
2004; 279: 41368–41376. Article CAS PubMed Google Scholar * Barbu A, Welsh N, Saldeen J . Cytokine-induced apoptosis and necrosis are preceded by disruption of the mitochondrial membrane
potential (Deltapsi(m)) in pancreatic RINm5F cells: prevention by Bcl-2. _Mol Cell Endocrinol_ 2002; 190: 75–82. Article CAS PubMed Google Scholar * Marselli L, Trincavelli L,
Santangelo C, Lupi R, Del Guerra S, Boggi U _et al_. The role of peripheral benzodiazepine receptors on the function and survival of isolated human pancreatic islets. _Eur J Endocrinol_
2004; 151: 207–214. Article CAS PubMed Google Scholar * Fujimoto K, Chen Y, Polonsky KS, Dorn II GW . Targeting cyclophilin D and the mitochondrial permeability transition enhances
beta-cell survival and prevents diabetes in Pdx1 deficiency. _Proc Natl Acad Sci USA_ 2010; 107: 10214–10219. Article CAS PubMed Google Scholar * El-Mir MY, Nogueira V, Fontaine E,
Averet N, Rigoulet M, Leverve X . Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. _J Biol Chem_ 2000; 275: 223–228. Article
CAS PubMed Google Scholar * Dumas JF, Argaud L, Cottet-Rousselle C, Vial G, Gonzalez C, Detaille D _et al_. Effect of transient and permanent permeability transition pore opening on
NAD(P)H localization in intact cells. _J Biol Chem_ 2009; 284: 15117–15125. Article CAS PubMed PubMed Central Google Scholar * Moriscot C, Pattou F, Kerr-Conte J, Richard MJ, Lemarchand
P, Benhamou PY . Contribution of adenoviral-mediated superoxide dismutase gene transfer to the reduction in nitric oxide-induced cytotoxicity on human islets and INS-1 insulin-secreting
cells. _Diabetologia_ 2000; 43: 625–631. Article CAS PubMed Google Scholar * Tiedge M, Lortz S, Drinkgern J, Lenzen S . Relation between antioxidant enzyme gene expression and
antioxidative defense status of insulin-producing cells. _Diabetes_ 1997; 46: 1733–1742. Article CAS PubMed Google Scholar * Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z,
Perego C _et al_. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death
program. _Diabetes_ 2001; 50: 1290–1301. Article CAS PubMed Google Scholar * Murphy RC, Schneider E, Kinnally KW . Overexpression of Bcl-2 suppresses the calcium activation of a
mitochondrial megachannel. _FEBS Lett_ 2001; 497: 73–76. Article CAS PubMed Google Scholar * Roy SS, Madesh M, Davies E, Antonsson B, Danial N, Hajnoczky G . Bad targets the permeability
transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. _Mol Cell_ 2009; 33: 377–388. Article CAS PubMed PubMed Central Google Scholar *
Zamzami N, El Hamel C, Maisse C, Brenner C, Munoz-Pinedo C, Belzacq AS _et al_. Bid acts on the permeability transition pore complex to induce apoptosis. _Oncogene_ 2000; 19: 6342–6350.
Article CAS PubMed Google Scholar * D'Alessandris C, Andreozzi F, Federici M, Cardellini M, Brunetti A, Ranalli M _et al_. Increased O-glycosylation of insulin signaling proteins
results in their impaired activation and enhanced susceptibility to apoptosis in pancreatic beta-cells. _FASEB J_ 2004; 18: 959–961. Article CAS PubMed Google Scholar * Bopassa JC,
Ferrera R, Gateau-Roesch O, Couture-Lepetit E, Ovize M . PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. _Cardiovasc Res_ 2006; 69:
178–185. Article CAS PubMed Google Scholar * Efanova IB, Zaitsev SV, Zhivotovsky B, Kohler M, Efendic S, Orrenius S _et al_. Glucose and tolbutamide induce apoptosis in pancreatic
beta-cells. A process dependent on intracellular Ca2+ concentration. _J Biol Chem_ 1998; 273: 33501–33507. Article CAS PubMed Google Scholar * Kahn SE, Haffner SM, Heise MA, Herman WH,
Holman RR, Jones NP _et al_. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. _N Engl J Med_ 2006; 355: 2427–2443. Article CAS PubMed Google Scholar * Sesti G,
Marini MA, Cardellini M, Sciacqua A, Frontoni S, Andreozzi F _et al_. The Arg972 variant in insulin receptor substrate-1 is associated with an increased risk of secondary failure to
sulfonylurea in patients with type 2 diabetes. _Diabetes Care_ 2004; 27: 1394–1398. Article CAS PubMed Google Scholar * Merani S, Shapiro AM . Current status of pancreatic islet
transplantation. _Clin Sci (Lond)_ 2006; 110: 611–625. Article CAS Google Scholar * Eriksson O, Eich T, Sundin A, Tibell A, Tufveson G, Andersson H _et al_. Positron emission tomography
in clinical islet transplantation. _Am J Transplant_ 2009; 9: 2816–2824. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by Grants from
INSERM and the Ministère de l'Enseignement de la Recherche et de la Technologie (MERT). SL was supported by fellowships from AGIR. We also thank Christophe Cottet for the English
corrections to this paper. AUTHOR INFORMATION Author notes * X Leverve: Professor X Leverve (1950–2010), in memoriam. AUTHORS AND AFFILIATIONS * Inserm, U1055, Grenoble, F-38041, France S
Lablanche, C Cottet-Rousselle, F Lamarche, P-Y Benhamou, X Leverve & E Fontaine * Joseph Fourier University, Grenoble, F-38041, France S Lablanche, C Cottet-Rousselle, F Lamarche, P-Y
Benhamou, X Leverve & E Fontaine * Grenoble University Hospital, Grenoble, F-38043, France P-Y Benhamou, S Halimi, X Leverve & E Fontaine Authors * S Lablanche View author
publications You can also search for this author inPubMed Google Scholar * C Cottet-Rousselle View author publications You can also search for this author inPubMed Google Scholar * F
Lamarche View author publications You can also search for this author inPubMed Google Scholar * P-Y Benhamou View author publications You can also search for this author inPubMed Google
Scholar * S Halimi View author publications You can also search for this author inPubMed Google Scholar * X Leverve View author publications You can also search for this author inPubMed
Google Scholar * E Fontaine View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to E Fontaine. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION Edited by M Federici RIGHTS AND PERMISSIONS This work is licensed under the Creative Commons
Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Lablanche, S., Cottet-Rousselle, C., Lamarche, F. _et al._ Protection of pancreatic INS-1 _β-_cells from glucose- and fructose-induced cell death by inhibiting
mitochondrial permeability transition with cyclosporin A or metformin. _Cell Death Dis_ 2, e134 (2011). https://doi.org/10.1038/cddis.2011.15 Download citation * Received: 27 January 2011 *
Revised: 09 February 2011 * Accepted: 10 February 2011 * Published: 24 March 2011 * Issue Date: March 2011 * DOI: https://doi.org/10.1038/cddis.2011.15 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * permeability transition * glucotoxicity * fructose * INS-1 * cyclosporin * metformin






