- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
In mammals, DNA methylation plays a crucial role in the regulation of gene expression, telomere length, cell differentiation, X chromosome inactivation, genomic imprinting and
tumorigenesis1. DNA methylation patterns are established _de novo_ by DNA methyltransferases (DNMTs) 3a and 3b, whereas DNMT1 maintains the parent-specific methylation from parental cells to
their progeny2. After DNA replication, the new DNA strand is unmethylated. Thus with the mother methylated strand, the DNA is hemimethylated. The protein UHRF1 (ubiquitin-like, containing
PHD and RING finger domains 1) recognizes and binds to the hemimethylated sites by its SRA domain. Then DNMT1 is recruited to the sites by the same domain, thereby it methylates the newly
synthesized DNA strand3, 4. Increased DNMT1 abundance has been seen in many human cancers, and the roles of DNMT1 in tumorigenesis have been shown by some genetic knockout and RNA
interference-mediated knockdown studies5, 6. Seeing that the mRNA abundance of DNMT1 contributes less to DNMT1 abundance, the stability of DNMT1 protein therefore plays an important role in
human cancers7, 8. Ubiquitin-proteasome pathway is significant in the stability of DNMT18, but ubiquitin-mediated protein degradation can be enhanced or attenuated by some modifications like
acetylation/deacetylation, protein methylation/demethylation, phosphorylation and _S_-nitrosylation9, 10, 11. Estève _et al_ demonstrated that SET7-mediated lysine methylation of DNMT1
decreased DNMT1 level by ubiquitin-mediated degradation10. Furthermore, an early study12 showed that HDAC inhibitors could induce degradation of DNMT1. These suggest that DNMT1-associated
proteins may play key roles in DNMT1 stability. In a recent issue of _Science Signaling_, Du _et al_ reported13 that some DNMT1-associated proteins can ubiquitinate/deubiquitinate and
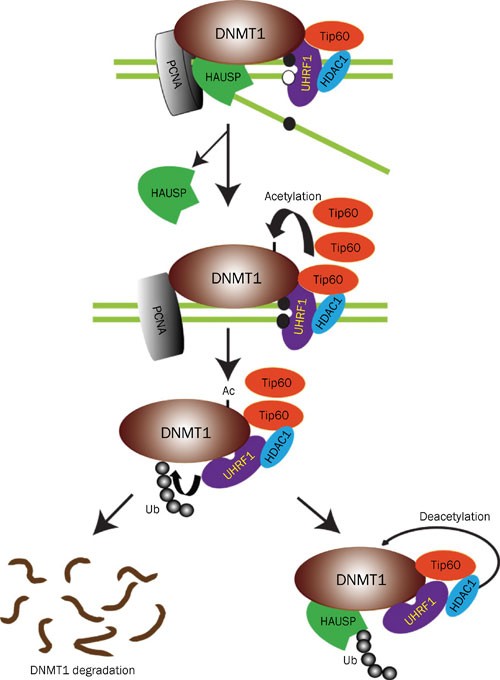
acetylate/deacetylate DNMT1. These modifications make the regulation of DNMT1 stability more coordinated (Figure 1). They identified deubiquitinase HAUSP (herpesvirus-associated ubiquitin
specific protease) as a DNMT1-associated protein. Knockout or knockdown of HAUSP increased DNMT1 ubiquitination and reduced its abundance, then they demonstrated the direct deubiquitination
of DNMT1 _in vitro_ by purified HAUSP recombinant proteins. They also found that overexpression of DNMT1-associated E3 ligase UHRF1 led to increased ubiquitination of DNMT1 and decreased
abundance of DNMT1 mutant lacking the HAUSP interaction domain, but not the full-length protein. These results show the coordination between ubiquitination of DNMT1 by UHRF1 and
deubiquitination by HAUSP. Furthermore, they found that knockdown of HDAC1 increased DNMT1 acetylation, and reduced DNMT1 abundance. Additionally, acetyltransferase Tip60 which was found to
acetylate DNMT1 promoted its ubiquitination, then destabilized it. At last, Tip60 and HAUSP were found to regulate DNMT1 protein stability during the cell cycle. The following clinical
samples of human colon cancer also revealed the correlation between the abundance of HAUSP and the abundance of DNMT1. Drugs targeting epigenetic modifications have been edging toward
anticancer therapies. DNMT1 also provides a candidate anti-cancer target14. Although global genomic DNA hypomethylation and tumor suppressor genes hypermethylation have been frequently
observed in different human cancers, this methylation change is not caused simply by increased levels of DNMT115. This paper shows that DNMT1, HAUSP, UHRF1, Tip60, HDAC1, and PCNA
(proliferating cell nuclear antigen) can interact with each other, thus they regulate DNMT1 stability and activity by a more coordinated way. Consequently, the current or future epigenetic
drugs such as 5-aza CdR (DNMT1 inhibitor), HDAC inhibitors, HAUSP inhibitors, and UHRF1 inhibitors may have a potential combination for effective cancer therapy. And equally, the drug
interactions and side effects should be taken into account, as one of the inhibitors may affect the other targets. REFERENCES * Goll MG, Bestor TH . Eukaryotic cytosine methyltransferases.
_Annu Rev Biochem_ 2005; 74: 481–514. Article CAS PubMed Google Scholar * Ooi SK, Bestor TH . Cytosine methylation: remaining faithful. _Curr Biol_ 2008; 18: R174–6. Article CAS PubMed
Google Scholar * Bostick M, Kim JK, Estève PO, Clark A, Pradhan S, Jacobsen SE . UHRF1 plays a role in maintaining DNA methylation in mammalian cells. _Science_ 2007; 317: 1760–4. Article
CAS PubMed Google Scholar * Sharif J, Muto M, Takebayashi S, Suetake I, Iwamatsu A, Endo TA, _et al_. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to
methylated DNA. _Nature_ 2007; 450: 908–12. Article CAS PubMed Google Scholar * Robert MF, Morin S, Beaulieu N, Gauthier F, Chute IC, Barsalou A, _et al_. DNMT1 is required to maintain
CpG methylation and aberrant gene silencing in human cancer cells. _Nat Genet_ 2003; 33: 61–5. Article CAS PubMed Google Scholar * Brown KD, Robertson KD . DNMT1 knockout delivers a
strong blow to genome stability and cell viability. _Nat Genet_ 2007; 39: 289–90. Article CAS PubMed Google Scholar * De Marzo AM, Marchi VL, Yang ES, Veeraswamy R, Lin X, Nelson WG .
Abnormal regulation of DNA methyltransferase expression during colorectal carcinogenesis. _Cancer Res_ 1999; 59: 3855–60. CAS PubMed Google Scholar * Agoston AT, Argani P,
Yegnasubramanian S, De Marzo AM, Ansari-Lari MA, Hicks JL, _et al_. Increased protein stability causes DNA methyltransferase 1 dysregulation in breast cancer. _J Biol Chem_ 2005; 280:
18302–10. Article CAS PubMed Google Scholar * Kruse JP, Gu W . Modes of p53 regulation. _Cell_ 2009; 137: 609–22. Article CAS PubMed PubMed Central Google Scholar * Estève PO, Chin
HG, Benner J, Feehery GR, Samaranayake M, Horwitz GA, _et al_. Regulation of DNMT1 stability through SET7-mediated lysine methylation in mammalian cells. _Proc Natl Acad Sci USA_ 2009; 106:
5076–81. Article PubMed PubMed Central Google Scholar * Azad N, Iyer A, Vallyathan V, Wang L, Castranova V, Stehlik C, _et al_. Role of oxidative/nitrosative stress-mediated Bcl-2
regulation in apoptosis and malignant transformation. _Ann N Y Acad Sci_ 2010; 1203: 1–6. Article CAS PubMed Google Scholar * Zhou Q, Agoston AT, Atadja P, Nelson WG, Davidson NE .
Inhibition of histone deacetylases promotes ubiquitin-dependent proteasomal degradation of DNA methyltransferase 1 in human breast cancer cells. _Mol Cancer Res_ 2008; 6: 873–83. Article
CAS PubMed PubMed Central Google Scholar * Du Z, Song J, Wang Y, Zhao Y, Guda K, Yang S, _et al_. DNMT1 stability is regulated by proteins coordinating deubiquitination and
acetylation-driven ubiquitination. _Sci Signal_ 2010; 3: ra80. Article PubMed PubMed Central Google Scholar * Karberg S . Switching on epigenetic therapy. _Cell_ 2009; 139: 1029–31.
Article CAS PubMed Google Scholar * Szyf M . Towards a pharmacology of DNA methylation. _Trends Pharmacol Sci_ 2001; 22: 350–4. Article CAS PubMed Google Scholar Download references
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Breast Surgery, Breast Cancer Institute, Cancer Hospital, Institutes of Biomedical Science, Shanghai Medical College, Fudan
University, 200032, Shanghai, China Qi Hong & Zhi-ming Shao Authors * Qi Hong View author publications You can also search for this author inPubMed Google Scholar * Zhi-ming Shao View
author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Zhi-ming Shao. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Hong, Q., Shao, Zm. Ubiquitination/deubiquitination and acetylation/deacetylation: Making DNMT1 stability more coordinated. _Acta Pharmacol Sin_ 32, 139–140
(2011). https://doi.org/10.1038/aps.2011.3 Download citation * Published: 04 February 2011 * Issue Date: February 2011 * DOI: https://doi.org/10.1038/aps.2011.3 SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative