- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND: Gestational trophoblastic diseases (GTDs) are related to trophoblasts, and human chorionic gonadotropin (hCG) is secreted by GTDs as well as normal placentas. However,
the asparagine-linked sugar chains on hCG contain abnormal biantennary structures in invasive mole and choriocarcinoma, but not normal pregnancy or hydatidiform mole.
_N_-acetylglucosaminyltransferase-IV (GnT-IV) catalyses _β_1,4-_N_-acetylglucosamine branching on asparagine-linked oligosaccharides, which are consistent with the abnormal sugar chain
structures on hCG. METHODS: We investigated GnT-IVa expression in GTDs and placentas by immunohistochemistry, western blot, and RT–PCR. We assessed the effects of GnT-IVa knockdown in
choriocarcinoma cells _in vitro_ and _in vivo_. RESULTS: The GnT-IVa was highly expressed in trophoblasts of invasive mole and choriocarcinoma, and moderately in extravillous trophoblasts
during the first trimester, but not in hydatidiform mole or other normal trophoblasts. The GnT-IVa knockdown in choriocarcinoma cells significantly reduced migration and invasive capacities,
and suppressed cellular adhesion to extracellular matrix proteins. The extent of _β_1,4-_N_-acetylglucosamine branching on _β_1 integrin was greatly reduced by GnT-IVa knockdown, although
the expression of _β_1 integrin was not changed. _In vivo_ studies further demonstrated that GnT-IVa knockdown suppressed tumour engraftment and growth. CONCLUSION: These findings suggest
that GnT-IVa is involved in regulating invasion of choriocarcinoma through modifications of the oligosaccharide chains of _β_1 integrin. SIMILAR CONTENT BEING VIEWED BY OTHERS PD-L1 ENHANCES
MIGRATION AND INVASION OF TROPHOBLASTS BY UPREGULATING ARHGDIB VIA TRANSCRIPTION FACTOR PU.1 Article Open access 22 September 2022 DYSFUNCTION OF SHH SIGNALING ACTIVATES AUTOPHAGY TO
INHIBIT TROPHOBLAST MOTILITY IN RECURRENT MISCARRIAGE Article Open access 04 January 2021 DYSREGULATED GLUT1 RESULTS IN THE PATHOGENESIS OF PREECLAMPSIA BY IMPAIRING THE FUNCTION OF
TROPHOBLAST CELLS Article Open access 10 October 2024 MAIN Gestational trophoblastic diseases (GTDs) are a spectrum of cellular proliferations arising from placental villous trophoblasts and
include hydatidiform mole and tumours that arise from trophoblasts, such as invasive mole, choriocarcinoma, and placental site trophoblastic tumour (PSTT; World Health Organization., 1983).
Hydatidiform moles are not tumours but abnormal conceptuses caused by genetic fertilisation disorders, and are classified into two entities as follows: complete hydatidiform mole, which is
androgenetic in origin, and partial hydatidiform mole, which is mostly a dispermic triploid (Kajii and Ohama, 1977). Invasive mole is a benign tumour, which occurs after 10–20% of complete
hydatidiform mole and 1–2% partial hydatidiform mole (Goto et al, 1993). However, chemotherapy is needed for treatment of invasive mole because the tumour arises from myometrial invasion of
molar villi, and 15–40% of patients show metastasis to lung or vagina (Soper, 2006; Lurain, 2010). Choriocarcinoma is a malignant epithelial tumour that is associated with all kinds of
pregnancies, but tends to develop from hydatidiform mole more than normal delivery and abortion (Lurain, 2010). PSTT is a rare malignant tumour arising from intermediate trophoblasts in
placental site (Shih and Kurman, 1998). Although recently all invasive moles can be in primary remission, 1–3% of invasive moles have recurrence and the survival rate of choriocarcinoma and
PSTT are about 85% and 70%, respectively (Khan et al, 2003; Schmid et al, 2009). These indicates that invasive mole is a pre-malignant disease, and hydatidiform mole has a greater potential
to develop into a malignancy than normal pregnancy, but the mechanism by which trophoblasts become malignant remains unclear. Human chorionic gonadotropin (hCG) is a glycoprotein hormone
that is produced by syncytiotrophoblasts of human placentas as well as GTDs. Human chorionic gonadotropin is a heterodimer composed of the _α_hCG and _β_hCG subunits. The _α_hCG subunit is
asparagine-linked (_N_-linked) glycosylated at Asn-52 and Asn-78, and the _β_hCG subunit contains two _N_-linked sugar chains at Asn-13 and Asn-30, and four serine-linked (_O_-linked) sugar
chains at Ser-121, Ser-127, Ser-132, and Ser-138 (Cole et al, 1984; Kobata and Takeuchi, 1999). The _N_-linked sugar chains of hCG in normal pregnancy and hydatidiform mole contained
monoantennary, biantennary, and fucosylated biantennary, but abnormal biantennary sugar chains were added in choriocarcinoma and a triantennary sugar chain added in choriocarcinoma and
invasive mole (Mizuochi et al, 1983; Endo et al, 1987). _N_-acetylglucosaminyltransferase IV (GnT-IV), which transfers an _N_-acetylglucosamine (GlcNAc) group to the core _α_1,3mannose of
_N_-glycans forming a _β_1-4 linkage, can act on biantennary sugar chains and generate the triantennary sugar chains on hCG in invasive mole and choriocarcinoma. The activities and mRNA
expression levels of glycosyltransferases (GnT-I to -V, _β_1-4galactosyltransferase, and _α_-mannosidase II) were examined in placentas and choriocarcinoma, and the GnT-IV activities and the
GnT-IVa mRNA level in the choriocarcinoma cell lines were significantly higher than in the normal placentas (Takamatsu et al, 1999, 2004). However, no study has examined GnT-IVa protein
expression and localisation in GTDs compared with normal human placentas or the function of GnT-IVa in trophoblasts. In this study, we examined GnT-IVa expression in trophoblastic cells of
GTDs and normal placentas, and the role of GnT-IVa in trophoblastic cells, especially in choriocarcinoma. MATERIALS AND METHODS TISSUE COLLECTION AND PROCESSING Informed consent was obtained
from patients for the use of placental samples and GTD tissue specimens. First-trimester and early second-trimester placentas were obtained from women undergoing elective pregnancy
terminations. Full-term placental samples were collected during elective Caesarean sections before the onset of labour. The GTD tissues were obtained from patients who underwent surgical
treatment, and the specimens were classified based on their histopathological characteristics. None of the patients had received chemotherapy for the disease before surgery. All tissue
samples were fixed in 10% formaldehyde, embedded in paraffin, and routinely stained with haematoxylin and eosin for histological examination. Some of the tissue samples were washed with
phosphate-buffered saline (PBS), frozen in liquid nitrogen immediately after removal, and then stored at −80 °C until protein extraction. This study was approved by the ethics committee of
Nagoya University Graduate School of Medicine. IMMUNOHISTOCHEMISTRY Immunohistochemical staining was performed using the avidin−biotin immunoperoxidase technique. Sections (4-_μ_m-thick)
were immunostained as previously described (Yamamoto et al, 2005), using an anti-GnT-IVa Ab (M-71; Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1 : 400 and anti-hCG Ab (N1534;
DAKO, Carpinteria, CA, USA). To evaluate the expression level of GnT-IVa in GTDs, the specimens of hydatidiform mole (_n_=11), invasive mole (_n_=4), choriocarcinoma (_n_=8), and PSTT
(_n_=3) were used. The GnT-IVa expression levels were classified semiquantitatively, based on the total scores of the per cent positivity of stained tumour cells and the staining intensity.
Namely, the per cent positivity was scored as 0 if <5% (negative), 1 if 5–20%, 2 if 20–50%, 3 if 50–70%, and 4 if >70% of cells stained. CELL LINES AND CULTURE Human choriocarcinoma
cell lines (Jar, BeWo, and JEG-3) were purchased from the American Type Culture Collection (Manassas, VA, USA). The NaUCC (CC)-1, CC-3, CC-4, and CC-6 are human choriocarcinoma cell lines
that were previously established in our laboratory (Ino et al, 1991). The human extravillous trophoblast (EVT) cell line HTR-8/SVneo was kindly provided by Dr Charles H Graham (Graham et al,
1993). All cell lines were grown in RPMI 1640 (Sigma, St Louis, MO, USA), supplemented with 10% FCS, penicillin (100 U ml−1), streptomycin (100 _μ_g ml−1), and 2 mM glutamine. Cultures were
incubated at 37 °C in 5% CO2. WESTERN BLOT ANALYSIS Western blot analysis for GnT-IVa protein was performed as previously described (Yamamoto et al, 2007), with an anti-GnT-IVa mAb (M-71;
Santa Cruz Biotechnology) diluted 1 : 1000. Immunoreactive proteins were stained using a chemiluminescence detection system (ECL; Amersham, Arlington Heights, IL, USA). An Ab against
_β_-actin (AC-15; Sigma) was used to standardise the protein loading. RNA EXTRACTION AND QUANTITATIVE RT–PCR Total RNA extraction and quantitative real-time PCR were performed as previously
described (Mano et al, 2009). We used the following primers for _GnT-IVa_: forward primer, 5′-ACCAAGGGCATACGCTGGAG-3′; reverse primer, 5′-GTTCTTGGTTGCCGCTATGGA-3′, and the following primers
for GAPDH: forward primer, 5′-CGGGAAACTGTGGCGTGAT-3′; reverse primer, 5′-ATGCCAGTGAGCTTCCCGT-3'. The PCR profile was an initial incubation at 95 °C for 10 s, followed by 45 cycles of
denaturation at 95 °C for 5 s, and annealing and extension at 60 °C for 30 s. _DATURA STRAMONIUM_ AGGLUTININ BLOT ANALYSIS Lectin blot analysis was performed as previously reported (Yamamoto
et al, 2009), using HRP-labelled _Datura stramonium_ agglutinin (DSA; Seikagaku, Tokyo, Japan) diluted 1 : 2000, and DSA recognises _β_1-4GlcNAc branching. SILENCING OF GNT-IVA BY SMALL
INTERFERING RNA AND SHORT HAIRPIN RNA TRANSFECTION Small interfering RNAs (siRNAs) were designed and synthesised by Nippon EGT (Toyama, Japan) to target GnT-IVa (siRNA1:
5′-AGAUGGCUAUUUCAGAAUATT-3′ and 5′-UAUUUCUGAAAUAGCCAUCUTT-3′; siRNA2: 5′-GAAGAUGGCUAUUUCAGAATT-3′ and 5′-UUCUGAAAUAGCCAUCUUCTT-3′). The non-targeting siRNAs (Nippon EGT) were used as a
control (control siRNA: 5′-GGAUUAUUACGCAGUUAAATT-3′ and 5′-UUUAACUGCGUAAUAAUCCTT-3′). Jar cells were grown in 60-mm plates to 60% confluency and then transfected with 60 pmol of siRNAs using
Lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After treating Jar cells with siRNAs (siRNA1, siRNA2, and control
siRNA) for 8 h, the medium containing the siRNAs and transfection reagents were removed and cells were cultured in fresh culture medium for at least 24 h before each experiment. Transfected
cells were cultured for 24 h before adhesion assay and immunoprecipitation, and for 48 h before zymography and hCG assay. To produce stable GnT-IVa knockdown cells for _in vivo_ studies, the
oligonucleotide sequences designed by Block-it RNAi Designer (Invitrogen) in the construction of the short hairpin RNA (shRNA) vector were as follows:
5′-CACCGCTATTGTATGAGTCATAATTCGAAAATTATGACTCATACAATAGC-3′ and 5′-AAAAGVTATTGTATGAGTCATAATTTTCGAATTATGACTGATACAATAGC-3′. The shRNA vector was synthesised by Invitrogen using the
oligonucleotides and pENTR/H1/TO (Invitrogen). The vector plasmid was transfected into Jar cells using Lipofectamine 2000 reagent (Invitrogen) and selected by adding zeosin. The original
pENTR/H1/TO vector was used as a control shRNA. _IN VITRO_ CELL PROLIFERATION ASSAY Cells (5 × 103) were plated in 100 _μ_l of medium in 96-well plates and incubated for 72 h at 37 °C. Cell
viability was determined using the modified tetrazolium salt assay using the Cell Titer 96 Aqueous One Solution Proliferation Assay kit (Promega, Madison, WI, USA) according to the
manufacturer’s instructions. The mean values of three independent experiments performed in eight wells are shown. TRANSWELL MIGRATION AND INVASION ASSAY The migration and invasion assay was
performed as previously reported (Yamamoto et al, 2005). Jar cells were transfected with siRNA 24 h before seeding for the migration and invasion assays, and both assays were performed after
24 h incubation. The number of cells was counted under a microscope at × 200 magnification. Data were obtained from three individual experiments performed in triplicate. ZYMOGRAPHY AND CELL
ADHESION ASSAY Cells (1 × 105) were plated in a 24-well chamber and incubated with serum-free medium for 48 h after siRNA transfection. Zymography was performed as previously reported
(Yamamoto et al, 2009). Cells (4 × 104) were plated in 96-well plates coated with fibronectin, collagen type I or type IV (Becton, Dickinson and Company, Franklin Lakes, NJ, USA),
centrifuged at 1500 r.p.m. for 15 s, and allowed to attach to each matrix at 37 °C for 30 min. After washing with PBS, absorbance readings at 492 nm (A492 nm) were performed using a
microplate reader (Multiskan Bichromatic; Labsystems, Helsinki, Finland). The rate of cell adherence was calculated as follows: (A492 nm (matrix)−A492 nm (no matrix))/A492 nm (no matrix)
(Inamori et al, 2006; Yamamoto et al, 2009). Data were obtained from three individual experiments performed in eight wells. LECTIN BLOT ANALYSIS ON IMMUNOPRECIPITATED _Β_ HCG
Immunoprecipitation was performed using 1.5 mg of protein that was extracted from cells with an anti-human _β_hCG mAb (HCG-60; Santa Cruz Biotechnology) or _β_1 integrin mAb (BV7; Abcam,
Cambridge, UK), as previously described (Yamamoto et al, 2007). The anti-_β_hCG mAb and anti-_β_1 integrin mAb (MAB2247; Chemicon International, Temecula, CA, USA) were used at a dilution of
1 : 200 and 1 : 1000, respectively, and western blotting and DSA lectin blotting were performed using the above protocol. HUMAN CHORIONIC GONADOTROPIN ASSAY Hormone assays were performed on
culture supernatant. Cells (5 × 104) were plated in a 24-well chamber and incubated with 1 ml serum-free medium for 48 h after siRNA transfection. The total hCG levels were quantified in
triplicate by enzyme immunoassay using an _α_hCG mAb and a _β_hCG-CTP mAb (SRL Inc., Tokyo, Japan). _IN VIVO_ STUDIES Female BALB/c slc nu/nu mice (5 weeks old) were purchased from Japan SLC
(Nagoya, Japan).The treatment protocol followed the guidelines for animal experimentation adopted by Nagoya University. Cells (5 × 106) per 0.2 ml of PBS/mouse were injected subcutaneously
on the right flank to examine implantation and survival analysis by GnT-IVa stable knockdown. Each group consisted of seven mice. The overall survival was defined as the time between the
date of inoculation and the date of death due to tumour. STATISTICAL ANALYSIS The non-parametric Kruskal–Wallis test was performed to compare immunostaining scores among all histological
types. Data are expressed as the mean±s.d. For data of _in vitro_ experiments, statistical comparisons among groups were performed using the one-way ANOVA with Bonferroni corrections.
Overall survival curves were analysed by the log-rank test. Differences were considered significant when _P_<0.05. RESULTS IMMUNOHISTOCHEMICAL EXPRESSION OF GNT-IVA IN GTD AND PLACENTA We
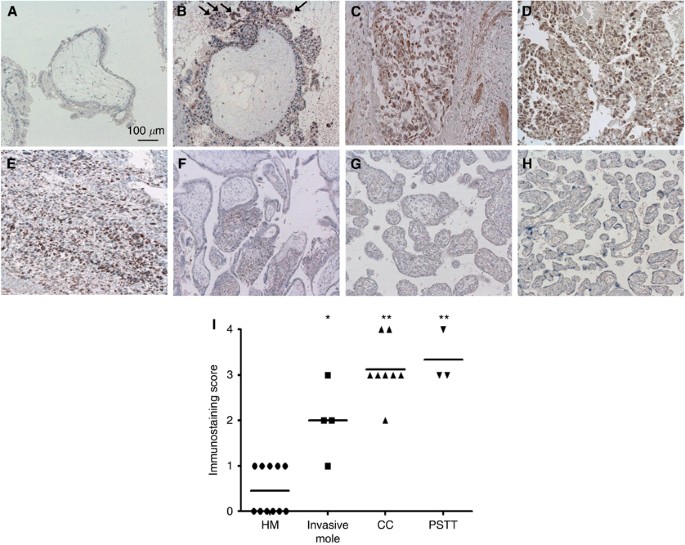
first examined GnT-IVa protein localisation in GTDs (hydatidiform mole, invasive mole, choriocarcinoma, and PSTT) and placentas by immunohistochemistry. The GnT-IVa was highly expressed in
trophoblasts in invasive mole, choriocarcinoma, and PSTT, but not or very weakly in hydatidiform mole (Figures 1A−E). The staining in trophoblasts of invasive mole was stronger at the edge
of the invasive site than other parts (arrows in Figure 1B). In placenta, there was no staining in trophoblasts after the second trimester, and only EVTs moderately expressed GnT-IVa in the
first trimester (Figures 1F−H). We examined the immunostaining score of 26 GTD patients, and the medians were 0.5 in hydatidiform mole, 2.0 in invasive mole, 3.1 in choriocarcinoma, and 3.0
in PSTT. These results showed that GnT-IVa expression is significantly higher in invasive mole (_P_=0.004), choriocarcinoma (_P_<0.001), and PSTT (_P_<0.001) compared with hydatidiform
mole (Figure 1I). THE GNT-IVA MRNA AND PROTEIN EXPRESSION IN VARIOUS TROPHOBLASTS The _GnT-IVa_ mRNA expression was examined in seven choriocarcinoma cell lines, one EVT cell line,
placental tissues, and hydatidiform mole tissues by quantitative PCR. The _GnT-IVa_ mRNA was expressed from 0.6- to 2.1-fold higher in choriocarcinoma cell lines compared with Jar cells but
0.02-fold higher in HTR-8/SVneo. In placenta at 6 weeks of gestation, the expression was almost the same in the first trimester as Jar, but the levels in placenta after the second trimester
and hydatidiform mole were weak (Figure 2A). In addition, western blot analysis demonstrated that the GnT-IVa protein was detected as a 75-kDa band (Figure 2B) in all choriocarcinoma cell
lines but not in hydatidiform mole. HTR-8/SVneo and placental tissues showed very weak expression at the protein level. These results were the same as the results of immunohistochemical
analysis. _Β_ 1-4GLCNAC BRANCHING IN TROPHOBLAST CELL LINES AND HUMAN PLACENTA We performed lectin blot analysis on total cellular proteins using DSA to determine the levels of _β_1-4GlcNAc
branching. Glycoproteins from choriocarcinoma cell lines and HTR-8/SVneo stained more strongly with DSA than samples from placenta and hydatidiform mole, and the molecular sizes of the major
glycoproteins recognised by DSA were distributed over approximately 40–150 kDa (Figure 2C). This analysis revealed that GnT-IVa target proteins exist in all kinds of trophoblasts, and
GnT-IVa produces more _β_1-4GlcNAc branching in choriocarcinoma and EVT than placenta and hydatidiform mole. KNOCKDOWN OF GNT-IVA EXPRESSION IN JAR CELLS Our results showed that GnT-IVa was
expressed strongly in trophoblasts of choriocarcinoma and invasive mole, and moderately in EVTs, and the common characteristic of these cells is invasiveness. To investigate the function of
GnT-IVa in trophoblasts, we used siRNA to establish a GnT-IVa knockdown model using Jar cells. The GnT-IVa protein expression in GnT-IVa siRNA transfectants (siGnT-IV1 and siGnT-IV2) was
effectively decreased compared with the parental or control siRNA transfectants (si-control) at 24 and 48 h after transfection (Figure 3A). Quantitative RT–PCR analysis also indicated that
siGnT-IV1 and siGnT-IV2 suppressed _GnT-IVa_ mRNA expression to 20.3% and 20.9%, respectively, compared with si-control (Figure 3B). EFFECTS OF GNT-IVA KNOCKDOWN ON CELL PROLIFERATION,
MIGRATION, AND INVASION We assessed the effects of GnT-IVa knockdown on cell proliferation by modified tetrazolium salt assay. After GnT-IVa siRNAs treatment, the cell number increased at 72
h of incubation; however, this was not significantly different from the si-control (Figure 3C). The migration assay revealed that decreased GnT-IVa expression reduced the migratory ability
in both siGnT-IV1 and siGnT-IV2 cells compared with si-control (41.5±14.0% and 37.7±13.0%, respectively; Figure 3D). In the invasion assay, we confirmed that siGnT-IV1 and siGnT-IV2 had a
significantly lower potential to invade compared with the si-control (33.6±9.4% and 31.7±10.6%, respectively; Figure 3E). These results showed that decreased GnT-IVa expression reduced the
invasive and migratory capabilities of choriocarcinoma. EFFECTS OF GNT-IVA KNOCKDOWN ON GELATINASE ACTIVITY AND CELL ADHESION TO THE EXTRACELLULAR MATRIX Invasion with trophoblasts and
cancer cells is a multistep process involving attachment to a basement membrane or extracellular matrix (ECM) components, followed by degradation and subsequent migration through the
degraded components. Type IV collagenases, such as MMP-2 and MMP-9, are thought to be the principal mediators of trophoblast invasion as well as cancer invasion. Hence, we performed gelatin
zymography and found that Jar predominantly secrete MMP-2. The GnT-IVa knockdown did not significantly affect the expression of either the pro form (72 kDa) or the active form (62 kDa) of
this enzyme (Figure 4A). Next we examined the adherence potential of Jar to ECM proteins after GnT-IVa knockdown, and the rate of cell adherence was calculated as shown in previous reports
(Inamori et al, 2006; Yamamoto et al, 2009). The rate of cell attachment to fibronectin, collagen type I, and collagen type IV decreased approximately 0.3-fold by GnT-IVa knockdown (Figure
4B). THE GNT-IVA KNOCKDOWN REDUCED _Β_ 1-4GLCNAC BRANCHING OF _Β_ HCG BUT NOT HCG SECRETION As GnT-IVa is an enzyme that catalyses _β_1-4GlcNAc addition to hCG sugar chains, we investigated
the affect of GnT-IVa knockdown on its abundance in hCG. First we examined the difference in the levels of secreted hCG by enzyme immunoassay, and there were no differences in hCG secretion
by Jar cells after GnT-IVa siRNA transfection as well as control siRNA transfection (Figure 4C). Next we examined the level of _N_-glycan sugar chains by immunoprecipitating with the _β_hCG
antibody followed by lectin blot with DSA, which recognises _β_1-4GlcNAc branching. The levels of _β_1-4GlcNAc branching on _β_hCG in siGnT-IV1 and siGnT-IV2 decreased significantly compared
with si-control (Figure 4D, upper panel); however, _β_hCG expression was not affected by GnT-IVa knockdown (Figure 4D, lower panel). THE GNT-IVA KNOCKDOWN REDUCED _Β_ 1-4GLCNAC BRANCHING OF
_Β_ 1 INTEGRIN To investigate a new target molecule for GnT-IVa, which affected the invasive ability in choriocarcinoma, we examined the glycosylation on _β_1 integrin because GnT-IVa
knockdown decreased the adhesion abilities to ECMs and integrin is a major carrier of _N_-glycans (Gu et al, 2009). The levels of _β_1-4GlcNAc branching on _β_1 integrin were decreased
significantly by GnT-IVa knockdown (Figure 4E, upper panel), although _β_1 integrin expression was the same level in siGnT-IV1 and siGnT-IV2 as si-control (Figure 4E, lower panel). These
results suggest that diminished _β_1-4GlcNAc branching on _β_1 integrin as a result of GnT-IVa knockdown might be associated with the decreased invasive capacity of choriocarcinoma. _IN
VIVO_ EFFECTS OF GNT-IVA KNOCKDOWN ON CHORIOCARCINOMA ENGRAFTMENT AND GROWTH To investigate whether the suppression of GnT-IVa expression in Jar could influence the invasive ability _in
vivo_, we established a stable GnT-IVa knockdown model of choriocarcinoma, using an shRNA vector, and analysed the effects on tumorigenicity in nude mice. The GnT-IVa protein expression in
GnT-IVa shRNA transfectants (shGnT-IV) was effectively decreased compared with the control shRNA transfectants (sh-control; Figure 5A). We inoculated the cells of sh-control or shGnT-IV
subcutaneously into seven nude mice for each group, and sh-control cells developed tumours in all cases. The tumour of sh-control rapidly generated lethally and the mice with sh-control died
at 31 days after the inoculation on average. On the other hand, shGnT-IV cells engrafted in only three cases and significantly suppressed growing tumours. The mice with shGnT-IV could
survive significantly longer than those with the sh-control (_P_=0.003, Figure 5B). Histopathological examination revealed that the engraftment tumours contained a two-cell pattern of
choriocarcinoma, consisting of syncytiotrophoblastic cells and cytotrophoblastic cells (Figure 5C, upper panels, arrows, and arrowheads), and were positive for hCG in both sh-control and
shGnT-IV; however, GnT-IVa staining in tumours of shGnT-IV was weaker than tumours of sh-control (Figure 5C). DISCUSSION The glycosylation of glycoproteins has a key role in a variety of
specific biological interactions (Hakomori, 1989). In particular, branching of _N_-linked oligosaccharides regulates the metastatic potential of cancer cells (Zhao et al, 2008; Gu et al,
2009). _N_-acetylglucosaminyltransferase-IV is one of the glycosyltransferases involved in _N_-glycan biosynthesis and has two isoenzymes, GnT-IVa and GnT-IVb. The GnT-IVb is broadly
expressed across various organs at almost the same level and exhibits housekeeping gene-like expression (Yoshida et al, 1999), whereas the _GnT-IVa_ gene is thought to have an essential role
in elevated GnT-IV activity. The enzymatic activities and expression levels of GnT-I to -V were examined in normal human placentas and three human choriocarcinoma cell lines, and _GnT-IVa_
mRNA, but not _GnT-IVb_ mRNA, was strongly expressed in all three choriocarcinoma cell lines (Takamatsu et al, 1999). In addition, previous reports have shown that structural alterations in
_N_-glycans of hCG produced by malignant (choriocarcinoma) and pre-malignant trophoblastic disease (invasive mole) could be catalysed by GnT-IV (Mizuochi et al, 1983; Endo et al, 1987). Our
results also show that GnT-IVa expresses strongly in malignant and pre-malignant trophoblastic cells, but not in normal or benign (hydatidiform mole) trophoblastic cells. Therefore, we
examined the role of GnT-IVa in trophoblastic diseases using choriocarcinoma cells. Immunoprecipitation with a _β_hCG antibody and subsequent DSA lectin blot showed that _β_hCG is a target
molecule of GnT-IVa in choriocarcinoma as previous reports suggested. The hCG has many important functions in pregnancy, including the promotion of progesterone production, implantation and
decidualisation, angiogenesis, cytotrophoblast differentiation, and immune cell regulation (Norris et al, 2011). Furthermore, there are many hCG variants, and hyperglycosylated-hCG (hCG-H),
which is a glycosylation variant of hCG, has been suggested to be involved in regulation of trophoblast invasion (Cole et al, 2006; Cole, 2010). Although regular hCG secreted by
syncytiotrophoblasts has monoantennary and biantennary _N_-linked oligosaccharides, and mostly trisaccharide _O_-linked oligosaccharides, hCG-H has predominantly larger fucosylated
triantennary _N_-linked oligosaccharides and/or double-sized hexasaccharide _O_-linked oligosaccharides (Cole, 2010). The _N_-linked and _O_-linked hCG-H were detected in the urine of
choriocarcinoma patients, but not in normal pregnant women in 1983 and 1985, respectively (Mizuochi et al, 1983; Cole et al, 1985). The function of _N_-linked hCG-H has not been
investigated, whereas it has been reported that _O_-linked hCG-H is produced in choriocarcinoma and during very early normal pregnancy, and promotes the invasion of choriocarcinoma and EVTs
at implantation sites (Cole, 2010). In our study, we showed that suppressing GnT-IVa reduced cell invasion in choriocarcinoma, although it did not affect the secretion of hCG or _O_-linked
hCG-H (data not shown). These results may suggest that both _N_-linked hCG-H and _O_-linked hCG-H have the same important function in promoting invasion that differs from the functions of
regular hCG. Our lectin blot experiments demonstrated that numerous proteins in choriocarcinoma exhibit _β_1-4GlcNAc glycosylation, which were approximately 40–150 kDa. In a study with
GnT-IVa knockout mice, glucose transporter 2 (Glut-2) was identified as a target glycoprotein of GnT-IVa in pancreatic _β_ cells, and loss of GnT-IVa attenuated the half-life of Glut-2 cell
surface expression and led to a metabolic dysfunction diagnosis of type 2 diabetes (Ohtsubo et al, 2005). Eight members of the Glut family have been described in human placental tissue and
trophoblasts, but Glut-2 is not expressed (Baumann et al, 2002). Other target molecules were suggested to be _γ_-glutamyltranspeptidase and carcinoembryonic antigen because the abnormal
biantennary structure by GnT-IV was detected on these proteins when they were purified from hepatocellular carcinoma and colon cancer, respectively (Yamashita et al, 1987, 1989). In mouse
hepatocarcinoma, it has been reported recently that GnT-IVa increases migration and metastasis capabilities through glycosylation of CD147, which is also named the ECM metalloproteinase
inducer, (Fan et al, 2012). CD147 stimulates the production of MMPs and has been reported to express strongly in choriocarcinoma by immunohistochemistry (Singh et al, 2012). However, GnT-IVa
knockdown did not affect MMP activities, but decreased the adherence potential of cells to ECM proteins in choriocarcinoma cells. These results suggest that one of the adhesion molecules is
modulated by GnT-IVa and involved in regulation of choriocarcinoma invasion. The staining levels in DSA lectin blot of choriocarcinoma cell lines and HTR-8/SVneo were not the same as the
levels of GnT-IVa expression in western blot. It may be because the _β_1-4GlcNAc branching on _N_-glycans can be the common substrate for other _N_-acetylglucosaminyltransferases, such as
GnT-III and GnT-V (Schachter et al, 1989). GnT-V catalyses the formation of _β_1-6GlcNAc branching structure, which can be recognised by leukoagglutinating phytohemagglutinin (L4-PHA)
lectin, and GnT-III reduces the level of _β_1-6GlcNAc branching by catalysing the addition of _β_1,4-bisecting-_N_-acetylglucosamine on _N_-glycans (Zhao et al, 2008; Gu et al, 2009). The
DSA lectin blot after immunoprecipitation for a specific molecule can show the level of _β_1-4GlcNAc branching on the molecule clearly as DSA lectin blots after immunoprecipitations for
Glut-2 by Ohtsubo et al (2005) and for _β_1 integrin in our study. We performed L4-PHA lectin blot with and without immunoprecipitation for _β_1 integrin, and there were no differences in
the levels of _β_1-6GlcNAc branching between si-control cells and siGnT-IV cells in both L4-PHA lectin blot (data not shown). Furthermore, suppression of GnT-V in Jar decreased _β_1-6GlcNAc
branching on _α_5_β_1 integrin and increased invasion ability and adhesion ability to ECMs (Yamamoto et al, 2009). These results suggest that GnT-V and GnT-III may not affect the functional
changes by glycosylation on _N_-glycans, including _β_1 integrin, by GnT-IV in Jar cells. In adhesion molecules, integrin and E-cadherin are major carriers of _N_-glycans. The changes of
biological functions of both molecules by _N_-glycosylation are considered to be associated with a carcinogenic process and tumourigenesis (Zhao et al, 2008; Gu et al, 2009). E-cadherin
mediates cell–cell adhesion, whereas integrins are _αβ_ heterodimers and the _N_-terminal domain of each subunit contains ECM binding site. Among the integrin superfamily, 12 members
containing _β_1 subunit and _β_1 integrin complexes, including _α_5_β_1 integrin (fibronectin receptor) and _α_3_β_1 integrin (laminin receptor), have been reported to change biological
functions in cancers by _N_-glycosylation by GnT-III and GnT-V (Isaji et al, 2004; Zhao et al, 2008; Gu et al, 2009). Our adhesion assay showed that GnT-IVa knockdown reduced adhesion of Jar
cells to collagen type I and type IV, as well as fibronectin. Therefore, we investigated the change of _β_1-4GlcNAc glycosylation level on _β_1 integrin, and our results suggest that
GnT-IVa might be involved in regulating trophoblast invasion through glycosylation of _β_1 integrin. However, more research is needed to confirm the relationship between invasion and
_β_1-4GlcNAc glycosylation on _β_1 integrin. _In vivo_ studies confirmed our findings that GnT-IVa stimulates the abilities of adhesion to ECMs and invasion in choriocarcinoma. The GnT-IVa
knockdown significantly reduced the potential for tumourigenesis and immunohistochemistry demonstrated that the cells of the tumour produced by shGnT-IV expressed GnT-IVa significantly
weakly compared with the tumour by sh-control, although the morphology and staining for hCG as choriocarcinoma were the same in these two groups. These results suggest that only
trophoblastic cells expressing GnT-IVa can adhere to ECMs and migrate by increasing of _β_1-4GlcNAc glycosylation on _β_1 integrin, which is consistent with the results that trophoblastic
cells of invasive mole, choriocarcinoma, and PSTT expressed GnT-IVa strongly, but not in hydatidiform mole in immunohistochemistry, western blot, and RT–PCR. We examined GnT-IVa localisation
in normal placentas during all trimesters, as well as in trophoblastic disease. In the human placenta, cytotrophoblastic stem cells at the basement of villi differentiate in two distinct
directions: syncytiotrophoblasts and EVTs (Damsky et al, 1992). Syncytiotrophoblasts originate from the syncytial layer in floating villi, which primarily manage transport and endocrine
functions. On the other hand, EVTs located in anchoring villi develop and invade the maternal decidua, myometrium, and uterine vasculature until the 10th gestational week. As trophoblast
cells migrate from the anchoring villus, they downregulate _α_6_β_4 integrin and upregulate _α_5_β_1 and _α_1_β_1 integrin (laminin/collagen receptor; Damsky et al, 1994), and _β_1-6
branching of _α_5_β_1 integrin by GnT-V is decreased (Yamamoto et al, 2009). When examining invasive function, Handschuh et al (2007) reported that invasive EVTs secreted hCG and expressed
the LH/CG receptor, and that hCG produced by invasive EVTs induced a 10-fold increase in EVT invasion, although hCG produced by syncytiotrophoblasts had no effect on invasion. They also
showed that _O_-linked hCG-H was located in invasive and endovascular EVTs, but not in syncytiotrophoblasts during the first trimester (Guibourdenche et al, 2010). Our results revealed that
only EVTs in the first trimester strongly expressed GnT-IVa in placentas. These results suggest that GnT-IVa may regulate the invasion of EVTs in the first trimester by glycosylation of the
_N_-glycan on hCG and _β_1 integrin, and that EVTs secrete _N_-linked hCG-H. Western blot and RT–PCR showed that mRNA and protein expression levels of GnT-IVa in placental tissues were weak,
except mRNA expression early in the first trimester. These tissues are villi, which consist of cytotrophoblasts and syncytiotrophoblasts. However, some parts of the tissues might have EVTs,
and these cells might affect the results of RT–PCR and western blot, especially in the first trimester. Further studies will be required to reveal the function of GnT-IVa and _N_-linked
hCG-H in implantation in early pregnancy. In summary, we have provided the first evidence of a functional role for GnT-IVa in choriocarcinoma migration and invasion, and a new target
molecule of GnT-IVa to be _β_1 integrin. Our results show that GnT-IVa is strongly expressed in trophoblastic cells that have invasive potential, including choriocarcinoma, invasive mole,
PSTT, and EVTs in the first trimester. These findings suggest that GnT-IVa can be a good marker for malignant/pre-malignant GTDs and is involved in regulating trophoblast invasion. CHANGE
HISTORY * _ 04 DECEMBER 2012 This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Baumann MU,
Deborde S, Illsley NP (2002) Placental glucose transfer and fetal growth. _Endocrine_ 19: 13–22 Article CAS Google Scholar * Cole LA (2010) Biological functions of hCG and hCG-related
molecules. _Reprod Biol Endocrinol_ 8: 102 Article Google Scholar * Cole LA, Birken S, Perini F (1985) The structures of the serine-linked sugar chains on human chorionic gonadotropin.
_Biochem Biophys Res Commun_ 126: 333–339 Article CAS Google Scholar * Cole LA, Dai D, Butler SA, Leslie KK, Kohorn EI (2006) Gestational trophoblastic diseases: 1. Pathophysiology of
hyperglycosylated hCG. _Gynecol Oncol_ 102: 145–150 Article CAS Google Scholar * Cole LA, Perini F, Birken S, Ruddon RW (1984) An oligosaccharide of the O-linked type distinguishes the
free from the combined form of hCG alpha subunit. _Biochem Biophys Res Commun_ 122: 1260–1267 Article CAS Google Scholar * Damsky CH, Fitzgerald ML, Fisher SJ (1992) Distribution patterns
of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. _J Clin
Invest_ 89: 210–222 Article CAS Google Scholar * Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ (1994) Integrin switching regulates
normal trophoblast invasion. _Development_ 120: 3657–3566 CAS PubMed Google Scholar * Endo T, Nishimura R, Kawano T, Mochizuki M, Kobata A (1987) Structural differences found in the
asparagine-linked sugar chains of human chorionic gonadotropins purified from the urine of patients with invasive mole and with choriocarcinoma. _Cancer Res_ 47: 5242–5245 CAS PubMed
Google Scholar * Fan J, Wang S, Yu S, He J, Zheng W, Zhang J (2012) N-acetylglucosaminyltransferase IVa regulates metastatic potential of mouse hepatocarcinoma cells through glycosylation
of CD147. _Glycoconj J_ 29: 323–334 Article CAS Google Scholar * Goto S, Yamada A, Ishizuka T, Tomoda Y (1993) Development of postmolar trophoblastic disease after partial molar
pregnancy. _Gynecol Oncol_ 48: 165–170 Article CAS Google Scholar * Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK (1993) Establishment and characterization of
first trimester human trophoblast cells with extended lifespan. _Exp Cell Res_ 206: 204–211 Article CAS Google Scholar * Gu J, Isaji T, Sato Y, Kariya Y, Fukuda T (2009) Importance of
N-glycosylation on alpha5beta1 integrin for its biological functions. _Biol Pharm Bull_ 32: 780–785 Article CAS Google Scholar * Guibourdenche J, Handschuh K, Tsatsaris V, Gerbaud P,
Leguy MC, Muller F, Brion DE, Fournier T (2010) Hyperglycosylated hCG is a marker of early human trophoblast invasion. _J Clin Endocrinol Metab_ 95: E240–E244 Article CAS Google Scholar *
Hakomori S (1989) Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. _Adv Cancer Res_ 52: 257–331 Article CAS Google Scholar * Handschuh K, Guibourdenche J,
Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T (2007) Human chorionic gonadotropin produced by the invasive trophoblast but not the villous trophoblast promotes cell
invasion and is down-regulated by peroxisome proliferator-activated receptor-gamma. _Endocrinology_ 148: 5011–5019 Article CAS Google Scholar * Inamori K, Gu J, Ohira M, Kawasaki A,
Nakamura Y, Nakagawa T, Kondo A, Miyoshi E, Nakagawara A, Taniguchi N (2006) High expression of N-acetylglucosaminyltransferase V in favorable neuroblastomas: involvement of its effect on
apoptosis. _FEBS Lett_ 580: 627–632 Article CAS Google Scholar * Ino K, Goto S, Kosaki A, Nomura S, Asada E, Misawa T, Furuhashi Y, Mizutani S, Tomoda Y (1991) Growth inhibitory effect of
bestatin on choriocarcinoma cell lines in vitro. _Biotherapy_ 3: 351–357 Article CAS Google Scholar * Isaji T, Gu J, Nishiuchi R, Zhao Y, Takahashi M, Miyoshi E, Honke K, Sekiguchi K,
Taniguchi N (2004) Introduction of bisecting GlcNAc into integrin alpha5beta1 reduces ligand binding and down-regulates cell adhesion and cell migration. _J Biol Chem_ 279: 19747–19754
Article CAS Google Scholar * Kajii T, Ohama K (1977) Androgenetic origin of hydatidiform mole. _Nature_ 268: 633–634 Article CAS Google Scholar * Khan F, Everard J, Ahmed S, Coleman
RE, Aitken M, Hancock BW (2003) Low-risk persistent gestational trophoblastic disease treated with low-dose methotrexate: efficacy, acute and long-term effects. _Br J Cancer_ 89: 2197–2201
Article CAS Google Scholar * Kobata A, Takeuchi M (1999) Structure, pathology and function of the N-linked sugar chains of human chorionic gonadotropin. _Biochim Biophys Acta_ 1455:
315–326 Article CAS Google Scholar * Lurain JR (2010) Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic
disease, and management of hydatidiform mole. _Am J Obstet Gynecol_ 203: 531–539 Article Google Scholar * Mano Y, Shibata K, Sumigama S, Hayakawa H, Ino K, Yamamoto E, Kajiyama H, Nawa A,
Kikkawa F (2009) Tocilizumab inhibits interleukin-6-mediated matrix metalloproteinase-2 and -9 secretions from human amnion cells in preterm premature rupture of membranes. _Gynecol Obstet
Invest_ 68: 145–153 Article CAS Google Scholar * Mizuochi T, Nishimura R, Derappe C, Taniguchi T, Hamamoto T, Mochizuki M, Kobata A (1983) Structures of the asparagine-linked sugar chains
of human chorionic gonadotropin produced in choriocarcinoma. Appearance of triantennary sugar chains and unique biantennary sugar chains. _J Biol Chem_ 258: 14126–14129 CAS PubMed Google
Scholar * Norris W, Nevers T, Sharma S, Kalkunte S (2011) Review: hCG, preeclampsia and regulatory T cells. _Placenta_ 32: S182–S185 Article Google Scholar * Ohtsubo K, Takamatsu S,
Minowa MT, Yoshida A, Takeuchi M, Marth JD (2005) Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. _Cell_ 123: 1307–1321
Article CAS Google Scholar * Schachter H, Brockhausen I, Hull E (1989) High-performance liquid chromatography assays for N-acetylglucosaminyltransferases involved in N- and O-glycan
synthesis. _Methods Enzymol_ 1989: 351–397 Article Google Scholar * Schmid P, Nagai Y, Agarwal R, Hancock B, Savage PM, Sebire NJ, Lindsay I, Wells M, Fisher RA, Short D, Newlands ES,
Wischnewsky MB, Seckl MJ (2009) Prognostic markers and long-term outcome of placental-site trophoblastic tumours: a retrospective observational study. _Lancet_ 374: 48–55 Article CAS
Google Scholar * Shih IM, Kurman RJ (1998) Epithelioid trophoblastic tumor: a neoplasm distinct from choriocarcinoma and placental site trophoblastic tumor simulating carcinoma. _Am J Surg
Pathol_ 22: 1393–1403 Article CAS Google Scholar * Singh M, Kindelberger D, Nagymanyoki Z, Ng SW, Quick CM, Yamamoto H, Fichorova R, Fulop V, Berkowitz RS (2012) Vascular endothelial
growth factors and their receptors and regulators in gestational trophoblastic diseases and normal placenta. _J Reprod Med_ 57: 197–203 CAS PubMed Google Scholar * Soper JT (2006)
Gestational trophoblastic disease. _Obstet Gynecol_ 108: 176–187 Article Google Scholar * Takamatsu S, Katsumata T, Inoue N, Watanabe T, Fujibayashi Y, Takeuchi M (2004) Abnormal
biantennary sugar chains are expressed in human chorionic gonadotropin produced in the choriocarcinoma cell line, JEG-3. _Glycoconj J_ 20: 473–481 Article CAS Google Scholar * Takamatsu
S, Oguri S, Minowa MT, Yoshida A, Nakamura K, Takeuchi M, Kobata A (1999) Unusually high expression of N-acetylglucosaminyltransferase-IVa in human choriocarcinoma cell lines: a possible
enzymatic basis of the formation of abnormal biantennary sugar chain. _Cancer Res_ 59: 3949–3953 CAS PubMed Google Scholar * World Health Organization (1983) _Gestational Trophoblastic
Diseases: Report of a WHO Scientific Group_ pp. 7–42. World Health Organization: Geneva * Yamamoto E, Ino K, Miyoshi E, Inamori K, Abe A, Sumigama S, Iwase A, Kajiyama H, Shibata K, Nawa A,
Kikkawa F (2009) N-acetylglucosaminyltransferase V regulates extravillous trophoblast invasion through glycosylation of alpha5beta1 integrin. _Endocrinology_ 150: 990–999 Article CAS
Google Scholar * Yamamoto E, Ino K, Miyoshi E, Shibata K, Takahashi N, Kajiyama H, Nawa A, Nomura S, Nagasaka T, Kikkawa F (2007) Expression of N-acetylglucosaminyltransferase V in
endometrial cancer correlates with poor prognosis. _Br J Cancer_ 97: 1538–1544 Article CAS Google Scholar * Yamamoto E, Ito T, Abe A, Sido F, Ino K, Itakura A, Mizutani S, Dovat S, Nomura
S, Kikkawa F (2005) Ikaros is expressed in human extravillous trophoblasts and involved in their migration and invasion. _Mol Hum Reprod_ 11: 825–831 Article CAS Google Scholar *
Yamashita K, Totani K, Iwaki Y, Takamisawa I, Tateishi N, Higashi T, Sakamoto Y, Kobata A (1989) Comparative study of the sugar chains of gamma-glutamyltranspeptidases purified from human
hepatocellular carcinoma and from human liver. _J Biochem_ 105: 728–735 Article CAS Google Scholar * Yamashita K, Totani K, Kuroki M, Matsuoka Y, Ueda I, Kobata A (1987) Structural
studies of the carbohydrate moieties of carcinoembryonic antigens. _Cancer Res_ 47: 3451–3459 CAS PubMed Google Scholar * Yoshida A, Minowa MT, Takamatsu S, Hara T, Oguri S, Ikenaga H,
Takeuchi M (1999) Tissue specific expression and chromosomal mapping of a human UDP-N-acetylglucosamine: alpha1,3-d-mannoside beta1, 4-N-acetylglucosaminyltransferase. _Glycobiology_ 9:
303–310 Article CAS Google Scholar * Zhao YY, Takahashi M, Gu JG, Miyoshi E, Matsumoto A, Kitazume S, Taniguchi N (2008) Functional roles of N-glycans in cell signaling and cell adhesion
in cancer. _Cancer Sci_ 99: 1304–1310 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank the laboratory of Dr Charles H Graham (Queen’s University, Kingston, ON,
Canada) for the generous gift of HTR-8/SVneo cells, and Ms Yuka Sakaguchi for her assistance with experiments. This work was supported by Grants-in-aid number 23592445 (to EY) from the
Japanese Ministry of Education, Culture, Sports, Science, and Technology. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Obstetrics and Gynecology, Nagoya University Graduate
School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan, K Niimi, E Yamamoto, S Fujiwara, K Shinjo, T Kotani, T Umezu, H Kajiyama, K Shibata & F Kikkawa * Department of
Obstetrics and Gynecology, Wakayama Medical University, 811-1 Kimiidera, Wakayama 641-0012, Japan, K Ino Authors * K Niimi View author publications You can also search for this author
inPubMed Google Scholar * E Yamamoto View author publications You can also search for this author inPubMed Google Scholar * S Fujiwara View author publications You can also search for this
author inPubMed Google Scholar * K Shinjo View author publications You can also search for this author inPubMed Google Scholar * T Kotani View author publications You can also search for
this author inPubMed Google Scholar * T Umezu View author publications You can also search for this author inPubMed Google Scholar * H Kajiyama View author publications You can also search
for this author inPubMed Google Scholar * K Shibata View author publications You can also search for this author inPubMed Google Scholar * K Ino View author publications You can also search
for this author inPubMed Google Scholar * F Kikkawa View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to E Yamamoto.
ADDITIONAL INFORMATION This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a
Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. RIGHTS AND PERMISSIONS From twelve months after its original publication, this work is licensed under the
Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Niimi, K., Yamamoto, E., Fujiwara, S. _et al._ High expression of _N_-acetylglucosaminyltransferase IVa promotes invasion of choriocarcinoma. _Br J
Cancer_ 107, 1969–1977 (2012). https://doi.org/10.1038/bjc.2012.496 Download citation * Received: 12 June 2012 * Revised: 09 October 2012 * Accepted: 18 October 2012 * Published: 20 November
2012 * Issue Date: 04 December 2012 * DOI: https://doi.org/10.1038/bjc.2012.496 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * beta1 integrin *
choriocarcinoma * human chorionic gonadotropin * invasion * _N_-acetylglucosaminyltransferase-IV






