- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND: Multiple early gastric cancers (EGCs) may develop in 6–14% of patients even after achieving curative endoscopic submucosal dissection (ESD); however, a useful biomarker
for predicting recurrence is not available. The present study investigated whether the expression of CD44 variant 9 (CD44v9), a functional cancer stem cell marker, in the primary gastric
cancer tissue represents an indicator of recurrence. METHODS: Eighty-eight patients who underwent ESD for EGC from 2008 to 2010 were enrolled and monitored for recurrence for 3 years. The
expression levels of CD44v9 in the tissue of initial EGCs were evaluated by immunohistochemistry, and the recurrence rate was compared between CD44v9-positive and CD44v9-negative groups. The
mucin phenotype and expression of microRNA-21 (_miR-21_) and programmed cell death protein 4 (PDCD4) were also analysed. RESULTS: The recurrence rate of EGC was significantly higher in the
CD44v9-positive group than in the CD44v9-negative group (hazard ratio (HR), 21.8; 95% confidence interval (CI), 5.71–83.1). However, mucin phenotypes and the expression of _miR-21_ and PDCD4
did not predict recurrence after ESD. Meanwhile, grade of gastric atrophy was also identified as a significant marker of multiple recurrence (HR, 4.95; 95% CI, 1.30–18.8). CONCLUSION: CD44
variant 9 expression represents a potential predictive marker for recurrence in EGC. SIMILAR CONTENT BEING VIEWED BY OTHERS THE UPREGULATION OF CIRCFNDC3B AGGRAVATES THE RECURRENCE AFTER
ENDOSCOPIC SUBMUCOSAL DISSECTION (ESD) IN EARLY GASTRIC CANCER (EGC) PATIENTS Article Open access 13 April 2022 LOSS OF CDX2 IN COLORECTAL CANCER IS ASSOCIATED WITH HISTOPATHOLOGIC SUBTYPES
AND MICROSATELLITE INSTABILITY BUT IS PROGNOSTICALLY INFERIOR TO HEMATOXYLIN–EOSIN-BASED MORPHOLOGIC PARAMETERS FROM THE WHO CLASSIFICATION Article Open access 06 October 2021 THE TUMOR
BIOLOGICAL SIGNIFICANCE OF RNF43 AND LRP1B IN GASTRIC CANCER IS COMPLEX AND CONTEXT-DEPENDENT Article Open access 23 February 2023 MAIN Endoscopic submucosal dissection (ESD) is a widely
used treatment for early gastric cancer (EGC) and is associated with good prognosis (Imaeda et al, 2006; Gotoda, 2007). However, multiple EGCs may develop even after achieving curative
resection, with previous reports showing that multiple primary gastric carcinomas developed in 6–14% of patients with gastric carcinoma (Nomura et al, 1991; Parsonnet et al, 1991; Huang et
al, 1998). Therefore, the optimal follow-up strategy to identify multiple recurrences of EGC after ESD is controversial because of the lack of a suitable biomarker for predicting recurrence.
_Helicobacter pylori_ (_H. pylori_) eradication has been found to reduce the recurrence rate of multiple EGC after ESD to one-third (Fukase et al, 2008), suggesting that concomitant _H.
pylori_ infection or inflammation potentially influences recurrence (Suzuki et al, 2009). In other words, one-third of the recurrent tumours cannot be prevented after _H. pylori_
eradication. In addition, because a consensus has not been reached regarding how often endoscopy follow-up should be performed after ESD, there is an important need for predictive markers to
identify patients with an increased risk of multiple recurrences of EGC. Recently, cancer stem cells (CSCs) that exhibit stem cell-like characteristics such as multilineage potential and
self-renewal potential have been identified in cancer tissue (Reya et al, 2001). Cancer stem cells are resistant to therapy because they have enhanced protection against reactive oxygen
species (ROS; Diehn et al, 2009), suggesting that a link exists between CSCs and tumour recurrence and metastasis. CD44, a major adhesion molecule for the extracellular matrix, is implicated
in a wide variety of physiological processes, including tumour cell invasion and metastasis (Nagano et al, 2004). Moreover, CD44 has been identified as one of the cell surface markers
associated with cancer stem-like cells in various solid tumours (Collins et al, 2005; Dalerba et al, 2007). We recently reported that CD44 variant 9 (CD44v9) interacts with and stabilises
xCT, a glutamate-cystine transporter, resulting in increased intracellular levels of reduced glutathione (GSH). CD44v9-positive cells demonstrate an enhanced ability to suppress the
production of ROS, resulting in subsequent therapeutic resistance, recurrence, and metastasis of tumours (Ishimoto et al, 2011; Tsugawa et al, 2012; Yae et al, 2012). These findings suggest
that CD44v9 has a specific function in the regulation of ROS defence and tumour growth. Therefore, if the primary EGC tissue is CD44v9 positive with the potential of maintaining such an
oxidative stress defense mechanism, multifocal recurrence may be more probable, even after curative resection is achieved by ESD. A previous report showed that mucin phenotype
subclassification is a predictive recurrence marker after surgical resection for advanced gastric cancer, defined as gastric cancer in which the depth of invasion extends beyond the
submucosa. Each tumour was phenotypically classified as an intestinal phenotype, a mixed phenotype, a gastric phenotype on the basis of the sum of their mucin phenotype immunohistochemistry
(IHC) scores for gastric markers (MUC5AC and MUC6) and intestinal markers (MUC2 and CD10; Tsukashita et al, 2001). The number of tumours classified as gastric phenotype was significantly
higher in tumours with peritoneal recurrence than in tumours without recurrence (Tajima et al, 2004). On the other hand, microRNAs (miRNAs) are small non-coding RNAs that can function as
endogenous silencers of target genes and have critical roles in human malignancies (Saito et al, 2006; Saito et al, 2009). MicroRNA-21 (_miR-21_) is an oncogenic miRNA that is overexpressed
in various human malignancies (Chan et al, 2005; Seike et al, 2009) and predominantly targets tumour-suppressor genes such as programmed cell death protein 4 (_PDCD4_; Fassan et al, 2011). A
previous report showed that _miR-21_ was significantly overexpressed in human gastric cancer tissues and cell lines (Zhang et al, 2008). In addition, the expression level of _miR-21_ was
associated with progression-related factors, such as depth of tumour invasion (Ueda et al, 2010). In addition, PDCD4 is strongly expressed in the nuclei of normal gastric mucosal cells,
whereas it is expressed at lower levels in the gastric adenocarcinoma (Kakimoto et al, 2011). Low PDCD4 expression was significantly correlated with poor clinical prognosis (Motoyama et al,
2010). These findings suggest that _miR-21_ and PDCD4 expression are biomarkers for gastric cancer recurrence (Wu et al, 2010). The aim of this prospective study was to identify a new
biomarker for predicting multiple recurrence of EGC after ESD. MATERIALS AND METHODS Additional details are included in the Supplementary Materials. PATIENTS AND SPECIMENS This prospective
study included patients who underwent ESD for EGC at the Keio University Hospital (Tokyo, Japan) between February 2008 and March 2010. Early gastric cancer was defined as an adenocarcinoma
confined to the mucosa or submucosa of the stomach. Patients who had a history of ESD and in whom multiple ESDs were performed were excluded. Tissue samples for RNA extraction were obtained
by endoscopic forceps biopsy. Following forceps biopsy, ESD was performed and resected specimens were obtained for IHC. The follow-up period was defined as the period from the time of
initial ESD to the time of the last oesophagogastroduodenoscopy (EGD). Follow-up EGDs were performed at ∼2, 6, 12, 18, 24, and 36 months after initial ESD (median follow-up period 32
months). Information on the clinical features of the patients such as gender, age, body mass index (BMI), _H. pylori_ infection status at the time of initial ESD, smoking history, tumour
location, tumour differentiation, and grade of gastric atrophy were obtained from medical records. _H. pylori_ infection status was determined using the 13C-urea breath test, serological
examination, and/or microaerobic bacterial cultivation. The grade of gastric atrophy was evaluated according to the endoscopic-atrophy-border scale described by Kimura and Takemoto (1969),
which correlates with the results of histological evaluation (Ito et al, 1996; Satoh et al, 1996). On the basis of this scale, the grade of atrophy was divided into two types:
closed-type/mildly extended atrophy and open-type/severely extended atrophy (Kimura and Takemoto, 1969; Ito et al, 1996; Satoh et al, 1996; Suzuki et al, 2004). The study protocol was
approved by the ethics committees of Keio University School of Medicine (Number 19-68-5), and written informed consent was obtained before subject enrolment. The UMIN Clinical Trials
Registry number for this study is UMIN000001057 (http://www.umin.ac.jp/ctr/). The study was performed in accordance with the principles of the declaration of Helsinki. IMMUNOHISTOCHEMISTRY
Following deparaffinisation and rehydration, antigens for anti-CD44v9 and anti-phospho-p38MAPK antibodies were retrieved by heating samples in citrate buffer (10 mM, pH 6.0) for 10 min at
105 °C. Antigens for anti-MUC2, anti-MUC5AC, and anti-MUC6 antibodies were retrieved by heating samples in citrate buffer (10 mM, pH 6.0) for 5 min at 121 °C. Antigens for anti-CD10
antibodies were retrieved by heating samples in citrate buffer (10 mM, pH 9.0) for 5 min at 121 °C. Antigens for anti-PDCD4 antibodies were retrieved by heating samples in citrate buffer (10
mM, pH 9.0) for 10 min at 121 °C. After antigen retrieval, endogenous nonspecific peroxidases were quenched with 0.3% hydrogen peroxide for 5 min. Nonspecific binding was blocked using
Protein block Serum-free (DAKO Japan, Kyoto, Japan), and each section was incubated overnight at 4 °C with the primary antibodies. CD44 variant 9 was detected with a rat monoclonal antibody
(1 : 200; Ishimoto et al, 2011). Phospho-p38MAPK was detected using a rabbit monoclonal antibody (Number 9211, Cell Signaling Technology, Beverly, MA, USA; 1 : 100). Monoclonal mouse
antibodies were used to detect MUC2 (NCL-MUC-2, Novocastra Laboratories, Newcastle-upon-Tyne, UK; 1 : 100), MUC5AC (NCL-MUC-5AC, Novocastra Laboratories; 1 : 50), MUC6 (NCL-MUC-6, Novocastra
Laboratories; 1 : 50), and CD10 (NCL-CD10-270, Novocastra Laboratories; 1 : 50). Programmed cell death protein 4 was detected using a rabbit monoclonal antibody (600-401-965, Rockland,
Gilbertsville, PA, USA; 1 : 100). Primary antibody binding was detected using a Vectastain Elite Kit (Vector Laboratories, Burlingame, CA, USA) and 3,3′-diaminobenzidine tetrahydrochloride
solution (DAKO Japan). Counterstaining was performed using Gill’s haematoxylin (DAKO Japan). The CD44v9 IHC score was defined as the proportion of the tumour area that showed CD44v9-positive
staining. The CD44v9 IHC score was calculated by using ImageJ software (US National Institutes of Health, Bethesda, MD, USA) and setting the threshold (0–120) after the red-green-blue
stack. The PDCD4 IHC score was defined as the average of the percentage of positive nuclei in each of three high-power fields of areas containing tumour cells. STATISTICAL ANALYSIS Clinical
factors were compared between the recurrence group and the non-recurrence group using the Student’s _t_-test for continuous variables (i.e., age, BMI, serum CEA, serum CA19-9, and follow-up
period) and the _χ_2-test for discrete variables (i.e., gender, _H. pylori_ infection status, smoking history, tumour location, tumour differentiation, and the type of gastric atrophy). The
optimal cut-off value for CD44v9 IHC scores, _miR-21_ expression levels, and PDCD4 IHC scores were determined using the receiver operating characteristic curve. Clinical factors were
compared between the CD44v9-positive and CD44v9-negative groups, _miR-21_-higher and _miR-21_-lower groups, and PDCD4-higher and PDCD4-lower groups using the Student’s _t_-test (continuous
variables) or the _χ_2-test (discrete variables). The differences in clinical factors among mucin phenotype subclassifications were analysed using one-way analysis of variance (ANOVA) for
continuous variables and the _χ_2-test for discrete variables. The Student’s _t_-test, _χ_2-test, and one-way ANOVA were applied after normality had been established using the Shapiro–Wilk
_W_-test. The associations of CD44v9 and the type of gastric atrophy with _miR-21_ and PDCD4 were evaluated using a linear regression model. Recurrence-free curves were calculated using the
Kaplan–Meier method and were compared using the log rank test. Univariate and multivariate Cox proportional hazard model analyses were performed to calculate hazard ratios (HRs). Statistical
significance was defined as a _P_-value of <0.05. The data are expressed as mean±s.d.. All statistical analyses were performed using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA).
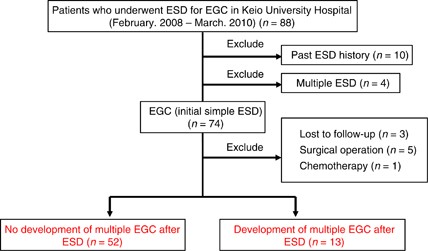
RESULTS PATIENT CHARACTERISTICS A total of 88 patients were enrolled (Figure 1). Samples from 10 patients who had a history of ESD and from 4 patients in whom multiple ESDs were performed
were excluded. Among the 74 initial simple EGC patients, 3 who had never undergone endoscopic examination after initial ESD, 5 who underwent gastrectomy after ESD because of a positive
margin, and 1 who was treated with chemotherapy for oesophageal cancer that developed after ESD were also excluded. Thirteen of the 65 remaining patients developed multiple recurrences
during the follow-up period, whereas recurrence was not identified in 52 patients. Clinically relevant background factors in the non-recurrence and recurrence group are shown in Table 1. A
significant difference in the grade of gastric atrophy was identified between these groups (_P_=0.046). EXPRESSION OF CD44V9 IN EGCS The CD44v9 IHC scores of all 65 EGCs are illustrated in
Figure 2. The average CD44v IHC score was 3.58±7.74%. Interestingly, the case that showed an extraordinarily high score (60.3%) was the only case that showed recurrence twice during the
follow-up period (22 and 40 months after the initial ESD). Subsequently, patients were allocated into the CD44v9-positive group (_n_=13) or the CD44v9-negative group (_n_=52) based on a
cut-off level of 3.55% (sensitivity, 76.9%; specificity, 93.8%, Supplementary Table 1). Representative CD44v9 immunohistochemical staining is shown in Figure 3. Although CD44v9 staining was
barely detectable in the CD44v9-negative group (Figure 3A), CD44v9 was expressed in the epithelium of tumour glands with a heterogeneous expression pattern in the CD44v9-positive group
(Figure 3B). In the high-power field, CD44v9 expression was identified along the cell membrane (Figure 3C). Significant correlations were not identified between CD44v9 positivity and any of
the clinical background factors (Supplementary Table 2). We examined the activation of p38MAPK, a major target of ROS, in cancer tissues to assess the role of CD44v9 in the regulation of
intracellular oxidative stress. Immunostains for CD44v9 and phospho-p38MAPK, the active form of p38MAPK, are shown in Supplementary Figures 1A and B, respectively. Phospho-p38MAPK staining
was barely detectable in the CD44v9-positive area but was apparent in the CD44v9-negative area. The immunostaining patterns of CD44v9 and phospho-p38MAPK were inversely correlated in the
tumourous glands of EGC, indicating that CD44v9 expression reduces oxidative stress in cancer cells. EXPRESSION OF EPITHELIAL MUCINS IN EGCS All 65 EGCs were classified into three mucin
phenotypes based on immunohistochemical staining against CD10, MUC2, MUC5AC, and MUC6 (Supplementary Figure 2): the gastric phenotype, 17 (26.2%) patients; the mixed phenotype, 23 (35.4%)
patients; and the intestinal phenotype, 25 (38.5%) patients. A significant association between mucin phenotypes and EGC location (_P_=0.022) was identified (Supplementary Table 2).
EXPRESSION OF MIR-21 AND PDCD4 IN EGCS The average _miR-21_ expression and PDCD4 IHC scores were 5.70%±5.45% and 20.2%±11.4%, respectively. Sufficient quantities of RNA could not be obtained
for four samples, and these were excluded from the _miR-21_ evaluation. Patients were allocated into the _miR-21_-lower group (_n_=27) or the _miR-21_-higher group (_n_=34) based on a
cut-off level of 3.58 (sensitivity, 53.8%; specificity, 43.8%), and the PDCD4-lower group (_n_=27) and the PDCD4-higher group (_n_=38) based on a cut-off level of 17.7% (sensitivity, 53.8%;
specificity, 43.8%; Supplementary Table 1). Significant correlations were not identified between the expression of _miR-21_ and clinical factors, but age was significantly associated with
PDCD4 expression (Supplementary Table 2). In addition, significant associations were not identified between CD44v9 expression or gastric atrophy grade and _miR-21_ or PDCD4 (CD44v9-_miR-21_:
_R_=0.162, _P_=0.236; CD44v9-PDCD4: _R_=0.035, _P_=0.791; atrophy-_miR-21_: _R_=0.136, _P_=0.297; atrophy-PDCD4: _R_=0.103, _P_=0.413). CD44V9 EXPRESSION IS A POTENTIAL MARKER FOR THE
DEVELOPMENT OF MULTIPLE CANCERS Kaplan–Meier curves for the development of EGC after ESD are shown in Figure 4. During the follow-up period, the development of EGC after ESD was identified
in 10 cases (76.9%) in the CD44v9-positive group and in 3 cases (5.8%) in the CD44v9-negative group. When the recurrence rate was analysed using the log rank test, the recurrence rate was
significantly higher in the CD44v9-positive group than in the CD44v9-negative group (_P_<0.001, Figure 4A). On the other hand, the recurrence rate was not significantly different when
patients were divided based on the subclassification of tumours into mucin phenotypes (_P_=0.94, Figure 4B), _miR-21_ expression (_P_=0.71, Figure 4C), or PDCD4 expression (_P_=0.67, Figure
4D). Moreover, the recurrence rate was significantly higher for open-type gastric atrophy than that for closed-type gastric atrophy (_P_=0.013, Figure 4E). The HRs are shown in Table 2. CD44
variant 9 positivity significantly correlated with multiple recurrences on using the univariate Cox proportional hazards model (_P_<0.001; HR, 19.7; 95% confidence interval (CI),
5.37–72.4) and the multivariate Cox proportional hazards model (_P_<0.001; HR, 21.8; 95% CI, 5.71–83.1). The type of gastric atrophy also significantly correlated with multiple
recurrences using the univariate Cox proportional hazards model (_P_=0.022; HR, 4.17; 95% CI, 1.23–14.1) and the multivariate Cox proportional hazards model (_P_=0.019; HR, 4.95; 95% CI,
1.30–18.8). However, no significant correlations were identified with regard to mucin phenotypes, _miR-21_ expression, or PDCD4 expression using the univariate Cox proportional hazard model.
DISCUSSION This study represents the first report that CD44v9 expression, a marker characteristic of cancer stem-like cells, in primary EGCs can clearly predict multiple recurrence of EGC
after ESD. Evaluation of CD44v9 expression represents a clinically ideal system, because it can be performed non-invasively on resected specimens when monitoring the clinical course after
endoscopic treatment for gastric cancer. The HR for multiple recurrences was 21.8 in patients with CD44v9-positive tumours in our study, suggesting that the follow-up after ESD should be
performed more frequently in patients with CD44v9-positive tumours than in patients with CD44v9-negative tumours. The present results support prophylactic CD44v9 monitoring following ESD
that would ultimately lead to reduced medical expenditure and mortality associated with EGC recurrence. Previously, we showed that CD44v9 expression maintains a high level of GSH, which
resulted in the suppression of ROS-p38MAPK signaling and subsequent resistance to intracellular ROS in gastric cancer (Ishimoto et al, 2011). In the present study, as well as in our previous
study, a reduced level of phospho-p38MAPK was identified in CD44v9-positive cells in EGC. Moreover, we have shown that CD44v9 expression was associated with the lung metastasis in the mouse
breast cancer cells (Yae et al, 2012). Furthermore, Chen et al. (2013) recently reported the existence of CD44+ cells within the tumours of gastric cancer patients that are endowed with
stem cells properties (Chen et al, 2013). These findings suggest that CD44v9-positive cells have CSC-like potential, and tumours with high CD44v9 levels have an increased risk of multiple
recurrences. On the other hand, CD44+ cells were detectable in human primary gastric cancer, although they did not express stem cell-like properties or exhibit tumour-initiating properties
in xenograft transplantation experiments (Rocco et al, 2012). However, the CD44v9 cell surface marker has the potential to identify CSCs in primary gastric cancer, whereas the general CD44
cell surface marker does not always identify gastric CSCs. It is also possible that CD44v9-positive cells may not necessarily represent true CSCs. In 1953, Slaughter _et al._ introduced the
concept of the field effect in cancer (also known as field defect or field cancerisation). The field effect of cancer refers to histologically abnormal epithelium adjacent to tumour tissue
within the aerodigestive region and was proposed to explain the occurrence of multiple primary tumours and locally recurrent cancer out of a ‘neoplastic tendency involving many cells at
once’ (Slaughter et al, 1953). It is possible that the background gastric mucosa of CD44v9-positive cancer has a neoplastic tendency, as has been observed in the case of multiple
hepatocellular carcinoma development from hepatic cirrhosis caused by hepatitis virus infection. A few studies have classified multiple recurrence as synchronous (<12 months) and
metachronous (>12 months; Nasu et al, 2005; Nakajima et al, 2006). However, it would not be appropriate to simply classify gastric cancer according to the time-to-recurrence in the
present study because of the field cancerisation concept. In present study, EGC was completely resected in all patients included in the study, and we excluded patients whose endoscopic
resected specimens had positive margins (cases of incomplete resection). According to the criteria used in the studies by Nakajima et al. (2006) and Nasu et al. (2005), nine patients
developed synchronous recurrence and four patients developed metachronous cancer in the present study. The development of metachronous multiple cancers was identified in 1 of 45 cases (2.2%)
in the CD44v9-negative group and in 3 of 6 cases (50.0%) in the CD44v9-positive group (_P_<0.001; Supplementary Figure 3). In a previous report, the severity of gastric atrophy was shown
to be a predictive factor for multiple gastric cancer (Shiotani et al, 2008; Hanaoka et al, 2010). The present study showed that the recurrence rate was higher in CD44v9-positive tumours
than in CD44v9-negative tumours, even when applying the multivariate Cox proportional hazard model adjusted for the type of gastric atrophy. Therefore, CD44v9 expression is an independent
predictive factor, irrespective of the severity of gastric atrophy. In addition, the recurrence rate was significantly higher for samples with open-type gastric atrophy than for samples with
closed-type gastric atrophy, and a significant difference was identified in the multivariate Cox proportional hazard model adjusted for CD44v9 positivity. The HR for CD44v9 positivity was
greater than that for the type of gastric atrophy, suggesting that CD44v9 expression may represent a more powerful predictive recurrence marker than the type of gastric atrophy. Although _H.
pylori_ infection clearly delineated EGC development when _H. pylori_-naive-negative and _H. pylori_-positive patients were compared (Hanaoka et al, 2010), _H. pylori_ eradication only
reduced the risk of EGC recurrence to one-third (Fukase et al, 2008). In the present study, CD44v9 positivity could predict EGC recurrence in a smaller population than was required for _H.
pylori_ infection status to predict recurrence. With regard to the infection status of _H. pylori_ at the initial ESD in all of the present cases (Supplementary Figure 4), 32 of 52 patients
in the CD44v9-negative group and 7 of 13 patients in the CD44v9-positive group were _H. pylori_ positive (Supplementary Table 2). After ESD, the success of _H. pylori_ eradication therapy
was confirmed in 21 of 32 (65.6%) patients in the CD44v9-negative group (5.6±6.1 months after ESD) and in all 7 patients (100%) in the CD44v9-positive group (6.1±3.0 months after ESD).
Therefore, _H. pylori_ positivity as a confounding factor could only have affected the CD44v9-negative group, suggesting that the present results were reliable even after accounting for the
_H. pylori_-associated effects. Thus, CD44v9 positivity can predict the development of multiple EGC even after accounting for the presence or absence of _H. pylori_ infection status.
Although the incidence of gastric mucin-producing tumours was significantly higher in cases of tumours with recurrence than in cases of tumours without recurrence of advanced gastric cancer
(Tajima et al, 2004), a correlation between the subclassification of mucin phenotypes and the intragastric recurrence rate was not found in the present study. Furthermore, _miR-21_ and PDCD4
expression did not predict the risk of developing multiple EGCs. A limitation of this study is that the cut-off values for CD44v9 and the other biomarkers were determined on the basis of
their optimal performance in this specific population of 65 patients. Risk prediction tools are known to suffer from optimism bias, and their performance in new populations does not always
match the success of that in the derivation population. Therefore, prospective validation of these biomarkers using separate patient populations is required. In conclusion, the data
presented in our study suggest that the recurrence rate of EGC following ESD is higher in cases of CD44v9-positive tumours than in cases of CD44v9-negative tumours. CD44 variant 9 expression
in primary EGC represents a useful biomarker for predicting patients’ potential risk of developing multiple EGCs after curative resection with ESD. CHANGE HISTORY * _ 23 JULY 2013 This
paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Chan JA, Krichevsky AM, Kosik KS (2005) MicroRNA-21
is an antiapoptotic factor in human glioblastoma cells. _Cancer Res_ 65 (14): 6029–6033. Article CAS PubMed Google Scholar * Chen W, Zhang X, Chu C, Cheung WL, Ng L, Lam S, Chow A, Lau
T, Chen M, Li Y, Nie Y, Wong BC, Pang R (2013) Identification of CD44+ cancer stem cells in human gastric cancer. _Hepatogastroenterology_ 60 (127): ): DOI:10.5754/hge12881. * Collins AT,
Berry PA, Hyde C, Stower MJ, Maitland NJ (2005) Prospective identification of tumorigenic prostate cancer stem cells. _Cancer Res_ 65 (23): 10946–10951. Article CAS PubMed Google Scholar
* Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF (2007) Phenotypic characterization of human
colorectal cancer stem cells. _Proc Natl Acad Sci USA_ 104 (24): 10158–10163. Article CAS PubMed PubMed Central Google Scholar * Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN,
Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF (2009) Association of
reactive oxygen species levels and radioresistance in cancer stem cells. _Nature_ 458 (7239): 780–783. Article CAS PubMed PubMed Central Google Scholar * Fassan M, Pizzi M, Giacomelli
L, Mescoli C, Ludwig K, Pucciarelli S, Rugge M (2011) PDCD4 nuclear loss inversely correlates with _miR-21_ levels in colon carcinogenesis. _Virchows Arch_ 458 (4): 413–419. Article CAS
PubMed Google Scholar * Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M (2008) Effect of eradication of _Helicobacter pylori_ on incidence
of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. _Lancet_ 372 (9636): 392–397. Article PubMed Google
Scholar * Gotoda T (2007) Endoscopic resection of early gastric cancer. _Gastric Cancer_ 10 (1): 1–11. Article PubMed Google Scholar * Hanaoka N, Uedo N, Shiotani A, Inoue T, Takeuchi Y,
Higashino K, Ishihara R, Iishi H, Haruma K, Tatsuta M (2010) Autofluorescence imaging for predicting development of metachronous gastric cancer after _Helicobacter pylori_ eradication. _J
Gastroenterol Hepatol_ 25 (12): 1844–1849. Article PubMed Google Scholar * Huang JQ, Sridhar S, Chen Y, Hunt RH (1998) Meta-analysis of the relationship between _Helicobacter pylori_
seropositivity and gastric cancer. _Gastroenterology_ 114 (6): 1169–1179. Article CAS PubMed Google Scholar * Imaeda H, Iwao Y, Ogata H, Ichikawa H, Mori M, Hosoe N, Masaoka T, Nakashita
M, Suzuki H, Inoue N, Aiura K, Nagata H, Kumai K, Hibi T (2006) A new technique for endoscopic submucosal dissection for early gastric cancer using an external grasping forceps. _Endoscopy_
38 (10): 1007–1010. Article CAS PubMed Google Scholar * Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K,
Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H (2011) CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc- and thereby
promotes tumor growth. _Cancer Cell_ 19 (3): 387–400. Article CAS PubMed Google Scholar * Ito S, Azuma T, Murakita H, Hirai M, Miyaji H, Ito Y, Ohtaki Y, Yamazaki Y, Kuriyama M, Keida Y,
Kohli Y (1996) Profile of _Helicobacter pylori_ cytotoxin derived from two areas of Japan with different prevalence of atrophic gastritis. _Gut_ 39 (6): 800–806. Article CAS PubMed
PubMed Central Google Scholar * Kakimoto T, Shiraishi R, Iwakiri R, Fujimoto K, Takahashi H, Hamajima H, Mizuta T, Ideguchi H, Toda S, Kitajima Y, Ozaki I, Matsuhashi S (2011) Expression
patterns of the tumor suppressor PDCD4 and correlation with beta-catenin expression in gastric cancers. _Oncol Rep_ 26 (6): 1385–1392. CAS PubMed Google Scholar * Kimura K, Takemoto T
(1969) An endoscopic recognition of the atrophic border and its significance in chronic gastritis. _Endoscopy_ 1 (3): 87–97. Article Google Scholar * Motoyama K, Inoue H, Mimori K, Tanaka
F, Kojima K, Uetake H, Sugihara K, Mori M (2010) Clinicopathological and prognostic significance of PDCD4 and microRNA-21 in human gastric cancer. _Int J Oncol_ 36 (5): 1089–1095. CAS
PubMed Google Scholar * Nagano O, Murakami D, Hartmann D, De Strooper B, Saftig P, Iwatsubo T, Nakajima M, Shinohara M, Saya H (2004) Cell-matrix interaction via CD44 is independently
regulated by different metalloproteinases activated in response to extracellular Ca2+ influx and PKC activation. _J Cell Biol_ 165 (6): 893–902. Article CAS PubMed PubMed Central Google
Scholar * Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, Saito D (2006) Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic
surveillance? _Gastric cancer_ 9 (2): 93–98. Article PubMed Google Scholar * Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I (2005) Characteristics of metachronous multiple early
gastric cancers after endoscopic mucosal resection. _Endoscopy_ 37 (10): 990–993. Article CAS PubMed Google Scholar * Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser
MJ (1991) _Helicobacter pylori_ infection and gastric carcinoma among Japanese Americans in Hawaii. _N Engl J Med_ 325 (16): 1132–1136. Article CAS PubMed Google Scholar * Parsonnet J,
Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK (1991) _Helicobacter pylori_ infection and the risk of gastric carcinoma. _N Engl J Med_ 325 (16): 1127–1131.
Article CAS PubMed Google Scholar * Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. _Nature_ 414 (6859): 105–111. Article CAS PubMed
Google Scholar * Rocco A, Liguori E, Pirozzi G, Tirino V, Compare D, Franco R, Tatangelo F, Palaia R, D'Armiento FP, Pollastrone G, Affuso A, Bottazzi EC, Masone S, Persico G, Nardone
G (2012) CD133 and CD44 cell surface markers do not identify cancer stem cells in primary human gastric tumors. _J Cell Physiol_ 227 (6): 2686–2693. Article CAS PubMed Google Scholar *
Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, Jones PA (2006) Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs
in human cancer cells. _Cancer Cell_ 9 (6): 435–443. Article CAS PubMed Google Scholar * Saito Y, Suzuki H, Tsugawa H, Nakagawa I, Matsuzaki J, Kanai Y, Hibi T (2009) Chromatin
remodeling at Alu repeats by epigenetic treatment activates silenced microRNA-512-5p with downregulation of Mcl-1 in human gastric cancer cells. _Oncogene_ 28 (30): 2738–2744. Article CAS
PubMed Google Scholar * Satoh K, Kimura K, Taniguchi Y, Yoshida Y, Kihira K, Takimoto T, Kawata H, Saifuku K, Ido K, Takemoto T, Ota Y, Tada M, Karita M, Sakaki N, Hoshihara Y (1996)
Distribution of inflammation and atrophy in the stomach of _Helicobacter pylori_-positive and -negative patients with chronic gastritis. _Am J Gastroenterol_ 91 (5): 963–969. CAS PubMed
Google Scholar * Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, Gemma A, Kudoh S, Croce CM, Harris CC (2009) MiR-21 is an EGFR-regulated
anti-apoptotic factor in lung cancer in never-smokers. _Proc Natl Acad Sci USA_ 106 (29): 12085–12090. Article CAS PubMed PubMed Central Google Scholar * Shiotani A, Uedo N, Iishi H,
Yoshiyuki Y, Ishii M, Manabe N, Kamada T, Kusunoki H, Hata J, Haruma K (2008) Predictive factors for metachronous gastric cancer in high-risk patients after successful _Helicobacter pylori_
eradication. _Digestion_ 78 (2–3): 113–119. Article PubMed Google Scholar * Slaughter DP, Southwick HW, Smejkal W (1953) Field cancerization in oral stratified squamous epithelium;
clinical implications of multicentric origin. _Cancer_ 6 (5): 963–968. Article CAS PubMed Google Scholar * Suzuki H, Masaoka T, Hosoda H, Nomura S, Ohara T, Kangawa K, Ishii H, Hibi T
(2004) Plasma ghrelin concentration correlates with the levels of serum pepsinogen I and pepsinogen I/II ratio: a possible novel and non-invasive marker for gastric atrophy.
_Hepatogastroenterology_ 51 (59): 1249–1254. CAS PubMed Google Scholar * Suzuki H, Iwasaki E, Hibi T (2009) _Helicobacter pylori_ and gastric cancer. _Gastric Cancer_ 12 (2): 79–87.
Article PubMed Google Scholar * Tajima Y, Yamazaki K, Nishino N, Morohara K, Yamazaki T, Kaetsu T, Suzuki S, Kawamura M, Kumagai K, Kusano M (2004) Gastric and intestinal phenotypic
marker expression in gastric carcinomas and recurrence pattern after surgery-immunohistochemical analysis of 213 lesions. _Br J Cancer_ 91 (7): 1342–1348. Article CAS PubMed PubMed
Central Google Scholar * Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, Nagano O, Matsuzaki J, Hibi T (2012) Reactive oxygen species-induced autophagic degradation of
_Helicobacter pylori_ CagA is specifically suppressed in cancer stem-like cells. _Cell Host Microbe_ 12 (6): 764–777. Article CAS PubMed Google Scholar * Tsukashita S, Kushima R, Bamba
M, Sugihara H, Hattori T (2001) MUC gene expression and histogenesis of adenocarcinoma of the stomach. _Int J Cancer_ 94 (2): 166–170. Article CAS PubMed Google Scholar * Ueda T, Volinia
S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, Yoshida K, Sasaki H, Nomura S, Seto Y, Kaminishi M, Calin GA, Croce CM (2010) Relation between microRNA
expression and progression and prognosis of gastric cancer: a microRNA expression analysis. _Lancet Oncol_ 11 (2): 136–146. Article CAS PubMed Google Scholar * Wu WK, Lee CW, Cho CH, Fan
D, Wu K, Yu J, Sung JJ (2010) MicroRNA dysregulation in gastric cancer: a new player enters the game. _Oncogene_ 29 (43): 5761–5771. Article CAS PubMed Google Scholar * Yae T,
Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ, Wada T, Masuko T, Mogushi K, Tanaka H, Osawa T, Kanki Y, Minami T, Aburatani H, Ohmura M, Kubo A, Suematsu M, Takahashi K,
Saya H, Nagano O (2012) Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. _Nat Commun_ 3: 883. Article PubMed Google Scholar * Zhang Z, Li
Z, Gao C, Chen P, Chen J, Liu W, Xiao S, Lu H (2008) miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. _Lab Invest_ 88 (12): 1358–1366. Article CAS PubMed Google
Scholar Download references ACKNOWLEDGEMENTS This study was supported by a Grant-in-Aid for Young Scientists (B) (40570932 to KH) and a Grant-in-Aid for Scientific Research (B) (22300169,
to HSu) from the Japan Society for the Promotion of Science (JSPS), a grant from the Smoking Research Foundation (to HSu), and the Keio Gijuku Academic Development Fund (to HSu). AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, 35 Shinanomachi, Tokyo, 160-8582,
Japan K Hirata, H Suzuki, J Matsuzaki, H Tsugawa & T Hibi * Department of General Internal Medicine, Saitama Medical University, 38 KeroHongo, 350-0495, Saitama, Japan H Imaeda *
Division of Gene Regulation, Institute for Advanced Medical Research, Keio University School of Medicine, 35 Shinanomachi, Tokyo, 160-8582, Japan O Nagano & H Saya * Interfaculty
Initiative in Information Studies, The University of Tokyo, 7-3-1 Hongo, Tokyo, 113-0033, Japan K Asakura Authors * K Hirata View author publications You can also search for this author
inPubMed Google Scholar * H Suzuki View author publications You can also search for this author inPubMed Google Scholar * H Imaeda View author publications You can also search for this
author inPubMed Google Scholar * J Matsuzaki View author publications You can also search for this author inPubMed Google Scholar * H Tsugawa View author publications You can also search for
this author inPubMed Google Scholar * O Nagano View author publications You can also search for this author inPubMed Google Scholar * K Asakura View author publications You can also search
for this author inPubMed Google Scholar * H Saya View author publications You can also search for this author inPubMed Google Scholar * T Hibi View author publications You can also search
for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to H Suzuki. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL
INFORMATION This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative
Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. Supplementary Information accompanies this paper on British Journal of Cancer website SUPPLEMENTARY INFORMATION
SUPPLEMENTARY FIGURES (PPT 2840 KB) SUPPLEMENTARY FIGURE LEGENDS (DOC 24 KB) SUPPLEMENTARY TABLES (DOC 89 KB) SUPPLEMENTARY TABLE LEGENDS (DOC 26 KB) SUPPLEMENTARY METHODS (DOC 27 KB) RIGHTS
AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a
copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hirata, K., Suzuki, H., Imaeda, H. _et al._ CD44
variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. _Br J Cancer_ 109, 379–386 (2013). https://doi.org/10.1038/bjc.2013.314 Download citation *
Received: 07 May 2013 * Accepted: 29 May 2013 * Published: 18 June 2013 * Issue Date: 23 July 2013 * DOI: https://doi.org/10.1038/bjc.2013.314 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * gastric cancer * CD44 variant 9 * cancer stem cell * recurrence * biomarker * endoscopic procedure







:max_bytes(150000):strip_icc():focal(691x396:693x398)/Olivia-Munn-070123-split-e2bf6c2d8773448a81f93b6e3bb78626.jpg)
