- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Tobacco use is estimated to be the largest single cause of premature death in the world. Nicotine is the major addictive substance in tobacco products. After cigarette smoking,
nicotine quickly acts on its target, nicotinic acetylcholine receptors (nAChRs), which are widely distributed throughout the mammalian central nervous system and are expressed as diverse
subtypes on cell bodies, dendrites and/or nerve terminals. Through the nAChRs in brain reward circuits, nicotine alters dopaminergic (DA) neuronal function in the ventral tegmental area
(VTA) and increases dopamine release from VTA to nuclear accumbens (NA), which leads to nicotine reward, tolerance and dependence. After quitting smoking, smokers experience withdrawal
symptoms, including depression, irritability, difficulty concentrating or sleeping, headache, and tiredness. Recently, evidence has been accumulated to reveal the molecular and cellular
mechanisms of nicotine reward, tolerance and dependence. The outcomes of these investigations provide pharmacological basis for smoking cessation. Here, I briefly summarize recent
advancements of our understanding of nicotine reward, tolerance and dependence. Based on these understandings, I propose a double target hypothesis, in which nAChRs and dopamine release
process are two important targets for smoking cessation. Dysfunction of nAChRs (antagonism or desensitization) is crucial to abolish nicotine dependence and the maintenance of an appropriate
level of extracellular dopamine eliminates nicotine withdrawal syndromes. Therefore, the medications simultaneously act on these two targets should have the desired effect for smoking
cessation. I discuss how to use this double target concept to interpret recent therapies and to develop new candidate compounds for smoking cessation. SIMILAR CONTENT BEING VIEWED BY OTHERS
TOBACCO AND NICOTINE USE Article 24 March 2022 VALIDATION OF A NICOTINE VAPOR SELF-ADMINISTRATION MODEL IN RATS WITH RELEVANCE TO ELECTRONIC CIGARETTE USE Article 16 June 2020 REPURPOSING
DEXTROMETHORPHAN AND METFORMIN FOR TREATING NICOTINE-INDUCED CANCER BY DIRECTLY TARGETING CHRNA7 TO INHIBIT JAK2/STAT3/SOX2 SIGNALING Article Open access 18 February 2021 MAIN Nicotine is a
potent addictive substance in the tobacco that is thought to promote the use of tobacco products by about one-quarter of the world's population. Tobacco use is the leading preventable
cause of disease, disability, and death. Cigarette smoking results in more than 400 000 premature deaths each year — about 1 in every 5 US deaths. Economically, more than $75 billion per
year of total US healthcare costs is attributable directly to smoking. China is the world's largest producer and consumer of tobacco. It estimates that there are 0.35 billion cigarette
smokers in China. Economically, more than $166 billion per year of total Chinese healthcare costs are attributable directly to smoking-associated diseases. Therefore, there is a considerable
need to reduce the population of smokers. Unfortunately, nicotine addiction severely confounds attempts to end tobacco product use. Nicotine addiction has been clinically delineated into
two specific diagnosable disorders: dependence and withdrawal symptoms. Nicotine dependence refers to the maladaptive and chronic use of tobacco that meets the same types of criteria that
are applied to other forms of drug addiction. Recent research has revealed two important features of nicotine addiction: (1) nAChRs play a critical role in developing nicotine addiction ,
and (2) nicotine addiction is a dynamic process including different stages such as nicotine-induced reward, tolerance, dependence and withdrawal-relapse symptoms1. Nicotine reward means that
nicotine, acting on brain nAChRs, stimulates brain reward-associated circuits, which allows the smoker to be in a euphoric state. Dopamine is one of the key neurotransmitters actively
involved within the reward circuits in the brain. Accumulating lines of evidence indicate that nicotine increases DA release from VTA to NA, which represents its nature of reward and intense
addictive qualities. Mounting evidence demonstrates that nAChR subtypes with different distributions within reward circuits mediate nicotine reward. Dopaminergic (DA) neurons in the VTA
express diverse nAChR subunits, including α3–α7 and β2–β42, 3, 4, 5, 6, 7, which can combine to form at least two pharmacologically distinct nAChR subtypes. One of these functionally
distinct nAChRs is homomeric α7-nAChRs, which are mainly expressed on glutamatergic presynaptic terminals, where they mediate nicotine-induced increase of glutamate release onto DA neurons6.
Another group of nAChRs is non-α7-nAChRs, which seems to be more complex. For example, electrophysiological studies, combined with single-cell RT-PCR technique, show α4α6α5(β2)2 or
α4α5(β2)2 combinations of nAChRs in midbrain reward center6. Immunoprecipitation approaches using nAChR subunit knockout mice show that functional α6β2-nAChRs are mainly located on DA
neuronal terminals, whereas α4β2-nAChRs represent the majority of functional heteromeric nAChRs on DA neuron somata8. Interestingly, GABAergic neurons located in the VTA likely express
relatively-simple nAChR subtypes, mainly (α4)2(β2)3-nAChRs6 and these α4β2nAChRs contribute to cholinergic modulation of GABA tonic release onto DA neurons9. Recently, we have characterized
three functional subtypes (ID, IID, IIID) of nAChRs in VTA DA neurons7 that may mediate nicotine reward and dependence. For example, nicotine, in the same concentrations and time ranges as
obtained from cigarette smoking, enhances glutamatergic excitation in the VTA DA neurons by increasing glutamate release via stimulating presynaptic α7-nAChRs10. Nicotine also activates then
desensitizes α4β2-nAChRs on both DA and GABAergic neurons9. Since cholinergic innervations in GABAergic neurons have much higher density than that in DA neurons 11, the nicotine-induced
α4β2-nAChR desensitization (after brief activation) mainly decreases GABA release onto DA neurons, and consequently increases DA neuronal activity. It has been proposed that nicotine-induced
desensitization is at least one of the major mechanisms for nicotine addiction12, 13, 14. In the VTA, nicotine activates (α7-) and/or desensitizes (α4β2) nAChRs, and in turn increases DA
neuronal activity. The short-lived direct excitation of the DA neurons coupled with the enhanced glutamatergic afferent activity provides the presynaptic and postsynaptic coincidence
necessary to initiate synaptic potentiation and plasticity (LTP)15, 16. Taken together, these synaptic events lead to a relatively long-lasting heightened activity of midbrain DA neurons,
which release more DA to NA and results in positive reinforcement. The nicotine reward is an important early event to initiate and develop to nicotine dependence. As mention before,
nicotine, in addition to activating nAChRs, also desensitizes them 17, which plays an important role in the developing nicotine tolerance and dependence. After repetitive exposure to
nicotine, nAChR desensitization represents a low functional status of nAChRs in the presence of low level of nicotine, and the recovery from receptor desensitization is relative slow. Under
this desensitization condition, the same concentrations of nicotine are not enough to induce the same level of reward signals (the level of extracellular DA in the NA), which consequently
causes nicotine tolerance. On one hand, to overcome nicotine tolerance (to get the same level of reward stimulation), smokers increase cigarette smoked per day, which results in the nicotine
dependence. On the other hand, nAChR desensitization triggers receptor upregulation, and the upregulated nAChRs imbalances cholinergic signaling and modulation, which causes withdrawal
symptoms when smoker quits cigarette smoking. In summary, nicotine addiction is the major reason why smokers continue to rely on tobacco products. Nicotine dependence and withdrawal symptoms
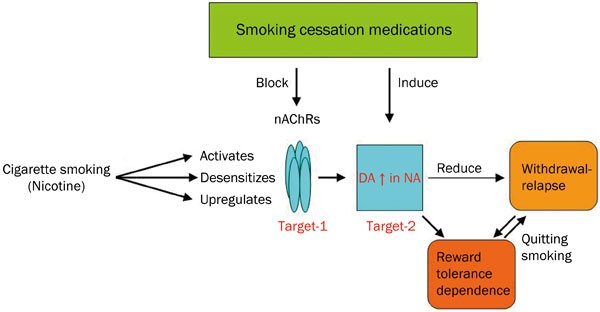
are two pathophysiological changes brought on by cigarette smoking. Based on these lines of evidence, I propose a “double target” hypothesis to interpret pharmacotherapuetic mechanisms of
smoking cessation. In this hypothesis, I assume that the medications that eliminate both nicotine dependence and withdrawal-relapse will exert more desired effect for smoking cessation
(Figure 1). To reduce nicotine dependence, medications should block nAChRs (in particular α4β2-nAChRs), while to eliminate withdrawal-relapse, they also should appropriately (mildly)
increase extracellular DA level in the NA. Using this double target concept, one can explain pharmacological mechanisms of existing smoking cessation medications. For instance, recent market
drugs available for smoking cessation are bupropion and varenicline18. Bupropion is used to treat mental depression, but it is also used as part of a support program to help people stop
smoking19. This medicine exhibits double target properties since it not only blocks dopamine transporter (maintain a mildly elevated level of extracellular DA in the NA), but also blocks
nAChRs, in particular α4β2-nAChRs19, 20, 21. Varenicline is another example of a smoking cessation medication22 that matches the double target concept. It is a partial agonist at
α4β2-nAChRs, and is also a full agonist at α7 nAChRs23. As a partial agonist of α4β2-nAChRs, varenicline mimics nicotinic effect to activate/desensitize its first target, α4β2-nAChR, and
diminishes nicotine dependence. In addition, as a full agonist at α7 nAChRs, it also mimics nicotinic stimulation on its second target, presynaptic α7-nAChR, to increase glutamate release
onto DA neurons in the VTA, which in turn appropriately increases DA release from the VTA to NA, and eliminates withdrawal symptoms. Based on this double target concept, one also can develop
new medications for smoking cessation. Recently, my laboratory has developed two different compounds that exhibit high potential to be novel smoking cessation medications. One group of
compounds is called tetrahydroprotoberberine analogs (THPBs) which include tetrahydroberberine (THB), l-stepholidine (l-SPD) and l-hydroparmatine (l-THP). THPBs are purified from several
Chinese herbs in the magnoliidae superorder. Mounting evidence indicates that THPBs exhibit dopamine receptor (D2) antagonist effects on sedation, hypnosis, antinociception,
anti-schizophrenia and anti-hypertension24, 25, 26, 27. The major pharmacological targets for THPBs are dopamine receptors, and THPBs exhibit D1 partial agonist and D2 antagonist effects. As
a D2 receptor antagonist in VTA DA neurons, systemic exposure to THPBs increase DA neuronal firing by blocking D2 auto-receptors on DA neurons and increase DA level in NA. It has been
reported that l-THP effectively eliminates heroin addiction in human28, suggesting that THPBs may have a potential for treating drug addiction. Recent research in my laboratory also shows
that THPBs potently inhibit either human α4β2-nAChRs heterologously expressed in SH-EP1 cell line or rodent α4β2-nAChRs in midbrain DA neurons. This double-target feature suggests that THPBs
are good candidates that can be developed into new smoking cessation medications. Another compound we are working on is iptakalim hydrochloride (Ipt). Ipt was initially designed and
synthesized as an antihypertensive drug29. It is a small, water soluble molecule that freely penetrates the blood-brain barrier and has minimal toxic side effects following long-term
systemic administration30. Possible pharmacological mechanisms underlying its antihypertensive action include KATP channel activation and endothelin antagonism. Tests in a variety of _in
vivo_ and _in vitro_ ischemia and Parkinson's disease models indicate that Ipt has neuroprotective effects30, 31, 32. Furthermore, Ipt can potentially prevent drug addiction since it
inhibits cocaine challenge-induced enhancement of dopamine release in rat NA33. The major pharmacological target of Ipi is thought to the cytoplasmic and/or mitochondrial KATP channels30. My
laboratory has evaluated the effects of Ipt on nAChR function and found that Ipt potently blocks α4β2-nAChRs heterologously expressed in human SH-EP1 cell line34 or natively expressed in
rat midbrain DA neurons35. Currently, we have also found that Ipt significantly prevents systemic nicotine-induced behavioral (locomotor activity) sensitization, further confirmed its nAChR
antagonism (Wu _et al_, unpublished data). Interestingly, systemic injections of Ipt along (30 mg·kg−1·day−1, ip for 7 days) increase rat locomotor activity (Wu _et al_, unpublished data),
suggesting that on one hand, Ipt can block α4β2-nAChR function, and on the other hand, it also can appropriately increase DA level at NA although the reason and mechanisms of this effect are
still unclear. Nevertheless, Ipt also exhibits double-target feature and it likely can be developed as a novel smoking cessation medication. In conclusion, nicotine addiction is a complex
brain disorder, which involves functional alterations in multiple brain circuits and exhibits different stages. Based on the new concept of network pharmacology36, 37, I propose a double
target hypothesis, which may help to understand the pharmacotherapeutic mechanisms of smoking cessation, and also help to develop new medications for quitting tobacco use. REFERENCES *
Brennan K, Lea R, Fitzmaurice P, Truman P . Review: Nicotinic receptors and stages of nicotine dependence. _J Psychopharmacol_ 2010; 24: 793–808. Article CAS PubMed Google Scholar *
Sargent PB . The diversity of neuronal nicotinic acetylcholine receptors. _Annu Rev Neurosci_ 1993; 16: 403–43. Article CAS PubMed Google Scholar * Charpantier E, Barneoud P, Moser P,
Besnard F, Sgard F . Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. _Neuroreport_ 1998; 9: 3097–101. Article
CAS PubMed Google Scholar * Sgard F, Charpantier E, Barneoud P, Besnard F . Nicotinic receptor subunit mRNA expression in dopaminergic neurons of the rat brain. _Ann N Y Acad Sci_ 1999;
868: 633–5. Article CAS PubMed Google Scholar * Le Novere N, Corringer PJ, Changeux JP . The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and
pharmacologic consequences. _J Neurobiol_ 2002; 53: 447–56. Article CAS PubMed Google Scholar * Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP . Molecular and physiological
diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. _J Neurosci_ 2001; 21: 1452–63. Article CAS PubMed PubMed Central Google Scholar * Yang K, Hu J,
Lucero L, Liu Q, Zheng C, Zhen X, _et al_. Distinctive nicotinic acetylcholine receptor functional phenotypes of rat ventral tegmental area dopaminergic neurons. _J Physiol_ 2009; 587:
345–61. Article CAS PubMed Google Scholar * Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, _et al_. Subunit composition of functional nicotinic receptors in
dopaminergic neurons investigated with knock-out mice. _J Neurosci_ 2003; 23: 7820–9. Article CAS PubMed PubMed Central Google Scholar * Mansvelder HD, Keath JR, McGehee DS . Synaptic
mechanisms underlie nicotine-induced excitability of brain reward areas. _Neuron_ 2002; 33: 905–19. Article CAS PubMed Google Scholar * Wonnacott S, Kaiser S, Mogg A, Soliakov L, Jones
IW . Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. _Eur J Pharmacol_ 2000; 393: 51–8. Article CAS PubMed Google Scholar * Garzon M, Vaughan RA, Uhl GR,
Kuhar MJ, Pickel VM . Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter. _J Comp Neurol_ 1999;
410: 197–210. Article CAS PubMed Google Scholar * Hulihan-Giblin BA, Lumpkin MD, Kellar KJ . Acute effects of nicotine on prolactin release in the rat: agonist and antagonist effects of
a single injection of nicotine. _J Pharmacol Exp Ther_ 1990; 252: 15–20. CAS PubMed Google Scholar * Sharp BM, Beyer HS . Rapid desensitization of the acute stimulatory effects of
nicotine on rat plasma adrenocorticotropin and prolactin. _J Pharmacol Exp Ther_ 1986; 238: 486–91. CAS PubMed Google Scholar * Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK,
Kozikowski AP, _et al_. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. _Mol Pharmacol_ 2006; 70: 1454–60. Article CAS
PubMed Google Scholar * Dani JA, Heinemann S . Molecular and cellular aspects of nicotine abuse. _Neuron_ 1996; 16: 905–8. Article CAS PubMed Google Scholar * Dani JA, Ji D, Zhou FM
. Synaptic plasticity and nicotine addiction. _Neuron_ 2001; 31: 349–52. Article CAS PubMed Google Scholar * Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA . Differential
desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. _J Neurosci_ 2003; 23: 3176–85. Article CAS PubMed PubMed Central Google Scholar
* Fant RV, Buchhalter AR, Buchman AC, Henningfield JE . Pharmacotherapy for tobacco dependence. _Handb Exp Pharmacol_ 2009; (192): 487–510. Article Google Scholar * Paterson NE .
Behavioural and pharmacological mechanisms of bupropion's anti-smoking effects: recent preclinical and clinical insights. _Eur J Pharmacol_ 2009; 603: 1–11. Article CAS PubMed Google
Scholar * Sidhpura N, Redfern P, Wonnacott S . Comparison of the effects of bupropion on nicotinic receptor-evoked [(3)H]dopamine release from rat striatal synaptosomes and slices. _Eur J
Pharmacol_ 2007; 567: 102–9. Article CAS PubMed Google Scholar * Slemmer JE, Martin BR, Damaj MI . Bupropion is a nicotinic antagonist. _J Pharmacol Exp Ther_ 2000; 295: 321–7. CAS
PubMed Google Scholar * Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, _et al_. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related
to effects on reward, mood and cognition. _Biochem Pharmacol_ 2009; 78: 813–24. Article CAS PubMed Google Scholar * Mihalak KB, Carroll FI, Luetje CW . Varenicline is a partial agonist
at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. _Mol Pharmacol_ 2006; 70: 801–5. Article CAS PubMed Google Scholar * Bian CF, Duan SM, Xing SH, Yu YM, Qin W,
Jin GZ, _et al_. Interaction of analgesics and l-stepholidine. _Acta Pharmacol Sin_ 1986; 7: 410–3. CAS Google Scholar * Zhang ZD, Jin GZ, Xu SX, Yu LP, Chen Y, Jiang FY, _et al_. Effects
of l-stepholidine on the central nervous and cardiovascular systems. _Acta Pharmacol Sin_ 1986; 7: 522–6. CAS Google Scholar * Xiong ZL, Sun Z, Jin GZ, Chen Y . [Influence of
l-stepholidine on blood pressure and its relation to alpha-adrenoceptors]. _Acta Pharmacol Sin_ 1987; 8: 497–501. CAS Google Scholar * Chu H, Jin G, Friedman E, Zhen X . Recent development
in studies of tetrahydroprotoberberines: mechanism in antinociception and drug addiction. _Cell Mol Neurobiol_ 2008; 28: 491–9. Article CAS PubMed Google Scholar * Yang Z, Shao YC, Li
SJ, Qi JL, Zhang MJ, Hao W, _et al_. Medication of l-tetrahydropalmatine significantly ameliorates opiate craving and increases the abstinence rate in heroin users: a pilot study. _Acta
Pharmacol Sin_ 2008; 29: 781–8. Article CAS PubMed Google Scholar * Wang H . Cardiovascular ATP-sensitive K+ channel as a new molecular target for development of antihypertensive drugs.
_Acta Pharmacol Sin_ 1998; 19: 397–402. CAS Google Scholar * Wang H, Zhang YL, Tang XC, Feng HS, Hu G . Targeting ischemic stroke with a novel opener of ATP-sensitive potassium channels in
the brain. _Mol Pharmacol_ 2004; 66: 1160–8. Article CAS PubMed Google Scholar * Yang Y, Liu X, Long Y, Wang F, Ding JH, Liu SY, _et al_. Systematic administration of iptakalim, an
ATP-sensitive potassium channel opener, prevents rotenone-induced motor and neurochemical alterations in rats. _J Neurosci Res_ 2005; 80: 442–9. Article CAS PubMed Google Scholar * Chen
H, Yang Y, Yao HH, Tang XC, Ding JH, Wang H, _et al_. Protective effects of iptakalim, a novel ATP-sensitive potassium channel opener, on global cerebral ischemia-evoked insult in gerbils.
_Acta Pharmacol Sin_ 2006; 27: 665–72. Article PubMed Google Scholar * Liu Y, He HR, Ding JH, Gu B, Wang H, Hu G . Iptkalim inhibits cocaine challenge-induced enhancement of dopamine
levels in nucleus accumbens and striatum of rats by up-regulating Kir6.1 and Kir6.2 mRNA expression. _Acta Pharmacol Sin_ 2003; 24: 527–33. CAS PubMed Google Scholar * Hu J, Lindenberger
K, Hu G, Wang H, Lukas RJ, Wu J . Iptakalim as a human nicotinic acetylcholine receptor antagonist. _J Pharmacol Exp Ther_ 2006; 316: 914–25. Article CAS PubMed Google Scholar * Hu J,
DeChon J, Yan KC, Liu Q, Hu G, Wu J . Iptakalim inhibits nicotinic acetylcholine receptor-mediated currents in dopamine neurons acutely dissociated from rat substantia nigra pars compacta.
_Neurosci Lett_ 2006; 403: 57–62. Article CAS PubMed Google Scholar * Yildirim MA, Goh KI, Cusick ME, Barabasi AL, Vidal M . Drug-target network. _Nat Biotechnol_ 2007; 25: 1119–26.
Article CAS PubMed Google Scholar * Hopkins AL . Network pharmacology. _Nat Biotechnol_ 2007; 25: 1110–1. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS Work
toward this project was supported by grants from the Arizona Biomedical Research Commission, the Institute for Mental Health Research, and Philip Morris International through their External
Research Program. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Shantou University Medical College, Shantou, 515041, China Jie Wu * Barrow Neurological Institute, St Joseph's Hospital
and Medical Center, Phoenix, 85013, AZ, USA Jie Wu Authors * Jie Wu View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence
to Jie Wu. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wu, J. Double target concept for smoking cessation. _Acta Pharmacol Sin_ 31, 1015–1018 (2010).
https://doi.org/10.1038/aps.2010.137 Download citation * Received: 30 May 2010 * Accepted: 20 July 2010 * Published: 16 August 2010 * Issue Date: September 2010 * DOI:
https://doi.org/10.1038/aps.2010.137 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * nicotine * nicotine addiction * nicotine reward *
nicotine dependence * nicotine withdrawal syndromes * nicotinic acetylcholine receptor * smoking cessation








