- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND: Radiation-related heart disease and lung cancer can occur following radiotherapy for breast cancer but the duration of any mortality risk is uncertain. METHODS:
Mortality ratios, by laterality of breast cancer, were estimated using Poisson regression for 558 871 women recorded with breast cancer during 1973–2008 in the Surveillance, Epidemiology and
End Results (SEER) cancer registries and followed until 01 January 2009. RESULTS: For women diagnosed with breast cancer during 1973–1982 and given radiotherapy shortly afterwards, the
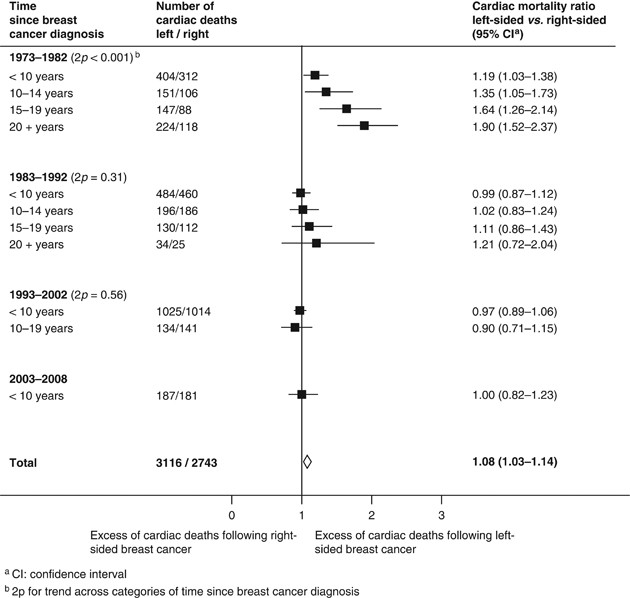
cardiac mortality ratios, left-sided _vs_ right-sided, were 1.19 (1.03–1.38), 1.35 (1.05–1.73), 1.64 (1.26–2.14) and 1.90 (1.52–2.37) at <10, 10–14, 15–19 and 20+ years since diagnosis
(2_p_ for trend: <0.001). The lung cancer mortality ratios, ipsilateral _vs_ contralateral, in these women were 1.05 (0.57–1.94), 2.04 (1.28–3.23) and 3.87 (2.19–6.82) at <10, 10–19
and 20+ years, respectively, (2_p_ for trend: 0.002). For women irradiated during 1983–92 there was evidence of radiation-related mortality for lung cancer, but not for heart disease. For
women irradiated since 1993 there is, as yet, little evidence of any radiation-related mortality. CONCLUSION: In this population, the radiation-related risks were larger in the third decade
after exposure than during the first two decades. SIMILAR CONTENT BEING VIEWED BY OTHERS HEART-RELATED MORTALITY AFTER POSTOPERATIVE BREAST IRRADIATION IN PATIENTS WITH DUCTAL CARCINOMA IN
SITU IN THE CONTEMPORARY RADIOTHERAPY ERA Article Open access 02 February 2021 RISK OF PRIMARY LUNG CANCER AFTER ADJUVANT RADIOTHERAPY IN BREAST CANCER—A LARGE POPULATION-BASED STUDY Article
Open access 01 June 2021 CONSEQUENCES OF IONIZING RADIATION EXPOSURE TO THE CARDIOVASCULAR SYSTEM Article 10 July 2024 MAIN In early breast cancer, radiotherapy can reduce the risk of death
from breast cancer itself (Early Breast Cancer Trialists' Collaborative Group (2011)). However, the treatment usually involves some incidental irradiation of the heart and lungs which
may increase the subsequent risks of heart disease and lung cancer. Mortality among women registered with breast cancer in the Surveillance, Epidemiology and End Results (SEER) cancer
registries during 1973–2001 has previously been reported, giving insight into radiation-related mortality during the first two decades after exposure (Darby et al, 2005). We now present
mortality up to 01 January 2009 providing, for the first time, information on the risks of death from radiation-related heart disease and lung cancer in this population during the third
decade after exposure. MATERIALS AND METHODS Information on cause-specific mortality in women registered with invasive breast cancer (localised or regional) during 1973–2008 was obtained
from the SEER public-use data set (Surveillance, Epidemiology and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2008), National Cancer Institute, DCCPS, Surveillance
Research Program, Cancer Statistics Branch (released January 2011)). Women aged 20–79 years at breast cancer diagnosis were included unless they had previously been registered with a
different cancer, their cancer was bilateral, of unknown laterality, metastatic, diagnosed at autopsy, or ascertained only from the death certificate. All deaths certified as due to heart
disease (ICD-10 I00–09, I11, I13, I20–51) were included in the analysis. Deaths from metastatic breast cancer can sometimes be registered as lung cancer. Therefore, deaths certified as due
to lung cancer (ICD-10 C34) were included only if microscopically confirmed lung cancer was recorded in the SEER data set. Women with breast cancer who are selected for radiotherapy may have
different mortality rates from those who are not selected for reasons unrelated to radiotherapy (McGale and Darby, 2008). Comparisons that avoid these selection effects can, however, be
performed, as the radiation dose to the heart is generally greater in left-sided than in right-sided breast cancer (Taylor et al, 2007). Therefore, a comparison of cardiac mortality in
irradiated women with left-sided cancer _vs_ irradiated women with right-sided cancer can give a valid indication of the extent of any radiation-related mortality (Vandenbroucke, 2004).
Similarly, the radiation dose to the ipsilateral lung from breast cancer radiotherapy is generally greater than to the contralateral lung. Thus a comparison of mortality from ipsilateral
lung cancer compared with contralateral lung cancer among irradiated women can be used to assess the presence of any radiation-related increase in lung cancer mortality. Each woman’s
contribution to the person-years at risk ran from the date of breast cancer diagnosis to the earliest of 1st January 2009, death, loss to follow-up, or 85th birthday. Mortality ratios were
estimated using Poisson regression with stratification by calendar year of diagnosis, time since diagnosis, age (all in 5-year groups), and race (white, black, other/unknown).
Over-dispersion tests were performed, but no adjustments were needed. Tests for trend were performed using variance-weighted least squares assuming Poisson variation. Significance tests were
two-sided. Calculations used Stata version 12. RESULTS A total of 558 871 women were included in the analysis. Similar proportions of women with left-sided and right-sided breast cancer
were recorded as receiving radiotherapy (left-sided: 45.8% (130 285 women), right-sided: 46.2% (126 691 women) (Table 1)). For women not recorded as receiving radiotherapy, there was little
evidence that mortality from heart disease differed in women with left-sided and right-sided cancer (cardiac mortality ratio, left-sided _vs_ right-sided: 1.02, 95% confidence interval (CI)
0.99–1.06, 2_p_=0.16). However, for women recorded as receiving radiotherapy, there was an excess of cardiac deaths following left-sided cancer (cardiac mortality ratio, left-sided _vs_
right-sided: 1.08, 95% CI 1.03–1.14, 2_p_=0.002). Considering only women recorded as receiving radiotherapy during 1973–1982, the cardiac mortality ratio, left-sided _vs_ right-sided, was
raised during the first decade after cancer diagnosis (1.19, 95% CI 1.03–1.38) and subsequently increased further, taking values 1.35 (1.05–1.73), 1.64 (1.26–2.14), and 1.90 (1.52–2.37),
respectively, in periods 10–14, 15–19 and 20+ years since breast cancer diagnosis (2_p_ for trend<0.001, see Figure 1). For women receiving radiotherapy after 1982, there was little
evidence of any radiation-related increase in heart disease mortality either during the first decade after breast cancer diagnosis or later, although follow-up is, as yet, incomplete. For
women not recorded as receiving radiotherapy, the lung cancer mortality ratio, ipsilateral _vs_ contralateral, was 0.97 (95% CI 0.89–1.06, 2_p_=0.50), while for irradiated women it was 1.30
(95% CI 1.16–1.45, 2_p_<0.001). For women diagnosed with breast cancer during 1973–1982 and irradiated, the lung cancer mortality ratio, ipsilateral _vs_ contralateral, increased with
time since breast cancer diagnosis, taking values 1.05 (95% CI 0.57–1.94), 2.04 (1.28–3.23), and 3.87 (2.19–6.82) in the first, second, and third decades since breast cancer diagnosis (2_p_
for trend=0.002, see Figure 2). For women diagnosed during 1983–92 and irradiated, the lung cancer mortality ratio, ipsilateral vs contralateral, also showed a substantial increase more than
20 years after exposure (3.87, CI 1.24–11.31, data not shown). DISCUSSION Several studies of mortality from radiation-related heart disease and lung cancer have suggested that where there
is a risk, it may continue for many years after exposure (Inskip et al, 1994; Preston et al, 2003; Early Breast Cancer Trialists' Collaborative Group 2005; Prochazka et al, 2005;
Bouillon et al, 2011). However, no study has demonstrated so clearly a progressive increase in risk with time since exposure lasting into the third decade. In this population, the
radiation-related increases in mortality are very clear for women irradiated during 1973–82. For women irradiated during 1983–92 there is evidence of radiation-related mortality for lung
cancer, but not for heart disease. Follow-up of those irradiated since 1993 reveals, as yet, no increase in risk. Individual radiation doses are not available for the women in this study.
Average mean doses to the heart and lungs for women diagnosed with breast cancer in a given decade depend mainly on the prescribed dose, the targets irradiated and the techniques used. There
has been little variation in prescribed dose in the United States over the past few decades (Solin et al, 1991; Shank et al, 2000). However, the radiotherapy targets and techniques have
changed. One of the main determinants of heart and lung doses has been the use of radiotherapy to the internal mammary nodes. In previous decades, internal mammary irradiation has delivered
around 13–17 Gy and 3–10 Gy to the heart for left and right-sided radiotherapy, respectively, and around 13–24 Gy and 3–11 Gy to the ipsilateral and contralateral lungs, respectively, where
the tumour dose is 50 Gy (Prochazka et al, 2005). The use of internal mammary radiotherapy has declined in the United States at least since the 1980s: in a survey of radiation oncology
practice, the internal mammary chain was irradiated in 62% of women after breast conserving surgery in the 1980s, but in only 1% of such women in the 1990s. This reduction may explain, at
least in part, the reductions in radiation-related cardiac and lung cancer mortality. The eventual risks for breast cancer patients given radiotherapy today are, as yet, unknown, but will
depend on the doses to the heart and lungs. The current average mean heart dose is likely to be around 2–7 Gy for left-sided (Taylor et al, 2008; Jagsi et al, 2010; Schubert et al, 2011) and
around 1.5 Gy for right-sided breast cancer radiotherapy (Taylor et al, 2008). Current average mean lung doses are around 7–18 Gy for the ipsilateral, and around 0.1–3 Gy for the
contralateral lung (Jagsi et al, 2010; Schubert et al, 2011). Therefore, the risks for women irradiated today are likely to be lower. REFERENCES * Bouillon K, Haddy N, Delaloge S, Garbay
J-R, Garsi J-P, Brindel P, Mousannif A, Le MG, Labbe M, Arriagada R, Jougla E, Chavaudra J, Diallo I, Rubino C, de Vathaire F (2011) Long-term cardiovascular mortality after radiotherapy for
breast cancer. _J Am Coll Cardiol_ 57: 445–452 Article Google Scholar * Darby SC, McGale P, Taylor CW, Peto R (2005) Long-term mortality from heart disease and lung cancer after
radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries. _Lancet Oncol_ 6: 557–565 Article Google Scholar * Early Breast Cancer
Trialists' Collaborative Group (EBCTCG) (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an
overview of the randomised trials. _Lancet_ 366: 2087–2106 Article Google Scholar * Early Breast Cancer Trialists' Collaborative Group (EBCTCG) (2011) Effect of radiotherapy after
breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. _Lancet_ 378: 1707–1716
Article Google Scholar * Inskip PD, Stovall M, Flannery JT (1994) Lung cancer risk and radiation dose among women treated for breast cancer. _J Natl Cancer Inst_ 86: 983–988 Article CAS
Google Scholar * Jagsi R, Moran J, Marsh R, Masi K, Griffith KA, Pierce LJ (2010) Evaluation of four techniques using intensity-modulated radiation therapy for comprehensive locoregional
irradiation of breast cancer. _Int J Radiat Oncol Biol Phys_ 78: 1594–1603 Article Google Scholar * McGale P, Darby SC (2008) Commentary: A dose-response relationship for radiation-induced
heart disease--current issues and future prospects. _Int J Epidemiol_ 37: 518–523 Article Google Scholar * Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K (2003) Studies of
mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. _Radiat Res_ 160: 381–407 Article CAS Google Scholar * Prochazka M, Hall P,
Gagliardi G, Granath F, Nilsson BN, Shields PG, Tennis M, Czene K (2005) Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer:
case-only design. _J Clin Oncol_ 23: 7467–7474 Article Google Scholar * Schubert LK, Gondi V, Sengbusch E, Westerly DC, Soisson ET, Paliwal BR, Mackie TR, Mehta MP, Patel RR, Tomé WA,
Cannon GM (2011) Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy, and topotherapy. _Radiother Oncol_
100: 241–246 Article Google Scholar * Shank B, Moughan J, Owen J, Wilson F, Hanks GE (2000) The 1993-94 patterns of care process survey for breast irradiation after breast-conserving
surgery – comparison with the 1992 standard for breast conservation treatment. _Int J Radiat Oncol Biol Phys_ 48: 1291–1299 Article CAS Google Scholar * Solin LJ, Fowble BL, Martz KL,
Goodman RL, Hanks GE (1991) Results of the 1983 patterns of care process survey for definitive breast irradiation. _Int J Radiat Oncol Biol Phys_ 20: 105–111 Article CAS Google Scholar *
Taylor CW, Nisbet A, McGale P, Darby SC (2007) Cardiac exposures in breast cancer radiotherapy: 1950s–1990s. _Int J Radiat Oncol Biol Phys_ 69: 1484–1495 Article Google Scholar * Taylor
CW, Povall JM, McGale P, Nisbet A, Dodwell D, Smith JT, Darby SC (2008) Cardiac dose from tangential breast cancer radiotherapy in the year 2006. _Int J Radiat Oncol Biol Phys_ 72: 501–507
Article Google Scholar * Vandenbroucke JP (2004) When are observational studies as credible as randomised trials? _Lancet_ 363: 1728–1731 Article Google Scholar Download references
ACKNOWLEDGEMENTS This work was funded by Cancer Research UK. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Clinical Trial Service Unit (CTSU), University of Oxford, Richard Doll Building,
Old Road Campus, Roosevelt Drive, Oxford OX3 7LF, UK, K E Henson, P McGale, C Taylor & S C Darby Authors * K E Henson View author publications You can also search for this author
inPubMed Google Scholar * P McGale View author publications You can also search for this author inPubMed Google Scholar * C Taylor View author publications You can also search for this
author inPubMed Google Scholar * S C Darby View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to K E Henson. RIGHTS AND
PERMISSIONS This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Henson, K., McGale, P., Taylor, C. _et al._ Radiation-related mortality from
heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. _Br J Cancer_ 108, 179–182 (2013). https://doi.org/10.1038/bjc.2012.575 Download citation * Received:
17 August 2012 * Revised: 13 November 2012 * Accepted: 20 November 2012 * Published: 20 December 2012 * Issue Date: 15 January 2013 * DOI: https://doi.org/10.1038/bjc.2012.575 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * epidemiology * breast cancer radiotherapy * radiation-related heart disease * radiation-related lung cancer *
long-term effects * mortality









