- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
We have discovered two types of 5′ intronic gene mutation that impair androgen receptor (AR) mRNA expression severely, and cause complete androgen insensitivity. Labium majus skin
fibroblasts (LMSF) hemizygous for each mutation had negligible specific androgen binding, and did not react to an antibody against an N-terminal peptide of the AR. Both mutations were
detected by direct sequencing of exons PCR-amplified with flanking primers. One mutation is an adenine to thymine transversion at position +3 of the intron 6 splice-donor site. Using LMSF
mRNA, RT-PCR of a portion of the AR androgen-binding domain yielded a small amount of a 302-bp mutant fragment instead of a 433-bp wild-type product. Sequencing established that exon 5 was
followed, out of frame, by exon 7: exon 6 was skipped. The other mutation is a thymine insertion at the +3 position of the intron 1 donor-splice site. RT-PCR and sequencing revealed a small
amount of normal-size mRNA with normal exon 1-exon 2 splicing. Quantitative RT-PCR on mutant LMSF showed AR mRNA levels were well below 10% of normal; hence, most of the aberrant AR mRNA
resulting from each mutation is probably unstable. The misbehavior caused by these two mutations indicates that in the AR the splice-donor site +3 adenine is critical; indeed, 57% of
eukaryotic introns have adenine in the +3 position, while only 2% have thymine.
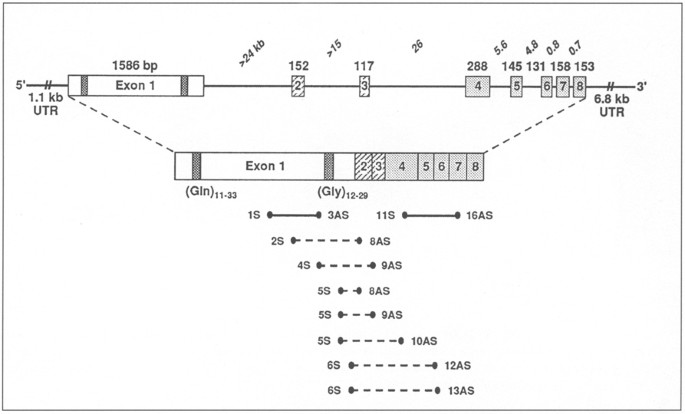
The AR gene and protein: untranslated regions (UTR), and intron-exon composition. Exon 1 encodes the transactivation modulatory domain; exons 2–3 and 4–8 form the DNA and androgen-binding
domains, respectively. Alternative 3′ processing yields transcripts of ∲ 8 and 11 kb. (Gln)n and (Gly)n denote the polymorphic polyglutamine and polyglycine tracts in the AR’s N-terminal
domain. Shown below are the primer combinations used for quantitative-competitive RT-PCR (solid lines) and for attempts to detect abnormal splicing in 926203 (dashed lines).
Subject 4479 (referred by Dr. H.N. Valentine, The University of Western Ontario, London, Ont., Canada) had a right inguinal herniorrhaphy at age 5 during which histologically identified
testicular tissue was removed. She had normal female external genitalia but lacked a uterus. The peripheral lymphocyte karyotype was 46,XY. The mother of 4479 had two maternal aunts with
primary amenorrhea; thus, we presumed she was an obligate heterozygote. Two skin fibroblast substrains, coded 7534 and 7534Q, were derived from a single expiant of her labium majus skin.
Subject 926203 presented at 15 years with primary amenorrhea and a height of 171.4 cm, well above the sex-adjusted genetic target height of 159 ± 8.5 cm. She had high blood levels of
luteinizing hormone and testosterone and well-developed breasts, but no pubic or axillary hair. The vagina was 4 cm and blind. The peripheral lymphocyte karyotype was 46,XY. In the family
history there were no maternally-related females with primary amenorrhea, delayed menarche, or sparse, delayed or asymmetrical sexual hair, and no maternally-related males with hypospadias,
gynecomastia, or unexplained infertility.
Replicate confluent monolayers in 6-cm Petri dishes were incubated with 3 nM [3H]mibolerone (MB) alone, in triplicate, to measure total binding, or together with 600 nM radioinert MB for 2 h
at 37 °C, in duplicate, to measure nonspecific binding [18]. Specific binding was determined by subtracting nonspecific from total binding, and dividing by protein concentration as
determined by the Lowry assay [19].
DNA sequencing ladders showing the AR splice-donor site mutations. Lower-case letters distinguish intronic from exonic bases. A An A to T transversion was identified at position +3 of intron
6 of subject 4479 [Int6(+3A>T)]; her mother (7534) was heterozygous for the same mutation. B A T insertion was found at the same position of intron 1 in subject 926203 [Int1(+3insT)].
Total RNA from 2080 (normal), 4479, 7534 and 7534Q fibroblasts was reverse-transcribed and PCR-amplified (RT-PCR) using exon 4- and exon 7-specific primers (1 IS and 16AS). The products were
analyzed on a 1.5% low-melt agarose gel with HaeIII-digested ΦX174RF markers (ΦX). The normal product expected is 433 bp; shorter fragments (302 bp) were observed for 4479 [Int6(+3A>T)],
and 7534Q.
DNA sequencing ladders of exon 4–7-specific RT-PCR products from normal and 4479. The normal fragment contained appropriate exon 5/6 and exon 6/7 junctions; the shorter fragment
Quantitative-competitive RT-PCR. A Exon 4-7-specific primers 11S and 16AS were used to determine the level of AR mRNA in 926203 fibroblasts relative to normal 2080. B The ethidium
bromide-stained gel containing digested PCR products whose negative was scanned to obtain the results plotted in A. M = HaeIII-ΦX174 markers; lanes 1–9 = the dilution series of the
competitor AR cDNA ranged from 400 to 1.6 pg/reaction for 2080 and from 50 to 0.2 pg/reaction for 926203. C The ethidium bromide-stained gel showing undigested exon 4–7 products of reverse
transcribed 4479 fibroblast mRNA (sample) and the same plasmid competitor AR cDNA used above (competitor). The competitor ranged from 50 to 0.4 pg/reaction (lanes 1–8). No competitor was
included in lane 9. HaeIII-ΦX174 marker sizes are indicated.
Total cell lysates from 926203, 7534Q (one of two substrains from the mother of 4479), 4479, 8812, 2200 and 2080 fibroblasts (350 µg protein each) were electrophoresed on a 7% SDS-PAGE gel.
AR genotype is indicated below: Intl(+3insT) and Int6(+3A>T) = +3 splice-donor site mutations; AR del = complete deletion of AR; + = normal. The blot was probed with (A) an AR-specific
monoclonal antibody F39.4.1, then (B) a 70-kD heat shock protein (hsp)-specific monoclonal antibody to control for amount of protein loaded. The blot was developed using the ECL Western
blotting chemiluminescence detection system (Amersham). The sizes of the Rainbow protein molecular weight markers (Amersham) are shown.
Extracts from the LMSF of 926203, 7534Q, 4479, and controls were prepared as described [18] and 350-µg protein samples subjected to electrophoresis on a 7% SDS-PAGE gel. After
electroblotting, the nitrocellulose filter was blocked in 5% skim milk powder in 0.5% Tween/10 mM Tris HCl pH 7.5, 150 mM NaCl (Tris-buffered saline (TBS)) and processed as described [25]
using the anti-AR monoclonal antibody F39.4.1 [26]. The filter was then washed in 0.5% Tween/TBS, reacted with a monoclonal antibody against constitutively expressed hsp 70 (Stressgen
Biotechnologies Corporation), diluted 1:1,000 in 0.5% Tween/TBS, and processed as above.
The consensus values (CV) for each normal and mutant splice-donor site were calculated according to the method of Shapiro and Senapathy [25] using primate values for nucleotide percentages
at each of eight splice-donor site positions. CV = 100 (t-mint)/(maxt-mint), where t = the total of the percentages for the eight positions of a single site and mint and maxt are the minimum
and maximum percentage totals possible for primate splice-donor sites.
We measured androgen-binding activity in LMSF of subjects 4479 and 926203, and in two LMSF substrains (7534 and 7534Q) derived from the mother of 4479. The cells of 4479 and 926203 had
negligible androgen-binding activity. However, one of the LMSF substrains derived from the mother of 4479 had very low specific androgen binding (2–5 fmol/mg protein; strain 7534Q), while
the other substrain had normal androgen binding (22 fmol/mg protein, strain 7534; normal range 15–40 fmol/mg protein). These results indicated cell mosaicism due to differential X-chromosome
inactivation, and strongly supported her presumptive diagnosis as an obligate heterozygote.
To determine if sequence alterations in the androgen-binding domain (ABD) of the AR were the cause of the negligible androgen binding in subjects 4479 and 926203, exons 4–8 were PCR
amplified from LMSF-derived genomic DNA and the sequences of the exons and exon/intron boundaries were determined. The only alteration found in subject 4479 was an A to T transversion in the
+3 position of intron 6 [Int6(+3A > T)], as shown in figure 2A. Coexistent mutations in and around exons 2 and 3 were excluded. Genomic sequencing (fig. 2A) proved that 7534, the mother of
4479, was heterozygous for Int6(+3A > T), as predicted by the androgen-binding assays. As no mutation was found in or around exons 2–8 of subject 926203, direct sequencing was extended to
the translated portion of exon 1 and its flanking sequences. Figure 2B shows the only mutation found: a T insertion at the +3 position of intron 1 [Int 1(+3insT)]. This mutation was not
present in the subject’s mother, as predicted from her family history.
Total RNA from 2080 (normal), 4479, 7534 and 7534Q fibroblasts was reverse transcribed (RT) and the cDNA subjected to PCR amplification using exon 4- and exon 7-specific primers within the
ABD. Figure 3 shows that the normal sample yields the expected 433-bp fragment, as does substrain 7534, while a shorter one (302 bp) is observed clearly for 4479, and for substrain 7534Q
from her heterozygous mother. Sequencing of a PCR product that included the 302-bp fragment shown in figure 3 revealed that the 131-bp exon 6 is missing (fig. 4); thereby fully accounting
for the reduced size of the mutant fragment (fig. 3). Furthermore, skipping of exon 6 with consequent exon 5-exon 7 fusion creates a frameshift that would result in 12 altered amino acids
followed by a premature stop codon (TGA). The predicted truncated AR would have 783 amino acids, a molecular weight of 82.6 kDa, and lack the portion of the ABD encoded by exons 6–8.
The normal and mutant fragments in substrains 7534 and 7534Q, respectively, indicated differential X-chromosome inactivation. This was confirmed by sequencing the glutamine [Gln;(CAG)n]
tracts of exon 1-specific RTPCR products from 4479, 7534 and 7534Q. The 7534 product had 28 Gln codons, while the 7534Q product had 21 Gln codons. The latter corresponded to the 21 Gln-codon
tract of the AR on the single X chromosome of 4479.
All the primer combinations used for RT-PCR of exon 1 and exons 2–8 of 926203’s AR mRNA produced greatly reduced quantities of normal-size fragments. Competitive RT-PCR using the primers
identified in figure 1 yielded a value of 6% of normal for exon 1 (results not shown) and 5% of normal for exons 4–7 (figs. 5A, B). Multiple primer pairs that spanned the exon 1/exon 2
junction were used in an attempt to isolate 926203 AR mRNA splicing variants. No products were seen however, except on one occasion with a combination of primers 2S and 8AS, when sequencing
showed the exon 1/exon 2 junction was normal, and the authenticity of the RT-PCR product was confirmed by sequencing the polyglycine (GGN)n tract. Both the RT-PCR product and 926203 genomic
DNA contained 23 glycine codons. Northern analysis of poly A+ mRNA from 926203 GSF also did not reveal any AR mRNA variants (results not shown).
AR mRNA expression in 4479 was found to be very low (fig. 5C). In a separate quantitative RT-PCR experiment, using 2080 RT-PCR product rather than plasmid DNA for competition, and no
restriction enzyme digestion, the level of the mutant transcript in 4479 was 4% of normal AR mRNA (results not shown).
To determine if AR proteins were produced by LMSF from 926203, 4479 and 7534Q, cell extracts were electrophoresed, transferred to a nitrocellulose filter and reacted with an anti-hAR
antibody (F39.4.1 [24]). No AR protein was detectable, either of normal size in 926203, or of reduced size in 4479 or 7534Q (fig. 6). Probing with an hsp 70 antibody showed equivalent
protein loading for all samples.
Cooper and Krawczak [26] estimated that point mutations altering mRNA splicing represent ∼ 15% of all those causing human genetic disease, and splice-donor sites are more often affected than
splice-acceptor sites [27]. As of October 1996, only 6 (including the 2 reported here) of the 143 (4.2%) point mutations or small deletions or insertions associated with androgen
insensitivity in the AR Gene Mutations Database [2] affect splicing; and, appropriately, 5 of the 6 are at splice-donor sites. Despite their infrequency, the analysis of natural splicing
mutations can illuminate basic mechanisms of mRNA transcription and processing. Three of the 4 AR splice mutations reported previously [3–5] caused complete androgen insensitivity by
substitution at the invariant +1 G position of the intron 3, 4, or 7 splice-donor sites. In the fourth, deletion of the intron 2 branch-point sequence caused partial androgen insensitivity
[28]. The 2 mutations described here affirm that alterations at the +3 position of a splice-donor site disrupt normal AR mRNA processing as much as alterations at the +1 position.
Splice-donor site +3 mutations have been incriminated as the cause of human genetic disease in 14 other cases [6–17 and references therein]. Of the total, 3 are +3 A > T [8, 12, 17], as in
our subject 4479, and 4 are +3insT [6, 9, 11, 14], as in our subject 926203.
The phenotypic consequence of the AR Int1(+3insT) or the Int6(+3A > T) mutation is complete androgen insensitivity. LMSF from both hemizygous subjects, and from one of two Int6(A > T)
heterozygous LMSF substrains (7534Q) had little androgen-binding activity and no AR protein detectable by Western blotting. AR mRNA was expressed at levels less than 10% of normal in both
cases, indicating that mRNA processing is adversely affected by both +3 splice-donor site mutations. Analysis of RT-PCR products from 4479 [Int6(A > T)] demonstrated that mutant mRNA was
produced; exon 6 was skipped. Exon skipping was also observed in the 7534Q substrain but not in the 7534 substrain. This may be explained by the chance development of two different LMSF
subpopulations with differential X-chromosome inactivation. The active X chromosome in the 7534 cells must bear the normal allele and thus produce normal AR protein capable of binding
androgen, while the 7534Q cells express the mutant allele, as confirmed by determination of polyglutamine-codon tract lengths in RT-PCR products.
We were not able to demonstrate aberrant splicing in LMSF of 926203 [Int1(+3insT)], despite the fact that splice-donor mutations can cause cryptic splice-site utilization or intron retention
in addition to exon skipping [29]. Presumably exon 1 skipping does not occur in strain 926203 because there is no upstream slice-donor site to which the splice-acceptor site of exon 2 can
be joined. In the single other case with a +3 splice-donor site mutation in intron 1, two mutant transcripts utilizing a cryptic splice site 20 bp into intron 1 were detected [14].
Atypically, in one of these transcripts, skipping of exon 2, but not of the exon upstream of the mutation (exon 1), occurred. With the exception of one pair of primers whose product
contained a normal junction, PCR amplification with multiple primer pairs spanning the exon1/exon 2 boundary of 926203 did not generate any DNA fragments. This indicates that cryptic splice
sites in the immediate vicinity of the normal splice-donor site of exon 1 in 926203 were not used. This also suggests that the low level AR mRNA measured by competitive RT-PCR in 926203 was
probably spliced normally. Retention of intron 1 would have resulted in addition of >24 kb to the already lengthy AR message (∼ 8 or 11 kb), but Northern analysis of 926203 poly A+ RNA also
failed to reveal any longer than normal AR mRNA species. Possibly, long-range PCR would be a more sensitive technique for revealing abnormal splicing patterns in 926203.
Base changes that alter the splice-donor site consensus sequence severely could hinder base-pairing between the 3′ UCCAUUCA 5′ sequence at the 5′ end of U1 snRNA and the 5′ AG:GUA/GAGU 3′
consensus sequence of a mammalian splice-donor site and therefore affect spliceosome assembly [30]. The consensus values calculated for the mutations described here indicate serious
alteration of the consensus sequence at the exon-intron borders 1 and 6. The consequence for Int1(+3insT) is a markedly reduced amount of normal mRNA; that for Int6(+3A > T), a markedly
reduced amount of aberrant mRNA, reinforces the exon definition mode of splice-site selection [31]. Exon skipping results when the splice-donor site of the previous exon is joined to the
splice-acceptor site of the following exon; this was demonstrated clearly for the Int6(+3A > T) mutation. Classical exon skipping also occurs with 12 of the 14 intron +3 mutations previously
recognized as causing human genetic disease; it was not sought in the 14th case [11].
As a consequence of exon skipping, the Int6(+3A > T) mutation yields a mutant transcript that is predicted to generate a premature stop codon (PSC) in the proximal portion of exon 7, the
penultimate exon of the AR. Such PSCs are usually associated with a reduced level of mRNA, rather than production of a truncated polypeptide. This is explainable by the ‘translational
translocation’ or ‘nuclear scanning’ models that link normal nuclear-cytoplasmic transport of mRNA to the integrity of mRNA translation [32]. Indeed, destabilization of the full-length mRNA
due to premature termination of translation has been reported for human β-globin trans-genes containing nonsense codons [33]. In any event, any protein translated from the mutant transcript
would be unable to bind androgen, and it is not sufficiently stable to be detectable by Western blotting. Likewise, the low amount of normal AR mRNA found in 926203 isinsufficient to support
any male sexual morphogenesis.
In conclusion, the abnormal mRNA expression caused by the two AR mutations reported herein demonstrates the critical role that the +3 position of a splice-donor site can play in mRNA
processing and development of human disease.
This work was supported by the Medical Research Council of Canada, the Cancer Research Society of Canada, the Fonds pour la Formation de Chercheurs et l’Aide à la Recherche du Québec, and
the Fonds de la Recherche en Santé du Québec. We are grateful to Mary Tomaras and Rhona Rosenzweig for faithful secretarial assistance.
Lady Davis Institute for Medical Research, Sir Mortimer B. Davis - Jewish General Hospital, 3755 Cote St. Catherine Road, Montreal, Que., H3T E21, Canada
Mark A. Trifiro, Rose Lumbroso, Lenore K. Beitel, D. Marie Vasiliou, Joanne Bouchard & Leonard Pinsky
Department of Pediatrics, McGill University, Montreal, Que., Canada
Anyone you share the following link with will be able to read this content:




