- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT FLIP is a potential anti-cancer therapeutic target that inhibits apoptosis by blocking caspase 8 activation by death receptors. We report a novel interaction between FLIP and the
DNA repair protein Ku70 that regulates FLIP protein stability by inhibiting its polyubiquitination. Furthermore, we found that the histone deacetylase (HDAC) inhibitor Vorinostat (SAHA)
enhances the acetylation of Ku70, thereby disrupting the FLIP/Ku70 complex and triggering FLIP polyubiquitination and degradation by the proteasome. Using _in vitro_ and _in vivo_ colorectal
cancer models, we further demonstrated that SAHA-induced apoptosis is dependant on FLIP downregulation and caspase 8 activation. In addition, an HDAC6-specific inhibitor Tubacin
recapitulated the effects of SAHA, suggesting that HDAC6 is a key regulator of Ku70 acetylation and FLIP protein stability. Thus, HDAC inhibitors with anti-HDAC6 activity act as efficient
post-transcriptional suppressors of FLIP expression and may, therefore, effectively act as ‘FLIP inhibitors’. SIMILAR CONTENT BEING VIEWED BY OTHERS CONVERSION OF KU80 K568 CROTONYLATION TO
SUMOYLATION FACILITATES DNA NON-HOMOLOGOUS END JOINING AND CANCER RADIORESISTANCE Article Open access 21 April 2025 SIRT1 DEACETYLATES WEE1 AND SENSITIZES CANCER CELLS TO WEE1 INHIBITION
Article 12 January 2023 DEUBIQUITINASE OTUD6A PROMOTES BREAST CANCER PROGRESSION BY INCREASING TOPBP1 STABILITY AND RENDERING TUMOR CELLS RESISTANT TO DNA-DAMAGING THERAPY Article 29 June
2022 MAIN FLIP is an anti-apoptotic protein that blocks the activation of apoptosis mediated by death receptors, such as Fas, TRAIL receptor 1 (TRAIL-R1/DR4) and TRAIL-R2 (DR5).1 By binding
to the adaptor protein FADD, FLIP inhibits apoptosis by blocking the processing and activation of procaspase 8 (FLICE) by death receptor complexes termed DISCs (death-inducing signalling
complexes).2 We previously reported that FLIP inhibits apoptosis induced by chemotherapeutic agents3 and that high FLIP expression is an independent adverse prognostic biomarker in
colorectal cancer (CRC).4 These and other studies have indicated that inhibition of FLIP constitutes a promising therapeutic strategy for the treatment of CRC. Ku70 and its binding partner
Ku80 are critical components of the non-homologous end joining (NHEJ) DNA repair machinery.5 Ku70 is regulated by acetylation, which is mediated by the histone acetyl transferases (HATs);
CREB-binding protein (CBP) and PCAF, and its acetylation can be enhanced by treating cells with histone deacetylase (HDAC) inhibitors.6 Ku70 acetylation disrupts its DNA-binding activity and
sensitises cells to DNA-damaging agents.7 In addition, cytoplasmic Ku70 binds to and regulates the pro-apoptotic Bcl-2 family member, Bax.6 Ku70 simultaneously inhibits Bax degradation via
the ubiquitin proteasome system (UPS) and prevents its translocation to the mitochondria.8 Moreover, it has been reported that Ku70 may have intrinsic de-ubiquitinating (DUB) activity.8 The
Ku70–Bax complex is disrupted by Ku70 acetylation, which promotes Bax translocation to mitochondria and apoptosis induction.6 Herein, we report a novel interaction between FLIP and Ku70 that
regulates FLIP stability. This interaction is acetylation-dependant and is disrupted by HDAC inhibitors with activity against HDAC6. Disruption of the Ku70–FLIP interaction subsequently
leads to FLIP degradation by the UPS and induction of caspase 8-dependant apoptosis. RESULTS FLIP INTERACTS WITH KU70 A yeast-2-hybrid screen was carried out to identify novel binding
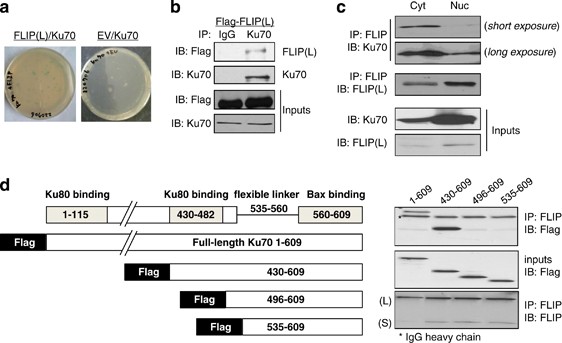
partners for the long FLIP splice variant (FLIP(L)). One of the novel proteins identified was Ku70. The interaction between FLIP(L) and Ku70 was confirmed by re-transformation assay (Figure
1a). Immunoprecipitation experiments confirmed that FLIP(L) interacts with Ku70 in CRC cells (Figure 1b). Despite the predominant site of Ku70 expression being nuclear, the interaction
between FLIP(L) and Ku70 was more prominent in cytosolic fractions (Figure 1c), suggesting that the primary site of the interaction is cytosolic. Ku70 also interacted with the short FLIP
splice form, FLIP(S) (Supplementary Figure S1A). To map Ku70's interaction with FLIP, we generated a series of Flag-tagged Ku70 constructs. The region from 430 to 609 amino acids
interacted strongly with FLIP, however, neither the 496–609 nor the 535–609 fragments interacted (Figure 1d). These mapping experiments indicate that Ku70 binds FLIP via a region between
amino acids 430 and 496, which encompasses one of its sites of interaction with Ku80. KU70 BINDS TO DED2 OF FLIP To identify the regions of FLIP that are important for the FLIP–Ku70
interaction, we used a FLIP peptide array of overlapping 25-mer peptides, each shifted by four amino acids, encompassing the entire FLIP(S) sequence.9 Duplicate membranes were overlaid with
lysates from cells transfected with Flag-Ku70, and the Flag epitope detected by immunoblotting. Strong binding of Ku70 to the array was detected in four regions (Figure 2a and table). To map
the FLIP–Ku70 interaction to specific amino acids, alanine substitution arrays were constructed for peptides 17, 30, 41 and 46. Several alanine substitutions in peptide 30 (but not the
other 3 peptides) were found to disrupt the binding of Ku70 (Figure 2b). By using viral FLIP MC159 as a template,10 a homology model of human FLIP(S) was generated. The model predicted that
two of the candidate residues identified in the alanine substitution array, Y119 and R122, are prominently surface exposed and, therefore, available for making protein–protein interactions
(Figure 2c); the other candidate residues were predicted to be buried. Mutation of FLIP(S) R122, but not Y119, to alanine significantly diminished binding to Ku70 (Figure 2d). Thus, these
studies identify R122 as a critical site of interaction between FLIP and Ku70. This region of FLIP death effector domain 2 (DED2) is present in all FLIP splice forms and is adjacent to the
‘hydrophobic patch’ region reported to be important for binding to FADD.11 Binding of FADD and procaspase 8 to FLIP was unaffected by R122A mutation indicating that FADD/procaspase 8 and
Ku70 interact with distinct regions of FLIP (Figure 2d). SAHA DISRUPTS THE FLIP–KU70 INTERACTION AND INDUCES FLIP DEGRADATION Treatment with the HDAC inhibitor Vorinostat (SAHA,
suberoylanilide hydroxamic acid) for 2 h disrupted the interaction between endogenous FLIP and Ku70 (Figure 3a), and increased Ku70 acetylation after 1 h (Figure 3b). Increased Ku70
acetylation following SAHA treatment was also observed in three other CRC cell lines (Supplementary Figure S1B). Although Ku70 was primarily expressed in the nucleus, acetylated Ku70 was
detectable at similar levels in the nucleus and the cytosol, and increased in both sub-cellular locations following SAHA treatment (Supplementary Figure S1C). We were unable to detect the
acetylation of FLIP following SAHA treatment (Supplementary Figure S1D), suggesting that it is not directly modified by acetylation. Ku70 acetylation following SAHA treatment decreased its
capacity to bind to FLIP (Figure 3b). Cohen _et al._6 demonstrated that mimicking the acetylation of lysine residues K539 or K542 in Ku70 by mutating them to glutamine disrupted its ability
to inhibit Bax-mediated apoptosis. We found that mutation of either of these residues to glutamine impaired the ability of Ku70 to interact with FLIP (Figure 3c). Collectively, these results
indicate that Ku70 acetylation inhibits its ability to interact with FLIP. Disruption of FLIP–Ku70 binding following SAHA treatment correlated with increased polyubiquitination of FLIP(L)
and FLIP(S) (Figure 3d) and rapid downregulation of both splice forms from ∼4 h following treatment (Figure 3e). SAHA treatment also downregulated FLIP protein expression in three other CRC
cell lines after 6 h at sub-IC50 doses (Supplementary Figures S2C and E). In contrast to other studies, which have found that HDAC inhibitors downregulate FLIP at the transcriptional
level,12, 13 we found no evidence of transcriptional suppression of FLIP in CRC cells (Supplementary Figures S2A and D). Moreover, co-treatment with the proteasome inhibitor MG132 attenuated
SAHA-induced FLIP downregulation (Supplementary Figure S2B), providing further evidence that FLIP downregulation is mediated by the UPS. KU70 POST-TRANSCRIPTIONALLY REGULATES FLIP
EXPRESSION Transfection with three different Ku70-targeted siRNAs resulted in Ku70 depletion and significant decreases in FLIP expression (Figure 4a). Similar effects were observed in three
other CRC cell lines (Figure 4b). However, Ku70 depletion had no effect on FLIP mRNA expression (Figure 4c). siRNA-mediated Ku70 downregulation resulted in apoptosis as indicated by
poly(ADP-ribose) polymerase (PARP) cleavage (Figure 4d), flow cytometry (Figure 4e) and caspase 3/7 activation (Supplementary Figure S3A). The FLIP downregulation observed in Ku70-depleted
cells was not an indirect result of caspase activation, as inhibition of apoptosis with the pan-caspase inhibitor z-VAD-fmk failed to prevent FLIP downregulation in Ku70-depleted cells
(Figure 4d). Furthermore, the half-lives of both FLIP(L) and FLIP(S) were significantly reduced in Ku70-depleted cells, with almost undetectable levels of FLIP 1 h after treatment with the
protein synthesis inhibitor cycloheximide (Figure 4f). Importantly, Ku70 downregulation resulted in increased levels of polyubiquitinated FLIP(L) and FLIP(S) (Figure 4g), suggesting that
Ku70 enhances the half-life of FLIP by inhibiting its degradation via the UPS. SAHA- AND KU70 SIRNA-INDUCED APOPTOSIS IS CASPASE 8-DEPENDANT Analysis of other apoptotic proteins indicated
that unlike FLIP, none were significantly affected by SAHA treatment after 6 h, however, Bax was upregulated and XIAP downregulated 24 h following SAHA treatment (Figure 5a). Despite being
acetylated, total Ku70 expression was not affected by SAHA treatment (Figure 5a). Expression of several BH3-only proteins was also assessed: NOXA and BID were not significantly altered
following SAHA treatment, whereas PUMA was downregulated and BIM upregulated (Figure 5b). As the upregulation of Bax and BIM at 24 h coincided with the onset of apoptosis as determined by
PARP cleavage, we directly assessed the roles of the initiator caspases, caspase 8 and caspase 9 during SAHA-induced apoptosis. siRNA-mediated inhibition of caspase 8 significantly inhibited
SAHA-induced apoptosis in each CRC cell line as assessed by flow cytometry (Figure 5c), PARP cleavage (Supplementary Figure S4A) and analysis of caspase 3/7 activity (Supplementary Figure
S4C). In contrast, caspase 9 silencing failed to inhibit SAHA-induced apoptosis (Figure 5c). Furthermore, caspase 8 activity increased following SAHA treatment and correlated with increased
activity of the executioner caspases 3/7 (Figure 5d). SAHA treatment also resulted in significant increases in caspase 8 activity in other CRC cell lines (Supplementary Figure S4B). Notably,
increased caspase 8 activity was detected in Ku70-depleted HCT116 cells (Supplementary Figure S3A), and the apoptosis induced by Ku70 silencing was caspase 8-dependant (Figure 4e). Similar
results were observed in HT29 and RKO cells, although not in H630 cells, in which Ku70 silencing failed to activate either caspase 8 or caspase 3/7 (Supplementary Figures S3B and S3C); this
may be because FLIP downregulation following Ku70 depletion was less pronounced in this cell line (Figure 4b). Interestingly, given Ku70's role in regulating Bax-mediated apoptosis,6
Ku70 depletion still resulted in significant (albeit reduced) levels of apoptosis in Bax-null HCT116 cells, and this was again caspase 8-dependant (Figure 4e). SAHA- AND KU70 SIRNA-INDUCED
APOPTOSIS IS FLIP-DEPENDANT Using an HCT116 FLIP(L) overexpressing cell line (FL17), we obtained direct evidence for the importance of FLIP downregulation during SAHA-induced apoptosis. SAHA
failed to downregulate FLIP(L) in this cell line (Figure 6a) as it activated transcription of the exogenous transgene (unpublished observations). Lack of FLIP(L) downregulation in this cell
line correlated with significantly reduced levels of SAHA-induced apoptosis compared with the parental cell line as determined by PARP cleavage (Figure 6a) and flow cytometry (Figure 6b).
Of note, FLIP(L) overexpression did not prevent SAHA-induced cell cycle arrest in G2/M phase (Figure 6b), indicating that FLIP regulates the cytotoxic rather than the cytostatic effects of
SAHA. SAHA also sensitised CRC cells to apoptosis induced by rTRAIL and chemotherapeutic agents in a FLIP-dependant manner (Supplementary Figures S5A-D). These results establish direct
evidence for FLIP downregulation as a major mechanism of apoptosis induction by SAHA alone and in combination with other anti-cancer agents. Ku70 siRNA-induced apoptosis was also
significantly reduced in FLIP(L)-overexpressing cells (Figure 6c). To assess the importance of FLIP downregulation for the _in vivo_ effects of SAHA, we used the FL17 FLIP(L)-overexpressing
model. In both parental and FL17 xenografts, treatment with SAHA resulted in increased acetylation of histone H4, a marker of HDAC inhibition (Figure 6d). SAHA treatment resulted in potent
FLIP(L) downregulation in the parental xenografts and also decreased FLIP(L) expression in the FL17 xenografts. However, the levels of FLIP(L) expression that were maintained in SAHA-treated
FL17 tumours were comparable to those in vehicle-treated parental xenografts. Notably, SAHA treatment failed to inhibit the growth of FL17 xenografts, whereas the growth of the parental
xenografts was significantly inhibited by SAHA (Figure 6e). These results are the first to demonstrate that SAHA downregulates FLIP _in vivo_ and that FLIP downregulation is important for
its anti-tumour activity. INHIBITORS WITH ANTI-HDAC6 ACTIVITY REGULATE FLIP EXPRESSION AND KU70 ACETYLATION To identify the HDACs involved in regulating Ku70 acetylation, we used the HDAC
inhibitor Droxinostat,14 which, although less potent, has a more restricted activity than SAHA: Droxinostat is an inhibitor of HDACs 6 and 8, and (to a lesser extent) HDAC3;14 SAHA inhibits
HDACs 1, 2, 3, 6 and (to a lesser extent) 8.15 In agreement with earlier studies,16 we found that Droxinostat potently downregulated FLIP expression and did so at early (6 h) time points
post-treatment (Supplementary Figure S6A). However, unlike these earlier studies, we found that this downregulation was post-transcriptional, as this agent had no effect on FLIP mRNA
expression (Supplementary Figure S6B). Droxinostat-induced apoptosis correlated with FLIP downregulation (Supplementary Figure S6C), and FLIP downregulation was not caspase dependant
(Supplementary Figure S6D). Like SAHA, Droxinostat-induced apoptosis was caspase 8-dependant (Supplementary Figures S6D and 7A). Using a ‘caspase-trap’ assay,17 we demonstrated that caspase
8 rather than caspase 9 was the first caspase activated in HCT116 cells in response to treatment with both SAHA and Droxinostat for 4 h (Figure 7b). In addition, Droxinostat-induced
apoptosis was inhibited in FL17 cells (Figures 7c and d). Given the reported importance of Bax for HDAC inhibitor-induced apoptosis,6 it was notable that the inhibitory effects of caspase 8
silencing or FLIP overexpression on HDAC inhibitor-induced apoptosis were more profound than loss of Bax (Figures 7a and d). Furthermore, in contrast to a previous study,18 we found that
HDAC inhibitor-induced apoptosis was not p53-dependant in HCT116 cells (Figure 7d). We previously reported that apoptosis induction following siRNA-mediated FLIP silencing is primarily
driven by the TRAIL receptor DR5.3 In agreement with this, SAHA- and Droxinostat-induced cell death were both significantly attenuated when DR5 was silenced, and this effect was further
enhanced when DR4 was co-silenced with DR5 (Figure 7e). As Droxinostat and SAHA both inhibit HDAC6, we hypothesised that HDAC6 may be involved in regulating FLIP expression. To test this, we
used an HDAC6-selective inhibitor Tubacin.19 Both Tubacin and Droxinostat increased Ku70 acetylation to the same extent as SAHA (Figure 8a). By comparison, acetylation of Ku70 in response
to Entinostat, which inhibits HDACs 1–3, but not HDAC6,15 was significantly less than that induced by the other HDAC inhibitors (Figure 8a). In addition, siRNA-mediated depletion of HDAC6
resulted in significantly increased Ku70 acetylation (Figure 8b). Like SAHA and Droxinostat, Tubacin treatment resulted in rapid FLIP protein downregulation (Figure 8c), but had no
significant effect on FLIP mRNA expression (Figure 8d). Tubacin also caused an increase in acetylation of _α_-tubulin, a marker of HDAC6 inhibition, whereas acetylation of histone H4, a
marker of nuclear HDAC inhibition, was significantly lower following Tubacin treatment than following SAHA treatment (Figure 8c). In contrast, Entinostat failed to downregulate FLIP
expression in HCT116 cells after 6 h at a concentration that potently increased acetylation of histone H4, but had no effect on acetylation of _α_-tubulin (Figure 8e). In addition,
Tubacin-induced apoptosis was highly FLIP-dependant as determined by flow cytometry (Figure 8f), consistent with FLIP downregulation being a primary mechanism of apoptosis induction in
response to this agent. DISCUSSION We previously demonstrated that FLIP is an important regulator of apoptosis induction and drug resistance in CRC. 3, 20 We carried out a yeast-2-hybrid
screen to identify novel FLIP-interacting proteins, with the aim of identifying novel regulators of FLIP expression or function. This screen identified Ku70, a DNA repair protein that was
initially characterised as being critical for NHEJ.21 Ku70 was subsequently shown to have an apoptosis-regulating function through its ability to bind to and sequester Bax.6 Both the DNA-
and Bax-binding activities of Ku70 are acetylation dependant.6, 7 Acetylation of Ku70 at residues K539 and K542 in its C-terminus by CBP and PCAF disrupt its ability to inhibit Bax-mediated
apoptosis.6 Moreover, Ku70 was reported to have intrinsic DUB activity that inhibits Bax degradation via the UPS.8 We found that FLIP interacts with Ku70 via a region in the DED2, using a
site (R122) adjacent to the region that is functionally important for FLIP binding to FADD.11 Binding of FADD and procaspase 8 to FLIP was unaffected by mutation of R122, indicating that
Ku70 interacts with a region of FLIP that is distinct from the region with which it interacts with these DISC components. Furthermore, Ku70 did not bind to FLIP via the same region it binds
Bax, but rather via a region previously shown to be important for dimerisation with Ku80 (amino acids 430–496).22 Therefore, FLIP interacts with the same region of Ku70 with which it engages
Ku80 and does so using a region in DED2 not involved in its DISC recruitment. In addition, fractionation experiments suggested that similar to Bax, the primary sub-cellular location of the
FLIP–Ku70 interaction is cytoplasmic. Ku70 depletion resulted in significant decreases in FLIP expression. Furthermore, the decrease in FLIP expression following Ku70 silencing was because
of a decrease in protein stability. Treatment with the HDAC inhibitor SAHA resulted in the acetylation of Ku70 and disrupted its interaction with FLIP. Moreover, mimicking Ku70 acetylation
by mutating the residues K539 and K542 to glutamine resulted in reduced Ku70–FLIP binding. These lysine residues are present in the flexible C-terminal linker region adjacent to the region
of Ku70, which binds FLIP (amino acids 430–496). It is possible that acetylation of this flexible linker region causes a conformational change of Ku70 that disrupts binding of FLIP in the
adjacent 430–496 region, or that acetylation disrupts binding of an as yet unidentified binding partner (or partners) that is important for maintaining the Ku70–FLIP complex. Although we
found no evidence of FLIP acetylation following SAHA treatment, polyubiquitination of both FLIP(L) and FLIP(S) was increased, and both splice forms were rapidly downregulated in a
proteasome-dependant manner. A direct link between Ku70 and FLIP ubiquitination status was indicated by the increased levels of polyubiquitinated FLIP(L) and FLIP(S) in Ku70-silenced cells.
In contrast to numerous other studies,12, 13 we found no evidence that SAHA downregulated FLIP expression at the transcriptional level. However, our findings agree with a recent study in
breast cancer models, which also found that SAHA-induced FLIP downregulation was post-transcriptional.23 In addition, a related pan-HDAC inhibitor Panobinostat (LBH589) was recently reported
to cause FLIP polyubiquitination and proteasomal degradation in pancreatic cancer cells.24 Collectively, these findings suggest that SAHA causes Ku70 acetylation, resulting in the
disruption of its interaction with FLIP leading to degradation of FLIP by the UPS. Although Ku70 is a substrate for the HATs CBP and PCAF,6 the HDACs that regulate Ku70 acetylation have not
been previously characterised. In this study, we provide evidence that HDAC6 is a key deacetylase for Ku70 as, in addition to Vorinostat (inhibitor of HDAC1-3, 6 and 8)15 and Droxinostat
(inhibitor of HDAC3, 6 and 8),14 the HDAC6-specific inhibitor Tubacin19 potently increased the acetylation of Ku70. Moreover, both Droxinostat and Tubacin also caused rapid FLIP
downregulation. In contrast, Entinostat, which inhibits HDAC1–3, but has no activity against HDAC6,15 failed to downregulate FLIP at early time points and had less impact on Ku70
acetylation. During revision of this manuscript, another study was published that also found that HDAC6 deacetylates Ku70.25 HDAC6 is a unique HDAC that has key roles in regulating protein
turnover by the proteasome through its regulation of HSP90 and by the aggresome through its ability to recruit polyubiquitinated proteins to the tubulin cytoskeleton.26 Thus, this study
identifies a further potential role for HDAC6 in regulating protein stability through its ability to deacetylate Ku70, thereby stabilising FLIP. By deacetylating Ku70, HDAC6 may also
regulate Bax and possibly other as yet unidentified Ku70-binding partners. Although a number of studies have shown that HDAC inhibitor-mediated downregulation of FLIP sensitises various
cancer cell lines to death ligands, such as TRAIL,23, 27, 28 to our knowledge, no previous studies have directly examined whether FLIP downregulation is a sufficient death signal for
apoptosis induction when these agents are administered as single agents. We found that FLIP downregulation in response to HDAC inhibitors results in caspase 8- and DR5-mediated apoptosis,
phenocopying the mechanism of apoptosis that we previously observed in CRC cells following siRNA-mediated downregulation of FLIP.3 We also demonstrate for the first time that SAHA
downregulates FLIP _in vivo_ and that FLIP(L) overexpression abrogates SAHA's _in vivo_ anti-tumour activity. FLIP(L) overexpression or caspase 8 downregulation had a greater inhibitory
effect on HDAC inhibitor-induced apoptosis than loss of Bax, suggesting that the extrinsic apoptotic pathway is at least as important as the intrinsic pathway in mediating the effects of
HDAC inhibitors in CRC cells. From a cancer therapeutics perspective, these results suggest that HDAC inhibitors with anti-HDAC6 activity act as efficient post-transcriptional suppressors of
FLIP expression in CRC. HDAC inhibitors are promising anti-cancer therapeutics that have demonstrated pre-clinical and clinical activities in haematological and solid cancers, and SAHA was
the first of this class of compound to be FDA approved.29, 30, 31, 32, 33 There have been several early phase clinical trials in advanced, chemotherapy-resistant CRC examining SAHA in
combination with standard 5-Fluorouracil (5-FU)-based chemotherapy.34, 35, 36 In one of these studies, 21/38 5-FU-refractory patients had stable disease and 1 had a partial response.34 The
plasma concentrations of SAHA achieved in these patients were similar to the concentrations that we find are sufficient to downregulate FLIP. Of note, we found that SAHA synergized with both
5-FU and oxaliplatin to induce apoptosis in CRC cells; however, this synergy was abolished in cells overexpressing FLIP(L). Thus, FLIP and components of the extrinsic pathway, such as
caspase 8, may be useful predictive biomarkers for the targeted use of HDAC inhibitors in CRC. In conclusion, we provide evidence that Ku70 regulates FLIP ubiquitination and that HDAC
inhibitor-mediated acetylation of Ku70 disrupts the interaction between FLIP and Ku70 resulting in degradation of FLIP via the UPS and induction of caspase 8-dependant apoptosis (summarised
in Figure 9). MATERIALS AND METHODS REAGENTS The following reagents were used: Vorinostat (Zolinza, SAHA; Selleck Chemicals, Suffolk, UK); Droxinostat
(4-(4-chloro-2-methylphenoxy)-N-hydroxybutanamide, compound #5809354) and its inactive analogue 4-(4-chloro-2-methylphenoxy)-N-(3-ethoxypropyl)butanamide (compound #7271570, ChemBridge
Corporation, San Diego, CA, USA). Tubacin was a kind gift from Dr. Ralph Mazitschek and Dr Stuart Schreiber (Howard Hughes Medical Institute, Broad Institute of Harvard and MIT). Entinostat
(MS-275) was synthesised in the laboratory as previously described.37, 38 Cycloheximide and MG132 (Sigma-Aldrich, Dorset, UK). Biotinylated z-VAD-fmk (Val-Ala-Asp-CH2F) and immobilised
Streptavidin slurry were purchased from MP Biomedicals (Cambridge, UK) and Thermo Fisher Scientific (Leicestershire, UK), respectively. CELL CULTURE HCT116, H630, HT29 and RKO human CRC cell
lines were used as previously described.3, 39 Matched isogenic p53 and Bax-null HCT116 cell lines were kindly provided by Professor B Vogelstein (Johns Hopkins University School of
Medicine, Baltimore). FL17 cells were generated and cultured as previously described.20 EXPRESSION CONSTRUCTS Flag-tagged FLIP(L), FLIP(S) and Ku70 expression constructswere generated in the
pCMV-3Tag-6 vector (Agilent Technologies, Berkshire, UK). The GST-tagged FLIP(S) expression construct was generated in the pGEX6P-3 vector (GE Healthcare, Herts, UK). Mutagenesis was
carried out using the KOD Extreme polymerase (Novagen, Nottingham, UK), with the template plasmid digested using _DpnI_ (New England Biolabs, Herts, UK). WESTERN BLOTTING AND SUB-CELLULAR
FRACTIONATION Western blots were carried out as previously described.3 Isolation of nuclear and cytosolic fractions was carried out by lysing the cells for 10 min on ice in buffer A (10 mM
HEPES, 1.5 mM MgCl2, 10 mM KCL, 0.5 mM DTT, 0.5% NP40, pH7.9) followed by centrifugation at 3000 r.p.m. for 10 min. The supernatants were retained as the cytosolic fractions, and the pellets
were subjected to further lysis in buffer B (5 mM HEPES, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 26% glycerol (v/v), pH 7.9). NaCl was added to a final concentration of 300 mM, and the
samples were homogenized using a Dounce homogenizer (VWR, Dublin, Ireland). After a 30-min incubation on ice, the lysates were centrifuged for 20 min at 24 000 _g_, and the resulting
supernatants collected as the nuclear fractions. ANTIBODIES _Mouse monoclonal (Western)_: FLIP (NF6; Alexis, San Diego, CA, USA), Caspase 8 (12F5; Alexis) and PARP (eBioscience, San Diego,
CA, USA), _β_-Actin and Flag-HRP (Sigma, Dorset, UK), Ku70, ITCH (both BD Transduction Laboratories, Oxfordshire, UK) and Noxa (Abcam, Cambridge, UK). _Rabbit polyclonal (Western and IP):_
Caspase 9, caspase 3, acetylated lysine, acetylated-_α_-tubulin, XIAP, Bcl-2, Bcl-XL, Mcl-1, Bax, Bak, Bid, Bim, PUMA (Cell Signaling Technology, Beverly, MA, USA), c-FLIP (H202), Ku70
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Hyperacetylated Histone H4 (Millipore, Watford, UK). Horseradish peroxidase-conjugated sheep anti-mouse and sheep anti-rabbit secondary
antibodies were used as appropriate (Amersham, Buckinghamshire, UK). YEAST-2-HYBRID The Matchmaker GAL4-based yeast-two-hybrid system was used (Clontech, Saint Germain en Laye, France).
Human FLIP(L) was used as the bait protein and expressed as a fusion to the GAL4 DNA-binding domain in pGBKT7 vector. A cDNA library generated from HCT116 cells was cloned into pGADT7 prey
vector. The yeast-two-hybrid screen for FLIP(L)-interacting proteins was carried out according to the manufacturer's instructions. IMMUNOPRECIPITATION (IP) REACTIONS Protein lysates
were prepared using CHAPS (10 mM HEPES, 150 mM NaCl, 1% CHAPS, pH 7.4) or NP-40 (0.2% NP-40, 20 mM Tris-HCL (pH 7.4), 150 mM NaCl, 10% glycerol) buffers. Lysates (0.5–2 mg) were pre-cleared
overnight with sheep anti-rabbit IgG Dynalbeads (Invitrogen, Paisley, UK). Antibodies (1–2 _μ_g) were conjugated with sheep anti-rabbit IgG Dynal beads for at least 1 h and then washed
before incubation with pre-cleared lysates for at least 4 h. After several washes, Dynal beads were resuspended in Laemmli loading buffer and heated at 95 °C for 5 min before immunoblot
analysis. Ubiquitin IP experiments were carried out using the Ubiquitin Enrichment kit (Thermo Scientific, Surrey, UK) according to the manufacturer's instructions. RECOMBINANT PROTEIN
PURIFICATION AND GST PULL-DOWNS GST and GST-tagged FLIP(S) were expressed in IPTG-stimulated BL21 bacteria. Bacterial cell lysates were purified using Glutathione-Sepharose beads (GE
Healthcare Bio-Sciences AB, Uppsala, Sweden) and dialysed using Slide-A-Lyzer Dialysis Cassettes (Thermo Scientific). For pull-downs, GST-tagged proteins (10 _μ_g) were conjugated to
Glutathione-Sepharose beads and incubated with pre-cleared protein lysates (500 _μ_g) for 2 h at 4 °C with constant mixing. Beads were washed several times and resuspended in Laemmli loading
buffer before western blot analysis. FLIP PEPTIDE ARRAY ANALYSIS FLIP peptide arrays were generated as previously described9 using the Genbank sequence U97074.1. The arrays were blocked in
5% milk/PBS/0.5% Tween-20 for 1 h and then overlaid with lysates prepared from the cells transfected with Flag-tagged Ku70 or EV. Following several washes (PBS/0.5% Tween-20), bound
Flag-tagged Ku70 was detected by immunoblotting. CASPASE ‘TRAP’ ASSAY This assay was performed as previously described.17 FLOW CYTOMETRY Apoptosis was determined using propidium iodide (PI)
staining to evaluate the percentage of cells with DNA content <2N as previously described.3 For annexin V/PI analysis, cells were harvested and analysed according to the
manufacturer's instructions (BD Biosciences, Oxford, UK). SIRNA TRANSFECTIONS The non-silencing control (SC) siRNA, FLIP-, caspase 8-, caspase 9, DR4- and DR5-targeted siRNAs were
obtained from Dharmacon (Chicago, IL, USA) and were the same as those previously described.3, 40 siRNAs targeting Ku70 and HDAC6 were obtained from Qiagen (West Sussex, UK). siRNA
transfections were carried out using OligofectAMINE (Invitrogen). QUANTITATIVE PCR (Q-PCR) RNA was isolated using the RNA STAT-60 reagent (Biogenesis, Poole, UK) and reverse transcribed
using Moloney murine leukemia virus-based reverse transcriptase kit (Invitrogen). Q-PCR analysis of the _FLIP_ gene expression was performed using the SYBR Green method or TaqMan Gene
Expression Assays (Applied Biosystems, Foster City, CA, USA). ANIMAL MODEL EXPERIMENTS Female BALB/c nude mice were maintained as previously described.3 All experiments were carried out in
accordance with the Animals (Scientific Procedures) Act, 1986 under a Project Licence (PPL 2590b) approved by the Department of Health, Social Services and Public Safety, Northern Ireland.
Mice were implanted sub-cutaneously on each flank with 2 × 106 HCT116 or FL17 cells using Matrigel (BD, Oxford, UK). After the tumours reached ∼100 mm3, mice were randomised to receive
vehicle control (10% DMSO/45% PEG400) or 100 mg/kg Vorinostat delivered IP on a 5-day on/2-day off schedule for 2 weeks. Tumour volumes were determined as previously described.3 To extract
protein, xenografts were homogenized in RIPA buffer. CASPASE ACTIVITY ASSAY Caspase-8 or caspase-3/7-GLO reagents (25 _μ_l) (Promega, Southampton, UK) were incubated with 1–10 _μ_g of
protein lysate diluted in cell culture medium in a total volume of 50 _μ_l for 1–2 h at room temperature. Luciferase activity was then determined using a luminometer. STATISTICAL ANALYSIS
Student's _t_-test was used for the statistical analysis; *denotes _P_<0.05;. **denotes _P_<0.01; ***denotes _P_<0.001; and ns denotes not significant compared with control.
ABBREVIATIONS * CBP: CREB-binding protein * CRC: colorectal cancer * DED: death effector domain * DISC: death-inducing signaling complex * DR4 and DR5: death receptor 4 and 5 * DUB:
de-ubiquitinating enzyme * FLIP: Fas-associated death domain (FADD)-like interleukin 1b-converting enzyme (FLICE)-inhibitory protein * 5-FU: 5-fluorouracil * HAT: histone acetyl transferase
* HDAC: histone deacetylase * HSP90: heat shock protein 90 * NHEJ: non-homologous end-joining * PARP: poly(ADP-ribose) polymerase * PCAF: P300/CBP-associated factor * Q-PCR: quantitative PCR
* SAHA: suberoylanilide hydroxamic acid * TRAIL: TNF-related apoptosis-inducing ligand * UPS: ubiquitin proteasome system * XIAP: X-linked inhibitor of apoptosis REFERENCES * Irmler M,
Thome M, Hahne M, Schneider P, Hofmann K, Steiner V _et al_. Inhibition of death receptor signals by cellular FLIP. _Nature_ 1997; 388: 190–195. Article CAS Google Scholar * Scaffidi C,
Schmitz I, Krammer PH, Peter ME . The role of c-FLIP in modulation of CD95-induced apoptosis. _J Biol Chem_ 1999; 274: 1541–1548. Article CAS Google Scholar * Wilson TR, McLaughlin KM,
McEwan M, Sakai H, Rogers KM, Redmond KM _et al_. c-FLIP: a key regulator of colorectal cancer cell death. _Cancer Res_ 2007; 67: 5754–5762. Article CAS Google Scholar * McLornan DP,
Barrett HL, Cummins R, McDermott U, McDowell C, Conlon SJ _et al_. Prognostic significance of TRAIL signaling molecules in stage II and III colorectal cancer. _Clin Cancer Res_ 2010; 16:
3442–3451. Article CAS Google Scholar * Featherstone C, Jackson SP . Ku, a DNA repair protein with multiple cellular functions? _Mutat Res_ 1999; 434: 3–15. Article CAS Google Scholar
* Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C _et al_. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. _Mol Cell_ 2004; 13:
627–638. Article CAS Google Scholar * Chen CS, Wang YC, Yang HC, Huang PH, Kulp SK, Yang CC _et al_. Histone deacetylase inhibitors sensitize prostate cancer cells to agents that produce
DNA double-strand breaks by targeting Ku70 acetylation. _Cancer Res_ 2007; 67: 5318–5327. Article CAS Google Scholar * Amsel AD, Rathaus M, Kronman N, Cohen HY . Regulation of the
proapoptotic factor Bax by Ku70-dependent deubiquitylation. _Proc Natl Acad Sci USA_ 2008; 105: 5117–5122. Article CAS Google Scholar * Kiely PA, Baillie GS, Lynch MJ, Houslay MD,
O’Connor R . Tyrosine 302 in RACK1 is essential for insulin-like growth factor-I-mediated competitive binding of PP2A and beta1 integrin and for tumor cell proliferation and migration. _J
Biol Chem_ 2008; 283: 22952–22961. Article CAS Google Scholar * Yang JK, Wang L, Zheng L, Wan F, Ahmed M, Lenardo MJ _et al_. Crystal structure of MC159 reveals molecular mechanism of
DISC assembly and FLIP inhibition. _Mol Cell_ 2005; 20: 939–949. Article CAS Google Scholar * Ueffing N, Keil E, Freund C, Kuhne R, Schulze-Osthoff K, Schmitz I . Mutational analyses of
c-FLIPR, the only murine short FLIP isoform, reveal requirements for DISC recruitment. _Cell Death Differ_ 2008; 15: 773–782. Article CAS Google Scholar * Guo F, Sigua C, Tao J, Bali P,
George P, Li Y _et al_. Cotreatment with histone deacetylase inhibitor LAQ824 enhances Apo-2 L/tumor necrosis factor-related apoptosis inducing ligand-induced death inducing signaling
complex activity and apoptosis of human acute leukemia cells. _Cancer Res_ 2004; 64: 2580–2589. Article CAS Google Scholar * Watanabe K, Okamoto K, Yonehara S . Sensitization of
osteosarcoma cells to death receptor-mediated apoptosis by HDAC inhibitors through downregulation of cellular FLIP. _Cell Death Differ_ 2005; 12: 10–18. Article CAS Google Scholar * Wood
TE, Dalili S, Simpson CD, Sukhai MA, Hurren R, Anyiwe K _et al_. Selective inhibition of histone deacetylases sensitizes malignant cells to death receptor ligands. _Mol Cancer Ther_ 2010; 9:
246–256. Article CAS Google Scholar * Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T _et al_. Chemical phylogenetics of histone deacetylases. _Nat Chem Biol_ 2010;
6: 238–243. Article CAS Google Scholar * Schimmer AD, Thomas MP, Hurren R, Gronda M, Pellecchia M, Pond GR _et al_. Identification of small molecules that sensitize resistant tumor cells
to tumor necrosis factor-family death receptors. _Cancer Res_ 2006; 66: 2367–2375. Article CAS Google Scholar * Tu S, McStay GP, Boucher LM, Mak T, Beere HM, Green DR . _In situ_ trapping
of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. _Nat Cell Biol_ 2006; 8: 72–77. Article CAS Google Scholar * Yamaguchi H, Woods NT, Piluso
LG, Lee HH, Chen J, Bhalla KN _et al_. p53 acetylation is crucial for its transcription-independent proapoptotic functions. _J Biol Chem_ 2009; 284: 11171–11183. Article CAS Google Scholar
* Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL . Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. _Proc Natl Acad
Sci USA_ 2003; 100: 4389–4394. Article CAS Google Scholar * Longley DB, Wilson TR, McEwan M, Allen WL, McDermott U, Galligan L _et al_. c-FLIP inhibits chemotherapy-induced colorectal
cancer cell death. _Oncogene_ 2006; 25: 838–848. Article CAS Google Scholar * Jeggo PA . Identification of genes involved in repair of DNA double-strand breaks in mammalian cells. _Radiat
Res_ 1998; 150: S80–S91. Article CAS Google Scholar * Wang J, Dong X, Myung K, Hendrickson EA, Reeves WH . Identification of two domains of the p70 Ku protein mediating dimerization with
p80 and DNA binding. _J Biol Chem_ 1998; 273: 842–848. Article CAS Google Scholar * Frew AJ, Lindemann RK, Martin BP, Clarke CJ, Sharkey J, Anthony DA _et al_. Combination therapy of
established cancer using a histone deacetylase inhibitor and a TRAIL receptor agonist. _Proc Natl Acad Sci USA_ 2008; 105: 11317–11322. Article CAS Google Scholar * Kauh J, Fan S, Xia M,
Yue P, Yang L, Khuri FR _et al_. c-FLIP degradation mediates sensitization of pancreatic cancer cells to TRAIL-induced apoptosis by the histone deacetylase inhibitor LBH589. _PLoS One_ 2010;
5: e10376. Article Google Scholar * Subramanian C, Jarzembowski JA, Opipari Jr AW, Castle VP, Kwok RP . HDAC6 deacetylates Ku70 and regulates Ku70-Bax binding in neuroblastoma.
_Neoplasia_ 2010; 13: 726–734. Article Google Scholar * Boyault C, Sadoul K, Pabion M, Khochbin S . HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and
ubiquitination. _Oncogene_ 2007; 26: 5468–5476. Article CAS Google Scholar * Schuchmann M, Schulze-Bergkamen H, Fleischer B, Schattenberg JM, Siebler J, Weinmann A _et al_. Histone
deacetylase inhibition by valproic acid down-regulates c-FLIP/CASH and sensitizes hepatoma cells towards CD95- and TRAIL receptor-mediated apoptosis and chemotherapy. _Oncol Rep_ 2006; 15:
227–230. CAS PubMed Google Scholar * Pathil A, Armeanu S, Venturelli S, Mascagni P, Weiss TS, Gregor M _et al_. HDAC inhibitor treatment of hepatoma cells induces both TRAIL-independent
apoptosis and restoration of sensitivity to TRAIL. _Hepatology_ 2006; 43: 425–434. Article CAS Google Scholar * Minucci S, Pelicci PG . Histone deacetylase inhibitors and the promise of
epigenetic (and more) treatments for cancer. _Nat Rev Cancer_ 2006; 6: 38–51. Article CAS Google Scholar * Kelly WK, O’Connor OA, Krug LM, Chiao JH, Heaney M, Curley T _et al_. Phase I
study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. _J Clin Oncol_ 2005; 23: 3923–3931. Article CAS Google Scholar *
Blumenschein Jr GR, Kies MS, Papadimitrakopoulou VA, Lu C, Kumar AJ, Ricker JL _et al_. Phase II trial of the histone deacetylase inhibitor vorinostat (Zolinza, suberoylanilide hydroxamic
acid, SAHA) in patients with recurrent and/or metastatic head and neck cancer. _Invest New Drugs_ 2008; 26: 81–87. Article CAS Google Scholar * Kelly WK, Richon VM, O’Connor O, Curley T,
MacGregor-Curtelli B, Tong W _et al_. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. _Clin Cancer Res_ 2003; 9:
3578–3588. CAS PubMed Google Scholar * Kavanaugh SM, White LA, Kolesar JM . Vorinostat: A novel therapy for the treatment of cutaneous T-cell lymphoma. _Am J Health Syst Pharm_ 2010; 67:
793–797. Article CAS Google Scholar * Fakih MG, Fetterly G, Egorin MJ, Muindi JR, Espinoza-Delgado I, Zwiebel JA _et al_. A phase I, pharmacokinetic, and pharmacodynamic study of two
schedules of vorinostat in combination with 5-fluorouracil and leucovorin in patients with refractory solid tumors. _Clin Cancer Res_ 2010; 16: 3786–3794. Article CAS Google Scholar *
Fakih MG, Pendyala L, Fetterly G, Toth K, Zwiebel JA, Espinoza-Delgado I _et al_. A phase I, pharmacokinetic and pharmacodynamic study on vorinostat in combination with 5-fluorouracil,
leucovorin, and oxaliplatin in patients with refractory colorectal cancer. _Clin Cancer Res_ 2009; 15: 3189–3195. Article CAS Google Scholar * Wilson PM, El-Khoueiry A, Iqbal S, Fazzone
W, LaBonte MJ, Groshen S _et al_. A phase I/II trial of vorinostat in combination with 5-fluorouracil in patients with metastatic colorectal cancer who previously failed 5-FU-based
chemotherapy. _Cancer Chemother Pharmacol_ 2010; 65: 979–988. Article CAS Google Scholar * Itoh Y, Suzuki T, Kouketsu A, Suzuki N, Maeda S, Yoshida M _et al_. Design, synthesis,
structure—selectivity relationship, and effect on human cancer cells of a novel series of histone deacetylase 6-selective inhibitors. _J Med Chem_ 2007; 50: 5425–5438. Article CAS Google
Scholar * Gediya LK, Belosay A, Khandelwal A, Purushottamachar P, Njar VC . Improved synthesis of histone deacetylase inhibitors (HDIs) (MS-275 and CI-994) and inhibitory effects of HDIs
alone or in combination with RAMBAs or retinoids on growth of human LNCaP prostate cancer cells and tumor xenografts. _Bioorg Med Chem_ 2008; 16: 3352–3360. Article CAS Google Scholar *
McDermott U, Longley DB, Galligan L, Allen W, Wilson T, Johnston PG . Effect of p53 status and STAT1 on chemotherapy-induced, Fas-mediated apoptosis in colorectal cancer. _Cancer Res_ 2005;
65: 8951–8960. Article CAS Google Scholar * Wilson TR, McEwan M, McLaughlin K, Le Clorennec C, Allen WL, Fennell DA _et al_. Combined inhibition of FLIP and XIAP induces Bax-independent
apoptosis in type II colorectal cancer cells. _Oncogene_ 2009; 28: 63–72. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by a grant from Cancer
Research UK. EK was supported by a studentship from the Department for Employment and Learning, NI. AUTHOR INFORMATION Author notes * E Kerr and C Holohan: These authors contributed equally
to this work. * P G Johnston and D B Longley: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Centre for Cancer Research and Cell Biology, School of Medicine,
Dentistry and Biomedical Science, Queen's University Belfast, 97 Lisburn Road, Belfast BT9 7BL, Northern Ireland, UK, E Kerr, C Holohan, K M McLaughlin, J Majkut, S Dolan, K Redmond, J
Riley, K McLaughlin, I Stasik, M Crudden, S Van Schaeybroeck, C Fenning, M Sgobba, D Haigh, P G Johnston & D B Longley * Department of Biochemistry, Cell Biology Laboratory, University
College Cork, Cork, Republic of Ireland R O'Connor & P Kiely Authors * E Kerr View author publications You can also search for this author inPubMed Google Scholar * C Holohan View
author publications You can also search for this author inPubMed Google Scholar * K M McLaughlin View author publications You can also search for this author inPubMed Google Scholar * J
Majkut View author publications You can also search for this author inPubMed Google Scholar * S Dolan View author publications You can also search for this author inPubMed Google Scholar * K
Redmond View author publications You can also search for this author inPubMed Google Scholar * J Riley View author publications You can also search for this author inPubMed Google Scholar *
K McLaughlin View author publications You can also search for this author inPubMed Google Scholar * I Stasik View author publications You can also search for this author inPubMed Google
Scholar * M Crudden View author publications You can also search for this author inPubMed Google Scholar * S Van Schaeybroeck View author publications You can also search for this author
inPubMed Google Scholar * C Fenning View author publications You can also search for this author inPubMed Google Scholar * R O'Connor View author publications You can also search for
this author inPubMed Google Scholar * P Kiely View author publications You can also search for this author inPubMed Google Scholar * M Sgobba View author publications You can also search for
this author inPubMed Google Scholar * D Haigh View author publications You can also search for this author inPubMed Google Scholar * P G Johnston View author publications You can also
search for this author inPubMed Google Scholar * D B Longley View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to D B
Longley. ETHICS DECLARATIONS COMPETING INTERESTS PGJ is a consultant for Merck. The other authors declare no conflict of interest. ADDITIONAL INFORMATION Edited by E Gottlieb Supplementary
Information accompanies the paper on Cell Death and Differentiation website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE S1-S6 (PPT 15373 KB) RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kerr, E., Holohan, C., McLaughlin, K. _et al._ Identification of an acetylation-dependant Ku70/FLIP complex that regulates FLIP expression
and HDAC inhibitor-induced apoptosis. _Cell Death Differ_ 19, 1317–1327 (2012). https://doi.org/10.1038/cdd.2012.8 Download citation * Received: 15 July 2011 * Revised: 21 December 2011 *
Accepted: 21 December 2011 * Published: 10 February 2012 * Issue Date: August 2012 * DOI: https://doi.org/10.1038/cdd.2012.8 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * Ku70 * FLIP * caspase 8 * HDAC inhibitors * colorectal cancer * apoptosis

![[withdrawn] forensic auditing: retailer voluntary commitment](https://assets.publishing.service.gov.uk/media/5ec780e6e90e0754d5088a59/s960_Slide11.jpg)

