- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND: Optimal cellular immunotherapy for cancer should ideally harness both the innate and adaptive arms of the immune response. Lymphokine-activated killer cells (LAKs) can
trigger early innate killing of tumour targets, whereas long-term adaptive-specific tumour control requires priming of CD8+ cytotoxic lymphocytes (CTLs) following acquisition of
tumour-associated antigens (TAAs) by antigen-presenting cells such as dendritic cells (DCs). As DCs stimulate both innate and adaptive effectors, combination cell therapy using LAKs and DCs
has the potential to maximise anti-tumour immune priming. METHODS: Reciprocal activation between human clinical grade LAKs and DCs on co-culture, and its immune consequences, was monitored
by cell phenotype, cytokine release and priming of both innate and adaptive cytotoxicity against melanoma targets. RESULTS: Co-culture of DCs and LAKs led to phenotypic activation of natural
killer (NK) cells within the LAK population, which was associated with increased production of inflammatory cytokines and enhanced innate cytotoxicity against tumour cell targets. The LAKs
reciprocally matured DCs, and the combination of LAKs and DCs, on addition of melanoma cells, supported priming of specific anti-tumour CTLs better than DCs alone. CONCLUSION: Clinical-grade
LAKs/DCs represents a practical, effective combination cell immunotherapy for stimulation of both innate and adaptive anti-tumour immunity in cancer patients. SIMILAR CONTENT BEING VIEWED
BY OTHERS DENDRITIC CELLS AS ORCHESTRATORS OF ANTICANCER IMMUNITY AND IMMUNOTHERAPY Article 07 February 2024 BCG PRIMING FOLLOWED BY A NOVEL INTERLEUKIN COMBINATION ACTIVATES NATURAL KILLER
CELLS TO SELECTIVELY PROLIFERATE AND BECOME ANTI-TUMOUR LONG-LIVED EFFECTORS Article Open access 07 June 2024 DENDRITIC CELLS IN CANCER IMMUNOLOGY Article Open access 03 September 2021 MAIN
Induction of efficient immunity against tumours requires a coordinated interplay between the innate and adaptive arms of the immune response. Dendritic cells (DCs) are components of the
early innate immune system and can also prime specific, adaptive responses. In particular, cross-presentation of tumour-associated antigens (TAAs) via MHC-I molecules on the surface of DCs
can expand specific cytotoxic lymphocytes (CTLs), potentially leading to a long-term anti-tumour memory response (Banchereau and Steinman, 1998). Natural killer (NK) cells are early innate
immune effectors that can exert direct nonspecific cytotoxicity against tumour cells (Srivastava et al, 2008). In addition to their role as initiators of antigen-specific responses, DCs also
support the tumouricidal activity of NK cells (Fernandez et al, 1999), and the extent of cross-talk between DCs and NK cells, leading to reciprocal activation of both cell subsets, has
become increasingly recognised (Kalinski et al, 2005; Semino et al, 2005). Significantly, NK/DC interactions can promote the generation of tumour-specific T-cell responses, whereby NK cells
function as nominal ‘helper’ cells to support adaptive anti-tumour immunity (Reschner et al, 2008). Lymphokine-activated killer cells (LAKs) are a heterogeneous population of cells
consisting primarily of NK, NKT and T cells, which are generated _in vitro_ by culture of peripheral blood mononuclear cells (PBMCs) in IL-2 (Grimm et al, 1982). The predominant effector
cells within LAKs are NK cells, which are mechanistically equivalent to peripheral blood NK cells, but are more cytotoxic against tumour cells, including otherwise NK-resistant targets
(Grimm et al, 1982). Moreover, LAKs are more readily cultured in large numbers for administration to patients than purified NK cells. LAKs have previously been shown to localise to tumour
sites in both mouse (Kjaergaard et al, 2000) and human systems (Keilholz et al, 1994), and therefore have the potential to access and lyse tumours in patients following systemic
administration _in vivo_. Although there was considerable clinical interest in LAKs for cancer therapy towards the end of the last century, their application for patients has not progressed,
in part due to concerns about the toxicity associated with IL-2, which had to be co-administered to maintain LAK activation _in vivo_ (Rosenberg, 1988). As with NK cells, there is evidence
that DCs can reciprocally activate LAKs _in vitro_ (Valteau-Couanet et al, 2002; Capobianco et al, 2006). A recent murine study has also shown that co-injection of LAKs and DCs into tumours
led to regression associated with protection against secondary rechallenge (Capobianco et al, 2008). These data suggest that combination LAK/DC therapy holds promise as a treatment for
cancer, based on the hypothesis that DC-activated LAKs will effectively kill tumour targets to liberate TAAs for uptake by DCs within an inflammatory tumour microenvironment. The TAA-loaded
mature DCs will then migrate to secondary lymphoid tissue and present TAAs to resident T cells for priming of an additional adaptive antigen-specific response. Moreover, activation of LAKs
by co-administered DCs may remove the need for IL-2 and hence reduce the toxicity associated with LAK therapy in the past. This preclinical study investigated the interaction between
clinically relevant LAKs and DCs, their ability to reciprocally activate each other, and the potential of the LAK/DC combination to prime both innate and adaptive immune responses against
melanoma in human _in vitro_ priming assays. We show that DCs are effectively matured by LAKs, while maintaining their phagocytic function for effective uptake of potential TAAs. In
parallel, LAK cytotoxicity is enhanced by co-culture with DCs, as is secretion of inflammatory IFN_γ_ and TNF_α_. Furthermore, the addition of LAKs to tumour cell co-cultures with DCs
increases specific CTL priming. Hence, LAKs/DCs have potential as a combination cell immunotherapy for priming of both innate and adaptive anti-tumour immunity. MATERIALS AND METHODS
DENDRITIC CELL CULTURE Buffy coats obtained from healthy donors or whole blood taken from melanoma patients (with written, informed consent in accordance with local institutional ethics
review and approval) were used to isolate PBMCs by Ficoll-Hypaque density centrifugation. Monocytes were isolated by MACS CD14+ selection (Miltenyi, Bergisch Gladbach, Germany) according to
manufacturer's instructions and were consistently found to be >95% pure. The CD14+ cells were additionally assessed for NK, NKT and T-cell contamination, and were found to be low
(average 2.6%, 1.5% and 4.8%, respectively). Immature DCs were generated from CD14+ monocytes in serum-free DC media (CellGro DC media; Cell Genix, Freiberg, Germany) supplemented with 800
IU ml−1 GMCSF (Peprotech, London, UK) and 500 IU ml−1 IL-4 (R&D Systems, Abingdon, UK) for 5 days. OK432 (Chugai Pharmaceutical Co., Tokyo, Japan) was used at 1000 IU ml−1 to generate
mature DCs (West et al, 2009). LYMPHOKINE-ACTIVATED KILLER CELL GENERATION CD14− PBMC, isolated following CD14 selection (as above), were routinely found to be negative for CD14+ cell
contamination (<5%). LAKs were generated from CD14− PBMC in LAK media (CellGro SCGM media; Cell Genix) supplemented with 5% (v/v) pooled human serum (Sera Labs, Haywards Heath, UK) and
1000 IU ml−1 IL-2 (Peprotech) for 5–7 days in non-coated tissue culture flasks (Berdeja et al, 2007). TUMOUR CELL LINES K562, Daudi and Skov3 cells were maintained in RPMI-1640 (Sigma,
Gillingham, UK) supplemented with 10% (v/v) FCS (BioSera, Ringmer, UK) and 1% (v/v) L-glutamine (Sigma). Melanoma cell lines (Mel888, Mel624, MeWo and SKMel-28) were maintained in DMEM
(Sigma) supplemented with 10% (v/v) FCS and 1% (v/v) L-glutamine. All cell lines were routinely tested for _mycoplasma_ and found to be free of infection. LYMPHOKINE-ACTIVATED KILLER CELL/DC
CO-CULTURES Freshly harvested LAKs and DCs were co-cultured together at a ratio of 10 : 1, in mixed culture media (50 : 50 LAK:DC media without cytokines) at a density of 2 × 106 ml−1 LAKs
for 48 h. Tumour cell lines were included at a 1 : 1 ratio with DCs at the onset of culture, as required. FLOW CYTOMETRY DCs and LAKs were analysed using the following antibodies with
appropriate isotype controls. _CD11c+ DCs_: anti-human CD14-PE, CD40-PE, CD83-PE, CD86-PE, HLA-DR-PE, MICA/B-PE, CD155-PE, MHC class 1-PE, ICAM-1-PE (BD Biosciences, Oxford, UK), CCR7-PE,
ULBP1-PE, ULBP2-PE, CD112-PE, CCR1-PE, CCR5-PE, CXCR1-PE and CXCR2-PE (R&D Systems). LAK (_CD3+CD56−, CD3-CD56+_ and _CD3+CD56+_): anti-human CD3-PerCP, CD56-FITC, CD8-FITC, CD16-FITC,
CD69-FITC, NKG2D-PE, CCR7-PE, DNAM-1-PE, NKp30-PE, NKp44-PE, LFA-1(CD18)-PE, CD40 L(CD154)-PE (BD Biosciences), CD56-PE (Serotec, Oxford, UK) and NKp46-PE (Miltenyi). Flow cytometry was
performed using a BD FACSCalibur and analysed using BD CellQuest Pro software. ELISA Supernatants from 48 h LAK/DC co-cultures were assessed for levels of IL-4, IL-5, IL-6, IL-8, IL-10,
IL-12 (p40 and p70), TNF_α_ and IFN_γ_ by ELISA using antibody matched pairs (all from BD Biosciences except TNF_α_, which was obtained from Invitrogen, Paisley, UK) according to the
manufacturer's instructions. DEGRANULATION ASSAY LAK degranulation as a response to target recognition was measured using CD107 surface expression as previously described (Prestwich et
al, 2008). Briefly, LAKs were co-cultured±DCs for 48 h before co-incubation with tumour targets (1 : 1 ratio LAK:tumour cell) for 4 h, before CD107 staining. LAKs were additionally stained
for CD3 and CD56. Analysis was performed by flow cytometry. CYTOTOXICITY ASSAY DC and LAK cytotoxicity was measured using a standard 4 h 51Cr release assay as previously described (Errington
et al, 2006). Briefly, 51Cr-labelled cell targets were incubated with LAKs, DCs or combination LAKs/DCs (pre-cultured together for 48 h) at varying effector to target (E:T) ratios. To
examine the perforin dependence of LAK cytotoxicity, E:T co-cultures were set up±2 mM EGTA (Sigma). Percent lysis was calculated using the following formula: % lysis=100 × (sample
c.p.m.−spontaneous c.p.m.)/(maximum c.p.m.−spontaneous c.p.m.). DENDRITIC CELL PHAGOCYTOSIS ASSAY DCs were pulsed with FITC-dextran (Sigma)±LAKs (1 : 10 ratio) for 60 min at 37°C (or 4°C
control). DCs were harvested at 15 min intervals, stained with CD11c-PE and then analysed by flow cytometry for FITC-dextran uptake. GENERATION OF TUMOUR-SPECIFIC CTL DCs were cultured with
Mel888 cells for 48 h, either in the presence or absence of LAKs, at 1 : 3 : 10 ratio, respectively, to permit loading of antigen on to the DCs, as previously described (Errington et al,
2006). These tumour-loaded DCs were then mixed with autologous PBMCs at a 1 : 30 ratio to generate tumour-specific CTLs. CTLs were re-stimulated 7 days later with a second population of
tumour-loaded DCs±LAKs. At 14 days, CTLs were harvested and used immediately in cytotoxicity studies against Mel888 and an irrelevant target (Skov3). STATISTICAL ANALYSES Statistical
analysis was performed using paired _t_-tests where _P_<0.05 denotes a significant result. RESULTS LYMPHOKINE-ACTIVATED KILLER CELLS ARE A HETEROGENEOUS POPULATION CONSISTING OF NK, NKT
AND T CELLS This preclinical study used Good Manufacturing Practice-compliant components to generate both LAKs and DCs, to ensure translational relevance for application in human clinical
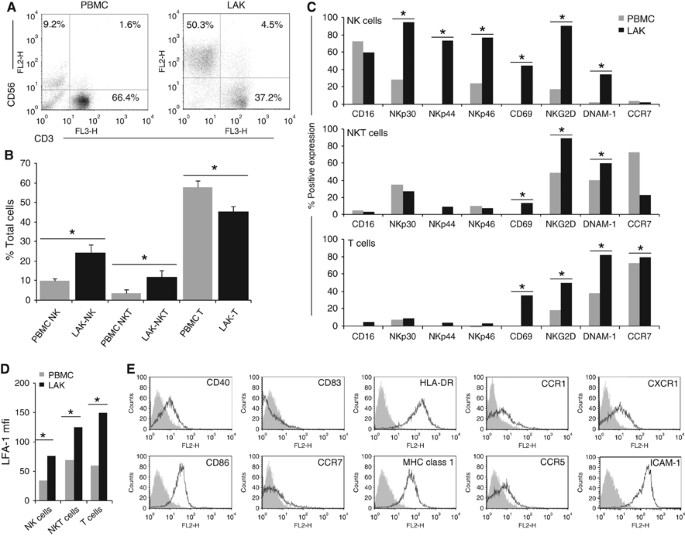
trials. Initial phenotypic analysis of LAKs alone revealed a mixed population consisting predominantly of CD3-CD56+ cells (NK-LAK), CD3+CD56+ cells (NKT-LAK) and CD3+CD56− cells (T-LAK). The
relative proportions of the three sub-populations significantly changed during LAK generation by culture of PBMCs in IL-2 (Figure 1A and B). The NK cells increased from 10.1±1.2% in PBMCs
to 24.2±4.1% in LAK, NKT cells from 3.9±1.9% to 11.7±3.5%, whereas the T-cell population reduced from 57.7±3.6% to 45.3±2.7%. Investigations into changes in expression of cell surface
molecules during LAK cell generation were performed on these three predominant cell types (Figure 1C and D). Cells of interest were initially identified by their CD3/CD56 expression, as
designated in Figure 1A, before analysis of specific activation markers. A significant increase in expression of both NK-specific activation markers (NKp30, NKp44 and NKp46) and general
activation markers (CD69, NKG2D and DNAM-1) in IL-2-activated NK-LAK, in comparison with resting NK cells in PBMCs, was observed. In agreement with other studies, NKp30 and NKp46 were
detected at low levels on resting NK cells and increased on activation, whereas NKp44 was found only on activated cells (Bryceson et al, 2006). The NK-LAK CD16 levels were high at the onset
of culture and remained so, whereas CCR7 expression was consistently low. CD40L staining was low or absent on NK-, NKT- and T-LAK (data not shown). The NKT-LAK increased expression of CD69,
NKG2D and DNAM-1 compared with NKT in PBMCs, although NK-specific markers remained low or absent (Figure 1C). As expected, the T-cell fraction of PBMCs or LAKs exhibited very low positivity
for all NK-specific receptors; however, other activation markers and CCR7 increased following LAK generation (Figure 1C). The adhesion molecule LFA-1 was found on all subsets, and its
expression uniformly increased following culture in IL-2 (Figure 1D). CLINICAL GRADE DCS EXHIBIT AN IMMATURE PHENOTYPE CD11c-positive clinical DCs were subjected to phenotype analysis of
cell surface markers and found to be positive for CD40, CD86, MHC-I and class II (HLA-DR) (Figure 1E), but negative for CD83, CCR7 and CD14 (data not shown): a phenotype indicative of
immature DCs (West et al, 2009). Other markers, including ligands for activating NK receptors (MICA/B, ULBP1 and 2, CD112), were not expressed (data not shown). ICAM-1 was highly expressed
(Figure 1E), correlating with high expression of its receptor, LFA-1, on LAK (Figure 1D). Staining for chemokine/cytokine receptors (CCR1, CCR5 and CXCR1) was also positive (Figure 1E).
DENDRITIC CELLS ACTIVATE LAKS Experiments were performed to explore potential reciprocal activation between clinical grade LAKs and DCs. Expression of NK-LAK cell surface markers is shown in
Figure 2. There was significant upregulation of CD16, NKp30, NKp44 and NKp46 on NK-LAK when co-cultured with DCs for 48 h. Stimulation of the early activation marker CD69 was also apparent,
as was upregulation of DNAM-1. However, levels of both NKG2D (already highly expressed) and CCR7 (consistently low) on NK-LAK were not significantly altered by DCs. A similar trend of
DC-induced activation was also seen on NKT-LAK and T-LAK (for non-NK specific markers only), although this did not reach statistical significance (Supplementary Figure 1). The LFA-1 on
NK-LAK was further enhanced by DCs (data not shown). LYMPHOKINE-ACTIVATED KILLER CELLS INDUCE DC MATURATION We next addressed the effects of LAKs on DCs. Although previous work has shown
that DCs can be lysed by autologous NK/LAK cells (Parajuli et al, 1999), there was no significant killing of DCs by LAK in our clinical grade system (Supplementary Figure 2). Analogous to
DC-induced LAK activation, LAKs effectively matured autologous DCs on co-culture (Figure 3B). Maturation was apparent from significant upregulation of CD83, CCR7 and MHC-I expression.
Enhancement of CD40 and CD86 was also seen, although this did not reach statistical significance over all six donors. An increase in the levels of the cytokine/chemokine receptors CCR1,
CCR5, CCR7, CXCR1 and CXCR2 on DCs in the presence of LAKs was also seen, as was upregulation of the activating NK receptor ligands, MICA/B, ULBP1 and ULBP2 (ligands for NKG2D), as well as
CD112 and CD155 (ligands for DNAM-1). In addition, we observed some increase in the already high levels of ICAM-1, although this was donor dependent (data not shown). To assess this
combination therapy within a clinical setting, we performed additional experiments with blood taken from melanoma patients with metastatic disease (stage IV), not on active current
treatment. The data demonstrate that LAKs and DCs can be successfully generated from patient PBMCs, and that they similarly cross-activate each other, as demonstrated by enhanced expression
of cell surface activation/maturation molecules (Supplementary Figure 3). We also tested the phagocytic capacity of DCs co-cultured with LAKs, to determine whether LAK-matured DCs retained
the ability to take up TAAs potentially released by LAK-lysed tumour cells. For this, uptake of FITC-dextran by DCs was measured±LAK. Figure 3C demonstrates that, even on co-culture with
maturation-inducing LAKs, DCs remained functional for uptake of exogenous material, an essential step for DC-mediated priming of an adaptive anti-tumour CTL response. RECIPROCALLY ACTIVATED
LAKS/DCS SECRETE INFLAMMATORY CYTOKINES The cytokine profile of LAK/DC co-culture supernatants was measured by ELISA (Figure 4). Increases in secretion of the inflammatory cytokines IL-6,
IL-8 and IFN_γ_ were observed, together with a nonsignificant trend for IL-12p40 and TNF_α_, in LAK/DC co-cultures compared with LAKs and/or DCs alone. However, IL-12p70 remained
undetectable (data not shown), whereas anti-inflammatory IL-10 production by DCs showed a trend to reduction on co-culture with LAKs, although this was donor dependent and not statistically
significant across multiple donors. MELANOMA CELLS DO NOT INHIBIT LAK/DC RECIPROCAL ACTIVATION Melanoma cells are known to suppress the function of immune cells, including DCs and NK cells
(Real et al, 2001). As we are proposing LAKs/DCs as a cytotoxic and immunogenic combination cell therapy on interaction with tumour cell targets, we next investigated whether the presence of
melanoma cells would have a deleterious effect on the cross-activation between LAKs and DCs described above. The LAKs/DCs were therefore co-cultured with the human melanoma cell lines
Mel888 or MeWo, which were found to have no significant effect on reciprocal LAK/DC activation, as demonstrated both by cell phenotype data and cytokine production (Supplementary Figures 4
and 5). DENDRITIC CELLS ENHANCE PERFORIN-DEPENDENT LAK CYTOTOXICITY AGAINST TUMOUR TARGETS To test whether DC-mediated activation of LAKs enhanced their killing of tumour targets, cytotoxic
activity was measured using standard 4 h 51Cr-release assays. The LAK, DC and LAK/DC killing of four melanoma cell lines (alongside NK-sensitive K562 and NK-resistant Daudi targets) is shown
in Figure 5A. As expected, DCs alone did not kill tumour targets. LAKs exhibited significant cytotoxicity against both K562 and Daudi cells, and were also variably active against the
different melanoma lines. Pre-activation of LAKs by DCs resulted in consistent and significantly enhanced killing of the majority of tumour targets (∼20% over that observed for LAKs alone at
all E:T ratios). Substantial killing of melanoma targets was also seen using patient-derived LAKs, which was enhanced by pre-incubation with DCs (Supplementary Figure 3). As 51Cr-release
assays measure target killing by LAKs as an entire population, to distinguish the relative cytolytic activity of the cell populations within LAKs, degranulation assays were used. The degree
of CD107 expression was measured on the surface of LAKs during exposure to Daudi and Mel888, following pre-culture±DCs for 48 h (Figure 5B and C). The results show the NK-LAK fraction
degranulated most following co-culture with targets. Although there was measurable background degranulation of both NK-LAK and NKT-LAK in the absence of targets, only CD107 on NK-LAK
increased against Daudi and Mel888 following previous activation of LAKs by DCs. T-LAKs did not degranulate under any conditions tested. To determine the mechanism of cytotoxicity utilised
by LAKs, we repeated Cr51-release assays in the presence of EGTA (Figure 5D). The reduction in LAK (and LAK/DC) cytotoxicity in the presence of EGTA, which chelates the calcium required for
perforin release, is consistent with our previous data demonstrating that DC-stimulated NK cell cytotoxicity is a perforin-dependent process (Errington et al, 2008). LYMPHOKINE-ACTIVATED
KILLER CELLS ENHANCE THE ABILITY OF DCS TO PRIME AN ANTIGEN-SPECIFIC CTL RESPONSE Data thus far has demonstrated an effective interaction between DCs and LAKs, resulting in reciprocal
activation and enhancement of nonspecific innate immune killing of melanoma targets, predominantly by NK-LAK. As DCs form a direct link between innate and adaptive immune responses, we
investigated the potential of LAKs/DCs to support adaptive priming of specific CTLs subsequent to early innate tumour killing. Tumour-specific CTLs were generated against Mel888 targets
using DCs alone or LAKs/DCs in an MHC class I-dependent _in vitro_ human CTLs priming assay (Errington et al, 2006). As anticipated, a positive control comprising DCs matured with the
bacterial adjuvant OK432 (West et al, 2009) induced an adaptive Mel888-specific response in autologous T cells, whereas CTL priming by immature DCs alone was ineffective (Figure 6). However,
the combination of LAKs and immature DCs resulted in potent specific CTL priming, with even higher levels of killing than those elicited by OK432-matured DCs. Hence, the ability of
co-cultured LAKs to kill targets and/or mature DCs improves adaptive anti-tumour immune priming. DISCUSSION The aim of this preclinical study was to investigate the innate and adaptive
immune anti-tumour potential of a deliverable, clinical grade human LAK/DC combination cell therapy for the treatment of cancer, in particular melanoma. LAKs are a clinically applicable
heterogeneous population of innate cytotoxic cells consisting predominantly of NK, NKT and T cells. On _ex vivo_ culture of human PBMCs in IL-2, we observed an expansion in NK and NKT cells,
with a corresponding reduction in T cells (Figure 1B). This is consistent with previously published data (Valteau-Couanet et al, 2002; Capobianco et al, 2006), suggesting that NK cells are
the main effectors within LAKs. The NK-LAKs were activated compared with ‘resting’ NK cells in PBMCs, as indicated by expression of both general surface activation markers (CD69, NKG2D and
DNAM-1) and the NK-specific natural cytotoxicity receptors (NCRs) NKp30, NKp44 and NKp46; NKT-LAKs and T-LAKs were also activated by IL-2 (Figure 1C). The DCs used in this study exhibited a
typical immature phenotype with low CD83 and CCR7 expression, high MHC-I and II and positive staining for CCR1, CCR5 and CXCR1 (Figure 1E). Notably, ligands for NK activating receptors were
initially present at low levels on DCs (Figure 3B), whereas the adhesion molecule ICAM-1 (the ligand for LFA-1 as expressed on LAKs, Figure 1D) was consistently expressed at high levels.
Mouse and human LAK/NK cells are known to activate DCs _in vitro_ (Valteau-Couanet et al, 2002; Capobianco et al, 2006) and vice versa (Kalinski et al, 2005; Yano et al, 2006), and we found
similar reciprocal activation in our clinical grade LAK/DC co-cultures. The DC-mediated LAK activation was evident from further increased expression of NCRs on NK-LAK, and CD69, DNAM-1 and
NKG2D on NK-, NKT- and T-LAK (Figure 2). In parallel, LAKs induced maturation of DCs (Figure 3B). Multiple mechanisms are thought to underlie NK/DC cross-talk, including contact-dependent
interactions involving CD40/CD40L (Yano et al, 2006), NKp30 (Vitale et al, 2005) and DNAM-1 (Chan et al, 2010) in cooperation with NKp30 (Balsamo et al, 2009). Although the detailed
interactions between LAKs/DCs are unknown, our data clearly show that following co-culture: (i) NCR on NK-LAK were upregulated (Figure 2) and (ii) known ligands for NK cell activating
receptors NKG2D (MICA/B, ULBP1 and ULBP2) and DNAM-1 (CD112 and CD155) were increased on DCs (Figure 3). LFA-1 present on LAKs, alongside ICAM-1 expression on DCs, was also further enhanced
during co-culture. LFA-1 and ICAM-1 are involved in cell–cell interaction during an inflammatory response, and LFA-1 is particularly important during NK interaction with antigen-presenting
cells and T cells (Borg et al, 2004). Taken together, these findings are consistent with LAK/DC co-culture enhancing the effector functions of NK-LAK via direct cellular cross-talk. In
addition to cell–cell contact, cytokines are also proposed to have a role in NK or LAK/DC cross-activation, and may also direct the nature of the innate and adaptive immune responses they
elicit _in vivo_. Enhanced levels of IL-6 (Figure 4) detected in LAK/DC co-cultures can stimulate NK and LAK cell proliferation and cytotoxicity (Iho and Shau, 1994) and neutralise
regulatory T-cell suppression to facilitate T-cell activation by DCs (Schmidt et al, 2004; Detournay et al, 2005). Immunosuppressive IL-10 production by DCs was variably inhibited in the
presence of LAKs (Figure 4), a finding consistent with previous data (Schmidt et al, 2004). Significant secretion of IL-8 by LAK/DC co-cultures (Figure 4) was detected alongside upregulated
CXCR1 and 2 expression on DCs (Figure 3B). As a major function of IL-8 is to induce chemotaxis of cells expressing its receptors (CXCR1 and CXCR2) to sites of inflammation in the initial
phases of an innate response (Mukaida, 2000), this data supports the potential for LAK/DC-derived IL-8 to sustain additional endogenous effector cell trafficking into targeted tumour sites
_in vivo_. Increased IFN_γ_ and TNF_α_ were also observed in LAK/DC co-cultures (Figure 4). The NK-secreted IFN_γ_ is postulated to be essential for DC maturation (Kalinski et al, 2005;
Capobianco et al, 2006), whereas both IFN_γ_ and TNF_α_ contribute to induction of stable type-1 polarised DC (so-called ‘DC1’) (Kalinski et al, 2005). Hence, if LAKs mature co-administered
and/or endogenous DCs _in vivo_ in the context of IFN_γ_/TNF_α_, these DCs have the potential to migrate to lymph nodes via CCR7-mediated chemotaxis (Figure 3B) to enhance Th1-adaptive
priming. Although LAKs matured DCs (Figure 3B), these DCs could still take up FITC-dextran (Figure 3C), illustrating their competence for acquiring exogenous material from their immediate
environment, an important function for cross-priming of TAAs potentially released by dying tumour cells _in vivo_. Moreover, the presence of melanoma cells did not inhibit cross-activation
between LAKs and DCs _in vitro_ in terms of phenotype and cytokine production (Supplementary Figures 4 and 5), suggesting that LAKs/DCs may be able to reverse immunosuppression to induce an
inflammatory microenvironment within tumours, appropriate for induction of therapeutic immune priming. Consistent with previous reports that DCs can stimulate NK cytotoxicity against tumour
targets (Valteau-Couanet et al, 2002; Capobianco et al, 2006), we found that DCs enhanced innate tumour cell killing by LAKs (Figure 5A). The NK cells were the predominant population within
DC-activated LAKs, which degranulated against targets (Figure 5B and C). Moreover, NK-LAK lysed archetypal NK-resistant Daudi cells, as well as classical NK-susceptible targets (K562;
Friberg et al, 1996), further demonstrating their potency over that of isolated NK cells. Consistent with our previous data on DC-activated NK cells (Errington et al, 2008), this killing was
perforin-mediated (Figure 5D). Hence, the phenotypic activation of LAKs by DCs (Figure 2) led to enhanced innate cytotoxicity. In addition to early direct tumour eradication by LAKs/DCs as
a combination cell therapy, we wished to address whether the presence of LAKs could enhance the anti-tumour CTL priming efficacy of DCs, thereby forming a link between the innate and
adaptive potential of cellular immunotherapy. DCs can modulate the innate responses of NK cells (Kalinski et al, 2005), NKT cells (Takahashi et al, 2002) and _γδ_T cells (Dieli et al, 2004)
to potentially connect with an adaptive anti-tumour memory immune response (Capobianco et al, 2008). Moreover, DC/NK cell interactions can circumvent the need for CD4+ T cell help during
induction of CTLs (Adam et al, 2005), further increasing the appeal of adding LAKs to DCs for enhanced adaptive priming. Figure 6 shows, in an established human _in vitro_ system for
assessing generation of specific anti-melanoma CTLs, that LAKs consistently improved DC-mediated CTL priming to a level greater than that of OK432-matured DCs. Although it is currently
unclear whether the benefit of LAKs in this context is due to their tumour-killing capability leading to enhanced antigen release, their ability to mature DCs, or a combination of the two,
this data suggests that a LAK/DC combination has the potential to enhance adaptive as well as innate anti-tumour effects in patients _in vivo_. In summary, this study shows that clinical
grade LAKs and DCs can be readily generated _in vitro_ in large numbers, both from healthy donors and patients with advanced melanoma, using established methodologies which have already been
applied separately and safely in the clinic. As DCs maintain and activate LAKs, the combination could be applied _in vivo_ without addition of toxic, systemic IL-2. Functionally, innate
direct cytotoxicity of LAKs is improved by DCs, and adaptive CTL priming by DCs is enhanced by the presence of LAKs. This data supports the development of LAKs/DCs as a practical, clinically
deliverable combination cellular immunotherapy for the treatment of cancer, and a clinical protocol in melanoma is currently in development. CHANGE HISTORY * _ 29 MARCH 2012 This paper was
modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Adam C, King S, Allgeier T, Braumuller H, Luking C,
Mysliwietz J, Kriegeskorte A, Busch DH, Rocken M, Mocikat R (2005) DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. _Blood_ 106 (1): 338–344
Article CAS Google Scholar * Balsamo M, Zambello R, Teramo A, Pedrazzi M, Sparatore B, Scordamaglia F, Pende D, Mingari MC, Moretta L, Moretta A, Semenzato G, Vitale M (2009) Analysis of
NK cell/DC interaction in NK-type lymphoproliferative disease of granular lymphocytes (LDGL): role of DNAM-1 and NKp30. _Exp Hematol_ 37 (10): 1167–1175 Article CAS Google Scholar *
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. _Nature_ 392 (6673): 245–252 Article CAS Google Scholar * Berdeja JG, Hess A, Lucas DM, O’Donnell P, Ambinder
RF, Diehl LF, Carter-Brookins D, Newton S, Flinn IW (2007) Systemic interleukin-2 and adoptive transfer of lymphokine-activated killer cells improves antibody-dependent cellular
cytotoxicity in patients with relapsed B-cell lymphoma treated with rituximab. _Clin Cancer Res_ 13 (8): 2392–2399 Article CAS Google Scholar * Borg C, Jalil A, Laderach D, Maruyama K,
Wakasugi H, Charrier S, Ryffel B, Cambi A, Figdor C, Vainchenker W, Galy A, Caignard A, Zitvogel L (2004) NK cell activation by dendritic cells (DCs) requires the formation of a synapse
leading to IL-12 polarization in DCs. _Blood_ 104 (10): 3267–3275 Article CAS Google Scholar * Bryceson YT, March ME, Ljunggren HG, Long EO (2006) Activation, coactivation, and
costimulation of resting human natural killer cells. _Immunol Rev_ 214: 73–91 Article CAS Google Scholar * Capobianco A, Manfredi AA, Monno A, Rovere-Querini P, Rugarli C (2008) Melanoma
and lymphoma rejection associated with eosinophil infiltration upon intratumoral injection of dendritic and NK/LAK cells. _J Immunother_ 31 (5): 458–465 Article Google Scholar * Capobianco
A, Rovere-Querini P, Rugarli C, Manfredi AA (2006) Melanoma cells interfere with the interaction of dendritic cells with NK/LAK cells. _Int J Cancer_ 119 (12): 2861–2869 Article CAS
Google Scholar * Chan CJ, Andrews DM, McLaughlin NM, Yagita H, Gilfillan S, Colonna M, Smyth MJ (2010) DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly
immunogenic melanoma metastases. _J Immunol_ 184 (2): 902–911 Article CAS Google Scholar * Detournay O, Mazouz N, Goldman M, Toungouz M (2005) IL-6 produced by type I IFN DC controls
IFN-gamma production by regulating the suppressive effect of CD4+ CD25+ regulatory T cells. _Hum Immunol_ 66 (5): 460–468 Article CAS Google Scholar * Dieli F, Caccamo N, Meraviglia S,
Ivanyi J, Sireci G, Bonanno CT, Ferlazzo V, La Mendola C, Salerno A (2004) Reciprocal stimulation of gammadelta T cells and dendritic cells during the anti-mycobacterial immune response.
_Eur J Immunol_ 34 (11): 3227–3235 Article CAS Google Scholar * Errington F, Jones J, Merrick A, Bateman A, Harrington K, Gough M, O’Donnell D, Selby P, Vile R, Melcher A (2006) Fusogenic
membrane glycoprotein-mediated tumour cell fusion activates human dendritic cells for enhanced IL-12 production and T-cell priming. _Gene Ther_ 13 (2): 138–149 Article CAS Google Scholar
* Errington F, Steele L, Prestwich R, Harrington KJ, Pandha HS, Vidal L, de Bono J, Selby P, Coffey M, Vile R, Melcher A (2008) Reovirus activates human dendritic cells to promote innate
antitumor immunity. _J Immunol_ 180 (9): 6018–6026 Article CAS Google Scholar * Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T,
Maraskovsky E, Zitvogel L (1999) Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses _in vivo_. _Nat Med_ 5 (4): 405–411 Article
CAS Google Scholar * Friberg DD, Bryant JL, Whiteside TL (1996) Measurements of natural killer (NK) activity and NK-cell quantification. _Methods_ 9 (2): 316–326 Article CAS Google
Scholar * Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA (1982) Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin
2-activated autologous human peripheral blood lymphocytes. _J Exp Med_ 155 (6): 1823–1841 Article CAS Google Scholar * Iho S, Shau H (1994) Role of enhanced cellular adhesion in
IL-6-augmented lymphokine-activated killer-cell function. _Scand J Immunol_ 39 (3): 233–240 Article CAS Google Scholar * Kalinski P, Mailliard RB, Giermasz A, Zeh HJ, Basse P, Bartlett
DL, Kirkwood JM, Lotze MT, Herberman RB (2005) Natural killer-dendritic cell cross-talk in cancer immunotherapy. _Expert Opin Biol Ther_ 5 (10): 1303–1315 Article CAS Google Scholar *
Keilholz U, Scheibenbogen C, Brado M, Georgi P, Maclachlan D, Brado B, Hunstein W (1994) Regional adoptive immunotherapy with interleukin-2 and lymphokine-activated killer (LAK) cells for
liver metastases. _Eur J Cancer_ 30A (1): 103–105 Article CAS Google Scholar * Kjaergaard J, Hokland ME, Agger R, Skovbo A, Nannmark U, Basse PH (2000) Biodistribution and tumor
localization of lymphokine-activated killer T cells following different routes of administration into tumor-bearing animals. _Cancer Immunol Immunother_ 48 (10): 550–560 Article CAS Google
Scholar * Mukaida N (2000) Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. _Int J Hematol_ 72 (4): 391–398 CAS PubMed Google Scholar * Parajuli P,
Nishioka Y, Nishimura N, Singh SM, Hanibuchi M, Nokihara H, Yanagawa H, Sone S (1999) Cytolysis of human dendritic cells by autologous lymphokine-activated killer cells: participation of
both T cells and NK cells in the killing. _J Leukoc Biol_ 65 (6): 764–770 Article CAS Google Scholar * Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T, Thompson J,
Morrison EE, Harrington KJ, Pandha HS, Selby PJ, Vile RG, Melcher AA (2008) Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. _Clin Cancer Res_ 14 (22): 7358–7366
Article CAS Google Scholar * Real LM, Jimenez P, Kirkin A, Serrano A, Garcia A, Canton J, Zeuthen J, Garrido F, Ruiz-Cabello F (2001) Multiple mechanisms of immune evasion can coexist in
melanoma tumor cell lines derived from the same patient. _Cancer Immunol Immunother_ 49 (11): 621–628 Article CAS Google Scholar * Reschner A, Hubert P, Delvenne P, Boniver J, Jacobs N
(2008) Innate lymphocyte and dendritic cell cross-talk: a key factor in the regulation of the immune response. _Clin Exp Immunol_ 152 (2): 219–226 Article CAS Google Scholar * Rosenberg
SA (1988) Immunotherapy of patients with advanced cancer using interleukin-2 alone or in combination with lymphokine activated killer cells. _Important Adv Oncol:_ 217–257 * Schmidt J,
Eisold S, Buchler MW, Marten A (2004) Dendritic cells reduce number and function of CD4+CD25+ cells in cytokine-induced killer cells derived from patients with pancreatic carcinoma. _Cancer
Immunol Immunother_ 53 (11): 1018–1026 Article CAS Google Scholar * Semino C, Angelini G, Poggi A, Rubartelli A (2005) NK/iDC interaction results in IL-18 secretion by DCs at the synaptic
cleft followed by NK cell activation and release of the DC maturation factor HMGB1. _Blood_ 106 (2): 609–616 Article CAS Google Scholar * Srivastava S, Lundqvist A, Childs RW (2008)
Natural killer cell immunotherapy for cancer: a new hope. _Cytotherapy_ 10 (8): 775–783 Article CAS Google Scholar * Takahashi T, Chiba S, Nieda M, Azuma T, Ishihara S, Shibata Y, Juji T,
Hirai H (2002) Cutting edge: analysis of human V alpha 24+CD8+ NK T cells activated by alpha-galactosylceramide-pulsed monocyte-derived dendritic cells. _J Immunol_ 168 (7): 3140–3144
Article CAS Google Scholar * Valteau-Couanet D, Leboulaire C, Maincent K, Tournier M, Hartmann O, Benard J, Beaujean F, Boccaccio C, Zitvogel L, Angevin E (2002) Dendritic cells for
NK/LAK activation: rationale for multicellular immunotherapy in neuroblastoma patients. _Blood_ 100 (7): 2554–2561 Article CAS Google Scholar * Vitale M, Della Chiesa M, Carlomagno S,
Pende D, Arico M, Moretta L, Moretta A (2005) NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. _Blood_ 106 (2):
566–571 Article CAS Google Scholar * West E, Morgan R, Scott K, Merrick A, Lubenko A, Pawson D, Selby P, Hatfield P, Prestwich R, Fraser S, Eves D, Anthoney A, Twelves C, Beirne D, Patel
P, O’Donnell D, Watt S, Waller M, Dietz A, Robinson P, Melcher A (2009) Clinical grade OK432-activated dendritic cells: _in vitro_ characterization and tracking during intralymphatic
delivery. _J Immunother_ 32 (1): 66–78 Article CAS Google Scholar * Yano Y, Ueda Y, Itoh T, Fuji N, Okugawa K, Naito K, Imura K, Kohara J, Hayashi T, Nakane K, Matsuura Y, Kawai K,
Yamagishi H (2006) A new strategy using autologous dendritic cells and lymphokine-activated killer cells for cancer immunotherapy: efficient maturation of DCs by co-culture with LAK cells
_in vitro_. _Oncol Rep_ 16 (1): 147–152 CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the Leeds Experimental Cancer Medicine Centre, the Leeds
Cancer Research UK Centre and the Leeds Cancer Vaccine Appeal. AUTHOR CONTRIBUTIONS EJW prepared the manuscript. AUTHOR INFORMATION Author notes * E J West and K J Scott: These authors
contributed equally to this work. AUTHORS AND AFFILIATIONS * Cancer Research UK Clinical Centre, St James's University Hospital, Beckett Street, Leeds, LS9 7TF, UK E J West, K J Scott,
V A Jennings & A A Melcher Authors * E J West View author publications You can also search for this author inPubMed Google Scholar * K J Scott View author publications You can also
search for this author inPubMed Google Scholar * V A Jennings View author publications You can also search for this author inPubMed Google Scholar * A A Melcher View author publications You
can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to A A Melcher. ADDITIONAL INFORMATION This work is published under the standard license to
publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies the paper on British Journal of Cancer website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 (PPT 180 KB) SUPPLEMENTARY FIGURE 2 (PPT 153 KB)
SUPPLEMENTARY FIGURE 3 (PPT 238 KB) SUPPLEMENTARY FIGURE 4 (PPT 187 KB) SUPPLEMENTARY FIGURE 5 (PPT 230 KB) SUPPLEMENTARY FIGURE LEGENDS (DOC 20 KB) RIGHTS AND PERMISSIONS From twelve months
after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE West, E., Scott, K., Jennings, V. _et al._ Immune activation by combination
human lymphokine-activated killer and dendritic cell therapy. _Br J Cancer_ 105, 787–795 (2011). https://doi.org/10.1038/bjc.2011.290 Download citation * Revised: 21 June 2011 * Accepted: 06
July 2011 * Published: 16 August 2011 * Issue Date: 06 September 2011 * DOI: https://doi.org/10.1038/bjc.2011.290 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative KEYWORDS * dendritic cells * lymphokine-activated killer cells * natural killer cells * melanoma * immunotherapy






