- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND: Advances in the treatment of metastatic colorectal cancer (mCRC) in the last decade have significantly improved survival; however, simple biomarkers to predict response
or toxicity have not been identified, which are applicable to all community oncology settings worldwide. The use of inflammatory markers based on differential white-cell counts, such as the
neutrophil/lymphocyte ratio (NLR), may be simple and readily available biomarkers. METHODS: Clinical information and baseline laboratory parameters were available for 349 patients, from two
independent cohorts, with unresectable mCRC receiving first-line palliative chemotherapy. Associations between baseline prognostic variables, including inflammatory markers such as the NLR
and tumour response, progression and survival were investigated. RESULTS: In the training cohort, combination-agent chemotherapy (_P_=0.001) and NLR⩽5 (_P_=0.003) were associated with
improved clinical benefit. The ECOG performance status ⩾1 (_P_=0.002), NLR>5 (_P_=0.01), hypoalbuminaemia (_P_=0.03) and single-agent chemotherapy (_P_<0.0001) were associated with
increased risk of progression. The ECOG performance status ⩾1 (_P_=0.004) and NLR>5 (_P_=0.002) predicted worse overall survival (OS). The NLR was confirmed to independently predict OS in
the validation cohort (_P_<0.0001). Normalisation of the NLR after one cycle of chemotherapy in a subset of patients resulted in improved progression-free survival (_P_=0.012).
CONCLUSION: These results have highlighted NLR as a potentially useful clinical biomarker of systemic inflammatory response in predicting clinically meaningful outcomes in two independent
cohorts. Results of this study have also confirmed the importance of a chronic systemic inflammatory response influencing clinical outcomes in patients with mCRC. SIMILAR CONTENT BEING
VIEWED BY OTHERS THE PROGNOSTIC AND DIAGNOSTIC SIGNIFICANCE OF THE NEUTROPHIL-TO-LYMPHOCYTE RATIO IN HEPATOCELLULAR CARCINOMA: A PROSPECTIVE CONTROLLED STUDY Article Open access 14 June 2021
CLINICAL SIGNIFICANCE OF BASELINE PAN-IMMUNE-INFLAMMATION VALUE AND ITS DYNAMICS IN METASTATIC COLORECTAL CANCER PATIENTS UNDER FIRST-LINE CHEMOTHERAPY Article Open access 27 April 2022 THE
COMBINATION OF PRE-NEOADJUVANT CHEMORADIOTHERAPY INFLAMMATION BIOMARKERS COULD BE A PROGNOSTIC MARKER FOR RECTAL CANCER PATIENTS Article Open access 11 March 2022 MAIN Colorectal cancer
(CRC) is the third leading cause of worldwide cancer mortality after lung and stomach cancer and is responsible for 639 000 deaths or 1.1% of total deaths (World Health Organisation, 2004).
There have been major advances in the treatment of metastatic CRC (mCRC) in the last 10–15 years, involving the introduction of new cytotoxic and molecular targeted therapies. However, use
of these newer treatments result in increased toxicities and are prohibitively expensive. Hence, there is a need for accurate predictors of outcomes from treatment, in particular, in
identifying those patients who are more likely to benefit by being assisted in rationalising increasingly expensive treatments, especially in under-resourced communities. Tumour development
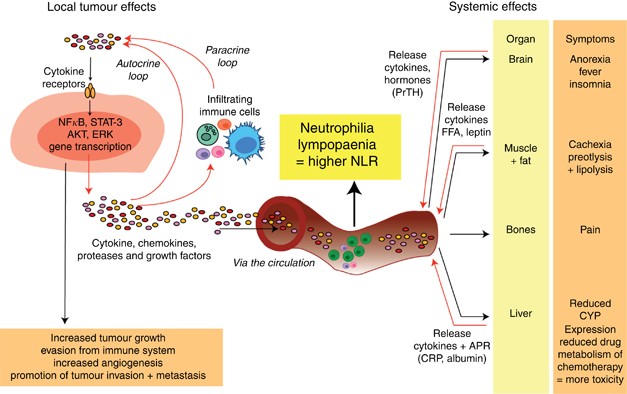
and growth occurs as a result of interactions among the tumour, host-derived stromal tissues including blood vessels and host immune/inflammatory cells (see Figure 1), with chronic
inflammation having an important role in cancer development and progression (Balkwill and Mantovani, 2010; Coussens and Werb, 2002). Lymphocytic infiltration in primary colorectal tumour
tissue with different lymphocyte subpopulations has been investigated as potential prognostic factors (Pagès et al, 2005; Galon et al, 2006). This chronic inflammatory state also has effects
on normal tissues, including the liver, resulting in an ongoing release of ‘acute-phase proteins’ that may be used to monitor this process. Current prognostication in advanced CRC, as in
other malignancies, involves a poorly defined combination of clinical experience with the use of relatively crude and subjective covariates, such as performance status, with few markers in
clinical practice apart from the use of _k-ras_ mutation status and treatment with epidermal growth-factor receptor inhibitors (Bokemeyer et al, 2009; Koopman et al, 2009; Van Cutsem et al,
2009; Chua et al, 2010). Over the last 10 years, laboratory markers of a systemic inflammatory response, including plasma C-reactive protein concentration (CRP), hypoalbuminaemia and Glasgow
Prognostic Score (GPS, which combines CRP and albumin), and absolute white cell or its components (neutrophils, neutrophil/lymphocyte ratios (NLRs) and platelet/lymphocyte ratios (PLRs))
have been investigated as prognostic and predictive markers in different cancer populations, with the best evidence for their use demonstrated in surgical patients with CRC (Roxburgh and
McMillan, 2010). Emerging evidence suggests that elevated baseline levels of CRP (Hilmy et al, 2005; Crumley et al, 2006a; Mitsuru et al, 2009; Johnson et al, 2010), abnormal GPS scores
(Forrest et al, 2003; Crumley et al, 2006b; McMillan, 2009) and elevated NLR (Yamanaka et al, 2007; Halazun et al, 2008, 2009; Liu et al, 2010) or PLR (Smith et al, 2009) are not only
negatively correlated with outcome after surgical resection but also in those with inoperable cancers. These inflammation scores based on readily available and inexpensive tests could
potentially be ideal biomarkers of outcome in patients with mCRC. Evidence for the use of these inflammatory markers as direct predictors of outcome in patients with advanced malignancy
receiving first-line chemotherapy is lacking. Two recent studies have highlighted the use of the systemic inflammatory response in predicting survival (Teramukai et al, 2009) and toxicity
(Arieta et al, 2010) in patients receiving chemotherapy for advanced non-small-cell lung cancer. Neutrophilia has been shown to be an adverse prognostic factor in patients receiving
first-line oxaliplatin-based chemotherapy for CRC (Michael et al, 2006). An elevated NLR in CRC patients with liver-only colorectal metastases receiving neoadjuvant chemotherapy before
surgical resection of liver metastases predicted worse survival (Kishi et al, 2009). In addition, those patients in whom NLR normalised after chemotherapy had significantly improved 1-, 3-
and 5-year survival similar to patients with NLR⩽5 at baseline. These data suggest that NLR may be a readily available and useful biomarker for monitoring early response and prognosis with
chemotherapy for CRC (Kishi et al, 2009). The aims of the current study were to investigate (1) NLR in predicting treatment response, toxicity and survival in medical patients receiving
first-line chemotherapy for advanced CRC in a training set; (2) validating the results from the training cohort in a separate community patient cohort; and (3) assessing the impact of
normalisation of NLR for monitoring early response during chemotherapy. PATIENTS AND METHODS STUDY POPULATION In total, there were 349 patients with available clinical information and
baseline laboratory parameters. The training set consisted of 171 patients enrolled in first-line chemotherapy trials at the Sydney Cancer Centre for advanced CRC between 1999 and 2007. The
independent validation set included 178 patients from a community-based clinical database in the province of Alberta and included patients referred to medial oncology units at the Cross
Cancer Institute who received first-line chemotherapy for advanced CRC between 2004 and 2007 (Prado et al, 2008). Table 1 lists the comparative baseline clinical information and laboratory
parameters for both cohorts before chemotherapy commencement. METHODS Baseline clinical information and biochemical evaluation, including full blood count (neutrophils, lymphocytes,
haemoglobin and platelets) and albumin before chemotherapy commencement, were collected in a database for patients in both the training and validation sets. Alkaline phosphatase was also
collected in the training set. Prognostic variables with >10% missing data were not included in the analysis. Differential white-cell counts (neutrophils and lymphocytes) were also
collected for patients before cycle 2 of chemotherapy. Response rates, dates of progression and survival were available for patients in the training set; however, only survival data were
available for patients in the validation cohort. Dates of death were followed up by the investigators through hospital records, local Cancer Registries or phone contact through patient
relatives, local medical practitioners and palliative-care services. Patients were consented to undergo analyses before commencing chemotherapy, and the study was approved by institutional
research ethics committees in both Sydney and Edmonton. STATISTICAL ANALYSIS Statistical analyses were performed using SPSS Graduate Version 17.0 (IBM Corporation 2010, Somers, NY, USA).
Response rates were determined according to criteria determined by individual clinical trials, RECIST criteria. Clinical response was defined as either complete or partial response and
non-response as either stable or progressive disease. Clinical benefit was defined as complete response, partial response and stable disease and no benefit as progressive disease alone.
Progression-free survival (PFS) was defined as the date of commencing protocol treatment to the date of first progression or death from any cause without progression. Overall survival (OS)
was defined as the date from the date of commencing protocol treatment to the date of death from any cause. The _χ_2-tests were used to test associations between variables of interest
(grouped using standard thresholds) and clinical response or benefit. Multivariate modelling was used for calculation of hazard ratios and clinical response and benefit. The follow-up period
commenced at the start of chemotherapy with the censor date of January 2010. Survival analysis was performed using the Kaplan–Meier method with log-rank test in univariate analyses. Cox
regression analysis was used for multivariate survival analysis and for calculation of hazard ratios. RESULTS PATIENT CHARACTERISTICS Baseline clinical demographics and laboratory values for
both training and validation sets are presented in Table 1. There were no differences in age and gender between the two cohorts. However, a significantly higher proportion of patients in
the validation cohort had rectal cancer as the primary tumour site and had ECOG PS⩾1. The majority of patients in both cohorts received combination chemotherapy±a biological agent.
PROGNOSTIC VARIABLES IN TRAINING SET Table 2 shows the univariate analyses between prognostic variables of interest and clinical benefit, PFS and OS in the training set. At the time of
analysis, all patients had progressed on chemotherapy and 169 patients were deceased. The overall clinical response (complete response and partial response) was 55% (93 out of 168 evaluable
patients) and clinical benefit (complete response, partial response and stable disease) was 75% (128 out of 168 evaluable patients). The median PFS was 6.7 months (95% CI 5.6–7.8 months) and
OS was 15.3 months (95% CI 12.4–18.2). CLINICAL BENEFIT AND RESPONSE Younger age (⩽65 years old), ECOG performance status 0, absence of hypoalbuminaemia, normal alkaline phosphatase, low or
normal neutrophil counts and NLR⩽5 were associated with improved clinical benefit (Table 2). Similarly, younger age (⩽65 years old), ECOG performance status 0 and NLR⩽5 were associated with
improved clinical response. In addition, combination-agent chemotherapy was also associated with improved clinical response. PFS AND OS Variables predicting improved PFS included younger
age, ECOG performance status 0, combination-agent chemotherapy, single site of metastasis, absence of neutrophilia or hypoalbuminaemia and NLR⩽5 (Table 2 and Figure 2A). The following
variables were associated with improved OS: younger age, ECOG PS 0, combination-agent chemotherapy, absence of neutrophilia or anaemia. Hypoalbuminaemia, elevated alkaline phosphatase and
NLR>5 were also significantly associated with worse OS (Table 2 and Figure 2B). MULTIVARIATE ANALYSIS In multivariate analysis performed in the training set (Table 3), combination-agent
chemotherapy and NLR⩽5 were associated with improved clinical benefit. The ECOG performance status ⩾1, NLR>5, hypoalbuminaemia and single-agent chemotherapy were associated with increased
risk of progression. The ECOG performance status ⩾1 and NLR>5 predicted worse OS. PROGNOSTIC VARIABLES ACCORDING TO NLR Table 4 summarises analysis of baseline characteristics and
prognostic variables according to NLR groups. Patients with NLR>5 were more likely to suffer from hypoalbuminaemia (_P_-level <0.0001) and elevated alkaline phosphatase (_P_-level
0.008). The association between NLR and gender (_P_-level 0.06) and number of metastatic sites (_P_-level 0.05) approached statistical significance. NLR IN VALIDATION COHORT At the time of
analysis, 82% (146 out of 178) of patients were deceased. The median OS in this cohort was 16.8 months (95% CI 13.1–20.4 months). Independent predictors of survival from the training cohort
(ECOG performance status and NLR) were tested in the validation cohort. The NLR was statistically significantly associated with OS (_P_-level <0.0001). Patients with NLR⩽5 had median OS
of 19.1 months (95% CI 15.3–22.8) compared with patients with NLR>5 (median OS 11.3 months; 95%CI 8.3–14.3; Figure 2C). The ECOG performance status was not predictive of survival in this
cohort (median OS for ECOG 0 was 21.5 months (95% CI 4.1–38.9) and PS⩾1 15.7 months (95% CI 13.1–18.3; _P_-level 0.15)). NORMALISATION OF NLR PRE-CYCLE 2 AND CORRELATION WITH PFS AND OS
(TRAINING COHORT) Patients were categorised into the following categories: (1) patients with NLR⩽5 at baseline (_n_=120; cohort 1), (2) NLR>5 at baseline and before cycle 2 of
chemotherapy (_n_=21; cohort 2) and (3) NLR>5 at baseline with normalisation of NLR⩽5 before cycle 2 of chemotherapy (_n_=21; cohort 3). Patients with normalisation of NLR before cycle 2
of chemotherapy (cohort 3) had an improved PFS of 5.8 months (95% CI 4.1–7.5) compared with patients without normalisation of NLR pre-cycle 2 (cohort 2; median PFS 3.7 months; 95% CI 0.6–6.8
months; _P_-level 0.012; Figure 3A). Normalisation of NLR improved median OS from 9.4 months (cohort 2; 95% CI 3.2–15.5) to 12.1 months (cohort 3; 95% CI 7.3–16.8) in patients with a
persistently elevated NLR, although this did not reach statistical significance (_P_-level 0.053; Figure 3B). Patients with normalised NLR before cycle 2 of chemotherapy (cohort 3) did not
have significantly different median PFS (5.8 months (95% CI 4.1–7.5) _vs_ 8.0 months (95% CI 6.9–9.0); _P_-level 0.37) or OS (12.1 months (95% CI 7.3–16.8) _vs_ 18.3 months (95% CI
16.2–20.4); _P_-level 0.77) in comparison with patients with NLR⩽5 before chemotherapy commencement (cohort 1; Figures 3A and B). Normalisation of NLR before cycle 2 of chemotherapy was not
performed in the validation cohort, as there was >10% of missing data for this patient group. DISCUSSION This is the first study, to our knowledge, to describe the use of NLR in a
non-selected unresectable mCRC setting for patients receiving first-line palliative chemotherapy to provide useful information regarding prognostication, and the data have been validated in
an independent community-based cohort. These results support the use of NLR as a marker of systemic inflammatory response and as an independent predictor of clinical benefit, progression and
survival in patients receiving chemotherapy for mCRC. An NLR cutoff >5 was able to identify a subset of patients least likely to respond to chemotherapy (40 _vs_ 16%) and those at higher
risk of progression and death (HR 1.6 and 1.7, respectively). A cutoff score of 5 was chosen on the basis of previous studies (Halazun et al, 2008, 2009; Kishi et al, 2009) and this
represents a simple measurement to use in clinical practice, although other cutoffs have been used (Duffy et al, 2006; Cho et al, 2009). This identifies ∼30% of CRC patients with a raised
NLR receiving first-line chemotherapy in both cohorts and associated with shorter survival of up to 8 months. These results are highly clinically relevant in this increasingly common
malignancy. In addition, evidence for significantly improved outcomes with normalisation of NLR after the first cycle is promising for possible manipulation of the systemic inflammatory
response through targeted anti-inflammatory mediators such as IL-6 blocking antibodies. If the use of NLR and normalistion of NLR after cycle 1 are confirmed, this would provide additional
prognostic information for clinicians at an earlier time point before conventional staging with computed tomography scans and potentially identify a proportion of patients in whom further
treatment may be futile. For example, in the training cohort, there was NLR normalisation after one cycle of chemotherapy in 50% (21 out of 42) of evaluable patients, which resulted in a
2-month PFS improvement (5.8 _vs_ 3.7 months) compared with patients without NLR normalisation. These data will permit not only retrospective evaluations of established large cohorts with
known outcome data to corroborate these findings but also to undertake correlation with molecular characteristics, such as microsatellite instability and _B-raf_ mutations, which are
associated with worse cancer outcomes. The strengths in our training cohort were that patient data were retrospectively analysed from robust prospectively collected data through entry into
clinical trials. As the patients were eligible for enrolment in a clinical trial, it is highly unlikely that the elevated NLR was due to other active inflammatory diseases or infection or
were requiring high doses of steroids; however, these issues should be specifically assessed in future studies. Other independent predictive variables identified from the training cohort,
such as performance status, use of combination chemotherapy and hypoalbuminaemia, have also been reported from previous studies and strengthens the case for this cohort being representative
of a palliative mCRC population. The median OS in both cohorts (15.3 and 16.8 months in training and validation cohorts, respectively) are shorter than those reported using modern
combination chemotherapy regimens, which have median OS upwards of 24 months. However, a significant proportion of the patients in both cohorts received single-agent chemotherapy, with
patients enrolled in chemotherapy trials from as early as 1999. There were also significant baseline differences in the types of chemotherapy regimens between the Australian and Canadian
cohorts. In the Canadian cohort, up to 29% of patients did not have the type of chemotherapy specified, which may account for some of the survival difference between the two cohorts. The
validation cohort in this study failed to identify performance status as an independent prognosticator, which, although surprising, may reflect the community-based origins of this group.
However, in both cohorts, the proportion of patients with NLR>5 was surprisingly consistent between the two cohorts (29 and 31%). In spite of differences between the cohorts, NLR remained
an independent prognosticator and may reflect that it is an even more robust and accurate prognosticator than performance status alone. The heterogeneity of treatment regimens used could be
criticised; however, this is probably more reflective of day-to-day clinical practice. The NLR is a simple, readily available and robust laboratory variable. Other authors have advocated
the use of GPS or a modified GPS, based on albumin and CRP levels, and validated its use as a prognostic variable particularly in the pre-operative setting. Two studies have reported the use
of GPS in patients receiving chemotherapy for mCRC and gastro-oesophageal malignancies (Crumley et al, 2008; Ishizuka et al, 2009). However, this assessment is complicated by the
requirement for an additional blood test to measure CRP levels, which may not be readily available as was in the case of both our training and validation sets. The NLR, as a continuous
variable, may also be a more accurate and dynamic variable reflecting acute changes in the inflammatory state of a patient rather than GPS, which is applied as a static, categorical
variable. The NLR and GPS have not been compared in the same population in CRC patients, and this comparison should be undertaken to discern whether these two indices are overlapping or
additive as indicators of cancer-associated inflammation. In CRC, the use of NLR has previously been confirmed as an independent prognostic factor in a cohort of patients with liver-only
colorectal metastases, the majority of whom proceeded to hepatic resection post chemotherapy (Kishi et al, 2009). Although this is an important subset of patients with mCRC, these patients
would have been highly selected for surgical intervention and not representative of the majority of patients with mCRC. The findings in our study are not only consistent with this earlier
report but also supports the use of NLR in a more generalised patient population receiving first-line chemotherapy both in a clinical trial and community setting. Although elevated NLR was
correlated with the presence of hypoalbuminaemia and elevated alkaline phosphatase in this study (Table 4), other prognostic variables, such as performance status, site or extent of disease,
were relatively well-balanced between the high- and low-NLR groups, suggesting that NLR provides additional information than these other variables. The association of both raised NLR and
hypoalbuminaemia is likely because of its role as a marker of systemic inflammation. The reasons for the correlation between alkaline phosphatase and NLR are unclear. Alkaline phosphatase
may be a more accurate marker of the extent of liver involvement or indirectly related to systemic inflammation. The NLR has also been previously shown to independently predict outcomes in
non-malignant disease, such as post-ST-segment elevation myocardial infarction (Núñez et al, 2008) and percutaneous coronary intervention (Duffy et al, 2006) in which the systemic
inflammation response has been implicated as a major contributing factor. This adds credibility for the use of NLR as a potential biomarker of the systemic inflammatory response. In recent
years, there have been significant developments and discoveries in cancer genomics. The development of gene-expression-based arrays or examining germline single-nucleotide polymorphisms for
defining prognosis or predicting response to therapy has limited clinical application even in the two most common malignancies, lung and breast cancers (Hartman et al, 2010; Subramanian and
Simon, 2010). For example, Wacholder et al (2010) discovered that the inclusion of 10 common breast cancer genetic variants only modestly improved the performance of existing risk-assessment
models in >11 000 patients, with little change in the predicted breast-cancer risk among most women, using currently available genetic information. These tests are also expensive and
confined to use in developed countries, with limited application in under-resourced communities. A useful biomarker needs to be not only accurate and reproducible but also easily accessible.
The prognostic importance of the systemic reaction to tumours has been relatively ignored in the quest for tumour-based molecular assessments of outcome. These data will encourage a
re-evaluation of that approach. These results have highlighted the use of a potential clinical biomarker of systemic inflammatory response in predicting clinically meaningful outcomes in two
independent cohorts. In addition, results of the study have also confirmed the importance of a chronic systemic inflammatory response influencing clinical outcomes in patients with mCRC.
Validation of these results in larger patient populations will allow many potential applications in the treatment of mCRC, a major cause of morbidity worldwide. Clinical applications include
(1) prognostication and in-patient stratification in clinical trials, (2) as a marker of response to chemotherapy treatment and, more excitingly, (3) in identifying patients for possible
interventions with anti-inflammatory mediators. The results of this study, we believe, strongly support the use of NLR in these settings, and more importantly, as a dynamic marker of
interactions among tumour, host and the systemic inflammatory response. CHANGE HISTORY * _ 29 MARCH 2012 This paper was modified 12 months after initial publication to switch to Creative
Commons licence terms, as noted at publication _ REFERENCES * Arieta O, Michel Ortega RM, Villaneuva-Rodriguez G, Serna-Thome MG, Flores-Estrada D, Diaz-Romero C, Rodriguez CM, Martinez L,
Sanchez-Lara K (2010) Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with
paclitaxel-cisplatin chemotherapy: a prospective study. _BMC Cancer_ 10: 50 Article Google Scholar * Balkwill F, Mantovani A (2010) Cancer and inflammation: implications for pharmacology
and therapeutics. _Clin Pharmacol Ther_ 87: 401–406 Article CAS Google Scholar * Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. _J Clin
Oncol_ 27: 663–671 Article CAS Google Scholar * Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, Lee K (2009) Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian
cancer and predicts survival after treatment. _Cancer Immunol Immunother_ 58: 15–23 Article CAS Google Scholar * Chua W, Kho PS, Moore MM, Charles KA, Clarke SJ (2010) Clinical,
laboratory and molecular factors predicting chemotherapy efficacy and toxicity in colorectal cancer. _Crit Rev Oncol Hematol_ [E-pub ahead of print 16 August 2010;
doi:10.101.6/j.critrevonc.2010.07.012] * Coussens LM, Werb Z (2002) Inflammation and cancer. _Nature_ 420: 860–867 Article CAS Google Scholar * Crumley AB, McMillan DC, McKernan M, Going
JJ, Shearer CJ, Stuart RC (2006a) An elevated C-reactive protein concentration, prior to surgery, predicts poor cancer-specific survival in patients undergoing resection for
gastro-oesophageal cancer. _Br J Cancer_ 94: 1568–1571 Article CAS Google Scholar * Crumley ABC, McMillan DC, McKernan M, McDonald AC, Stuart RC (2006b) Evaluation of an
inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. _Br J Cancer_ 94: 637–641 Article CAS Google Scholar * Crumley AB, Stuart RC, McKernan M,
McDonald AC, McMillan DC (2008) Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG-ps) in patients receiving palliative chemotherapy for
gastroesophageal cancer. _J Gastroenterol Hepatol_ 23: e325–e329 Article CAS Google Scholar * Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL (2006) Usefulness of an elevated
neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. _Am J Cardiol_ 97: 993–996 Article Google Scholar * Forrest LM, McMillan DC,
McArdle CS, Angerson WJ, Dunlop DJ (2003) Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. _Br J
Cancer_ 89: 1028–1030 Article CAS Google Scholar * Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F,
Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F (2006) Type, density, and location of immune cells within human colorectal tumours predict clinical outcome. _Science_ 313:
1960–1964 Article CAS Google Scholar * Halazun K, Hardy MA, Rana AA, Woodland DC, Luyten EJ, Mahadev S, WItkowski P, Stegel AB, Brown RS, Emond JC (2009) Negative impact of neutrophil
lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. _Ann Surg_ 250: 141–151 Article Google Scholar * Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad
KR, Toogood GJ, Lodge JP (2008) Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. _Eur J Surg Oncol_ 34:
55–60 Article CAS Google Scholar * Hartman M, Loy EY, Ku CS, Chia KS (2010) Molecular epidemiology and its current clinical use in cancer management. _Lancet Oncol_ 11: 383–390 Article
CAS Google Scholar * Hilmy M, Bartlett JMS, Underwood MA, McMillan DC (2005) The relationship between the systemic inflammatory response and survival in patients with transitional cell
carcinoma of the urinary bladder. _Br J Cancer_ 92: 625–627 Article CAS Google Scholar * Ishizuka M, Nagata H, Takagi K, Kubota K (2009) Influence of inflammation-based prognostic score
on mortality of patients undergoing chemotherapy for far advanced or recurrent unresectable colorectal cancer. _Ann Surg_ 250: 268–272 Article Google Scholar * Johnson TV, Abbasi A,
Owen-Smith A, Young A, Ogan K, Pattaras J, Nieh P, Marshall FF, Master VA (2010) Absolute pre-operative C-reactive protein predicts metastasis and mortality in the first year following
potentially curative nephrectomy for clear cell renal cell cancer. _J Urol_ 183: 480–485 Article CAS Google Scholar * Kishi Y, Kopetz S, Chun Y, Palavecino M, Abdalla E, Vauthey J-N
(2009) Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. _Ann Surg Oncol_ 16: 614–622 Article Google
Scholar * Koopman M, Venderbosch S, Nagtegaal ID, van Krieken JH, Punt CJ (2009) A review on the use of molecular markers of cytotoxic therapy for colorectal cancer, what have we learned?
_Eur J Cancer_ 45: 1935–1949 Article CAS Google Scholar * Liu H, Liu G, Bao Q, Sun W, Bao H, Bi L, Wen W, Liu Y, Wang Z, Yin X, Bai Y, Hu X (2010) The Baseline Ratio of neutrophils to
lymphocytes is associated with patient prognosis in rectal carcinoma. _J Gastrointest Cancer_ 41: 116–120 Article Google Scholar * McMillan DC (2009) Systemic inflammation, nutritional
status and survival in patients with cancer. _Curr Opin Nutr Metab Care_ 12: 223–226 Article Google Scholar * Michael M, Goldstein D, Clarke SJ, Milner AD, Beale P, Friedlander M, Mitchell
P (2006) Prognostic factors predictive of response and survival to a modified FOLFOX regimen: importance of an increased neutrophil count. _Clin Colorectal Cancer_ 6: 297–304 Article CAS
Google Scholar * Mitsuru I, Junji K, Mitsugi S, Kyu R, Masato K, Tokihiko S (2009) Systemic inflammatory response predicts postoperative outcome in patients with liver metastases from
colorectal cancer. _J Surg Oncol_ 100: 38–42 Article Google Scholar * Núñez J, Núñez E, Bodí V, Sanchis J, Miñana G, Mainar L, Santas E, Merlos P, Rumiz E, Darmofal H, Heatta AM, Llàcer A
(2008) Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. _Am J Cardiol_ 101: 747–752 Article Google Scholar
* Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J (2005)
Effector memory T cells, early metastasis and survival in CRC. _N Eng J Med_ 353: 2654–2666 Article Google Scholar * Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L,
Baracose VE (2008) Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. _Lancet
Oncol_ 9: 629–635 Article Google Scholar * Roxburgh CSD, McMillan DC (2010) Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. _Future
Oncol_ 6: 149–163 Article CAS Google Scholar * Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P (2009) Preoperative platelet-lymphocyte ratio is an
independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. _Am J Surg_ 197: 466–472 Article Google Scholar * Subramanian J, Simon R (2010) Gene
expression-based prognostic signatures in lung cancer: ready for clinical use? _J Natl Cancer Inst_ 102: 464–474 Article CAS Google Scholar * Teramukai S, Kitano T, Kishida Y, Kawahara M,
Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, Nakano K, Tsuboi M, Shibata K, Furuse K, Fukushima M (2009) Pretreatment neutrophil count as an independent prognostic factor in
advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. _Eur J Cancer_ 45: 1950–1958 Article Google Scholar * Van Cutsem E, Köhne CH, Hitre E,
Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and
chemotherapy as initial treatment for metastatic colorectal cancer. _N Eng J Med_ 360: 1408–1417 Article CAS Google Scholar * Wacholder S, Hartge P, Prentice R, Garcia-Closas M, Feigelson
HS, Diver WR, Thun MJ, Cox DG, Hankinson SE, Kraft P, Rosner B, Berg CD, Brinton LA, Lissowska J, Sherman ME, Chlebowski R, Kooperberg C, Jackson RD, Buckman DW, Hui P, Pfeiffer R, Jacobs
KB, Thomas GD, Hoover RN, Gail MH, Chanock SJ, Hunter DJ (2010) Performance of common genetic variants in breast-cancer risk models. _N Eng J Med_ 362: 986–993 Article CAS Google Scholar
* World Health Organisation (2004) Global burden of disease 2004. http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en * Yamanaka T, Matsumoto S, Teramukai S, Ishiwata
R, Nagai Y, Fukushima M (2007) The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. _Oncology_ 73: 215–220 Article Google
Scholar Download references ACKNOWLEDGEMENTS We thank Jenny Peat for assistance with statistical analysis. Wei Chua was supported by a NSW Cancer Institute Fellowship (Australia) and a
Pfizer Australia Cancer Research Grant. The research was facilitated by a Translational Colorectal Cancer Research Grant from the NSW Cancer Institute. This study was funded by the Canadian
Institute of Health Research. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Sydney Cancer Centre, Concord Repatriation General Hospital, Hospital Road, Concord, 2139, New South Wales,
Australia W Chua & S J Clarke * Faculty of Medicine, University of Sydney, Sydney, New South Wales, Australia W Chua & S J Clarke * School of Medical Sciences (Pharmacology) and
Bosch Institute, University of Sydney, Sydney, New South Wales, Australia K A Charles * Department of Oncology, University of Alberta, Edmonton, Alberta, Canada V E Baracos Authors * W Chua
View author publications You can also search for this author inPubMed Google Scholar * K A Charles View author publications You can also search for this author inPubMed Google Scholar * V E
Baracos View author publications You can also search for this author inPubMed Google Scholar * S J Clarke View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to S J Clarke. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. RIGHTS AND PERMISSIONS From twelve months after its
original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chua, W., Charles, K., Baracos, V. _et al._ Neutrophil/lymphocyte ratio
predicts chemotherapy outcomes in patients with advanced colorectal cancer. _Br J Cancer_ 104, 1288–1295 (2011). https://doi.org/10.1038/bjc.2011.100 Download citation * Received: 01
December 2010 * Revised: 15 February 2011 * Accepted: 25 February 2011 * Published: 29 March 2011 * Issue Date: 12 April 2011 * DOI: https://doi.org/10.1038/bjc.2011.100 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative KEYWORDS * colorectal cancer * prognosis * neutrophil/lymphocyte ratio * cancer-associated inflammation