- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Micro- and nanoscale structures on aluminium provide a superhydrophobic surface, making it resistant to corrosion. Corrosion of metals causes many problems, and much research is undertaken
to try and prevent it. Aluminium corrodes easily, especially when exposed to moist or acidic environments. Typical ways of preventing aluminium from corrosion involve creating a protective
barrier by coating its surface. Chromate is particularly effective for this purpose but the hexavalent chromium has carcinogenic properties and more inert coatings are sought. A
self-assembled monolayer of an environmentally friendly hydrophobic organic material can provide initial protection but it has limited thermal stability and has molecule-sized defects. Now,
as an alternative to conventional materials, Xue Duan, Fazhi Zhang and colleagues at Beijing University of Chemical Technology have incorporated a ‘laurate’— a derivative of lauric acid—in a
layered double hydroxide (LDH); a synthetic clay.1 Zhang says that, “LDHs are very versatile materials and the ability to vary their composition over a wide range allows materials with a
range of properties to be prepared.” The researchers first fabricated porous anodic alumina by anodizing an aluminium substrate and placing it in an alkaline solution of zinc nitrate with
excess nitrate ions, forming a film of ZnAl-LDH-NO3–. As the substrate was the source of the Al3+, a tightly bound film with good mechanical stability was formed. Immersion in an aqueous
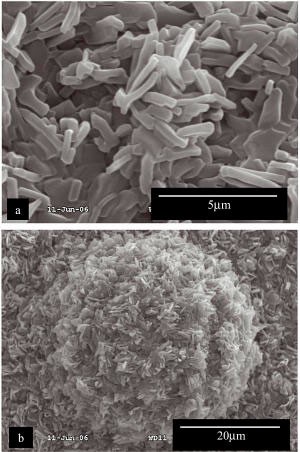
solution of sodium laurate resulted in a tilted bilayer of laurate anions between layers of the LDH. Scanning electron imaging showed the surface to be rough due to both nanoscale LDH
hexagonal platelets oriented perpendicular to the surface (Fig 1a), and macroscale protrusions with a similar surface morphology (Fig. 1b); the protrusions were assumed to result from
thickening of the LDH crystallites due to the incorporation of the laurate anions. Water droplets on this rough surface sit on a combination of the platelets/protrusions and air, with a
contact angle of 163°, which is within the realm of superhydrophobicity. Zhang and co-workers found that the films remained corrosion-resistant even after immersion in salt water for 21
days. This group is developing clays using different anions and hydrophobic moieties to produce films with industrially important properties. REFERENCES * Zhang, F., Zhao, L., Chen, H., Xu,
S., Evans, D. G. & Duan, X. Corrosion resistance of superhydrophobic layered double hydroxide films on aluminum. _Angew. Chem. Int. Ed._ 47, 2466–2469 (2008). Article CAS Google
Scholar Download references ADDITIONAL INFORMATION This research highlight has been approved by the author of the original article and all empirical data contained within has been provided
by said author. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hard skinned aluminium. _NPG Asia Mater_ (2008). https://doi.org/10.1038/asiamat.2008.34
Download citation * Published: 13 May 2008 * DOI: https://doi.org/10.1038/asiamat.2008.34 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
