- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The Atlantic–Mediterranean area has recently been proposed as a new phylogeographical area on the basis of concordance of genetic differentiation patterns observed in several marine
species. However, additional taxa need to be studied to establish the phylogeographical relationship between the Atlantic and Mediterranean. Eleven samples of the cuttlefish _Sepia
officinalis_ around the Iberian Peninsula, one from the Canary Islands, and another from Fiumicino (Italy) were screened for 33 allozyme loci. Genetic variability was low in all samples
(_H_e between 0.022 and 0.076). Intersample genetic differentiation was high (_F_ST=0.220), mainly because of genetic variation in the non-Iberian samples. One locus (_PEPD_*), diagnostic
between the Italian sample and all others, suggests the possible existence of hitherto unrecognized species or subspecies of _Sepia_ in the Mediterranean Sea. The 11 Iberian samples
exhibited moderate genetic differentiation (_F_ST=0.100), which could be explained on the basis of genetic differentiation between Atlantic and Mediterranean samples. Significant clines in
allele frequencies were observed for five out of six polymorphic loci. These results support a model of secondary intergradation (i.e. secondary contact of populations that were previously
differentiated in isolation) similar to that previously proposed for other marine species from the Atlantic–Mediterranean area. SIMILAR CONTENT BEING VIEWED BY OTHERS PHYLOGEOGRAPHY OF THE
VEINED SQUID, _LOLIGO FORBESII,_ IN EUROPEAN WATERS Article Open access 12 May 2022 MITOCHONDRIAL, NUCLEAR AND MORPHOLOGICAL DIFFERENTIATION IN THE SWIMMING CRAB _LIOCARCINUS DEPURATOR_
ALONG THE ATLANTIC-MEDITERRANEAN TRANSITION Article Open access 20 August 2024 SPATIAL AND TEMPORAL STABILITY IN THE GENETIC STRUCTURE OF A MARINE CRAB DESPITE A BIOGEOGRAPHIC BREAK Article
Open access 20 August 2022 INTRODUCTION Studies on several different marine fishes and molluscs (see Sanjuan et al., 1996a, 1997 and references therein; Roldán et al., 1998) have revealed
geographically concordant intersample genetic differentiation between Atlantic and Mediterranean samples. A hypothesis has been proposed to explain these patterns, wherein Mediterranean
populations were isolated from Atlantic populations during ice-ages (Pliocene and Quaternary), with subsequent genetic divergence, post ice-age secondary contact, and present-day gene flow
between populations restricted by the Straits of Gibraltar or the Almería-Oran oceanographic front. Nevertheless, additional studies using taxa with different biological characteristics are
needed to clarify and confirm the importance of this region as a new phylogeographical area. _Sepia officinalis_ (Linnaeus 1758; Cephalopoda: Sepiidae) is an important fishery resource for
European and North African countries (annual captures are around 70 000 metric tonnes; FAO, 1994). It is distributed along the NE Atlantic continental margin, from the Baltic Sea to Senegal,
and throughout the Mediterranean Sea (Guerra, 1992). This cephalopod fixes its eggs to the sea floor, a larval phase is lacking, and the adults have limited migratory capacity, so its
dispersive ability is presumed to be restricted (Guerra, 1992). Allozyme polymorphisms have proved to be effective for estimating population divergence and identifying discrete fish and
cephalopod stocks (Carvalho & Hauser, 1994 and references therein). The one previous allozyme-based study on _Sepia officinalis_ (Sanjuan et al., 1996b) found no significant genetic
differences between samples on either side of a subsurface oceanographic front off the NW Iberian Peninsula. The aim of the present study was to analyse the genetic structure, using allozyme
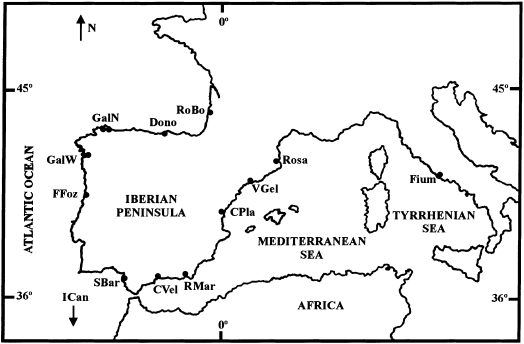
markers, of _S. officinalis_ populations from the Atlantic and Mediterranean Sea for geographical patterns of genetic variation. MATERIALS AND METHODS SAMPLING Thirteen samples of _Sepia
officinalis_ were collected from commercial catches in NE Atlantic and Mediterranean fishing ports between March 1993 and November 1997 (Table 1 and Fig. 1). Eleven samples were caught from
waters around the Iberian Peninsula, six from Atlantic waters (RoBo, Dono, GalN, GalW, FFoz and SBar), and five from the Mediterranean Sea (CVel, RMar, CPla, VGel and Rosa); one sample was
taken off the Canary Islands (ICan); and another off Fiumicino (Fium), Italy. The specimens were obtained on the date of capture and were immediately frozen in dry ice and stored at −72°C
until required. ELECTROPHORESIS Samples of mantle muscle were prepared for electrophoresis using methods previously described for _Sepia_ species (Pérez-Losada et al., 1996). Standard
horizontal starch gel electrophoresis was carried out (Murphy et al., 1996). Twenty-eight enzymes, yielding 33 putative enzyme coding loci, displayed adequate activity and resolution for
consistent interpretation and routine examination. Detailed electrophoretic conditions and histochemical staining recipes for most of the enzymes are described in Pérez-Losada et al. (1996).
For ALPDH, G6PDH and GAPDH the electrode buffer was Tris-Citrate pH 8.0 (gel buffer dilution 1:9), and histochemical staining recipes were as in Murphy et al. (1996). DATA ANALYSIS Genotype
frequencies at polymorphic loci were tested for agreement with Hardy–Weinberg equilibrium expectations by chi-squared tests, and the probability of the null hypothesis was estimated using
Monte Carlo simulation. The genetic structure of samples was analysed by means of _F_-statistics (Nei, 1987). The significance of _F_ST was calculated by a chi-squared test of homogeneity of
allele frequencies and the probability of the null hypothesis was estimated using Monte Carlo simulation. Mean expected heterozygosity per locus (unbiased estimate), observed
heterozygosity, mean number of alleles and proportion of polymorphic loci (Nei, 1987) were calculated for each sample. Nei’s (1978) (_D_N) and Cavalli-Sforza & Edwards’s (1967) arc
(_D_arc) genetic distances among samples were computed. These values were then used to construct UPGMA (unweighted pair-group method using arithmetic averages; Sneath & Sokal, 1973) and
neighbour-joining (Saitou & Nei, 1987) trees. The correlation coefficient between the matrix of Cavalli-Sforza & Edwards’s (1967) arc genetic distances and geographical distances was
calculated and its probability was estimated by means of a Mantel test based on 1000 permutations. Allele frequencies of the most polymorphic loci were plotted against geographical
distances among samples, and the significance of allozyme variation patterns was tested by correlation coefficients between the arcsine-squared-root-transformed allele frequencies and
geographical distances. The sequential Bonferroni technique (Rice, 1989) was used to adjust significance levels for multiple simultaneous comparisons. BIOSYS-1 (Swofford & Selander,
1981) and Zaykin & Pudovkin (1993) computer programs were used to perform most of the genetic analyses. The Mantel test was carried out using the NTSYS-PC computer program (Rohlf, 1994).
RESULTS GENETIC VARIABILITY Seventeen enzyme loci were monomorphic in all samples; allele frequencies at the 16 polymorphic loci are shown in Table 2Table 2. Eight loci showed one or two
rare alleles (allele frequency <0.05), and seven loci exhibited a moderate variability with two or three alleles per locus (_AAT-1_*, _ESTD_*, _IDDH_*, _IDHP_*, _MEP_*, _OPDH-1_* and
_PGDH_*). The _PEPD_* locus was diagnostic between the Fium sample (_N_=24) and all 12 other _Sepia_ samples (_N_=328). In addition, at locus _AAT-1_* the Italian sample had an _AAT-1_*_100_
allele frequency of only 0.396, whereas in the other 12 samples this allele was nearly fixed. The _IDDH_* locus showed a fixed allele (_IDDH_*_85_) in the ICan sample, whereas this allele
varied from 0.100 (VGel and Rosa) to 0.667 (RoBo) in the other 12 samples. The _F_-values between loci and samples showed great variability, although no significant deviations from
Hardy–Weinberg expectations were found. The estimates of genetic variability for all 13 _S. officinalis_ samples (Table 2) were low for all indices (_H_e ranged between 0.022 and 0.076) and
the _H_e over all samples was 0.057 ± 0.022. Mediterranean samples exhibited the highest values for the variability indices, although _H_e was not significantly different between
Mediterranean and Atlantic samples [Student’s _t_11=0.710, 0.40 < _P_ < 0.50; Nei (1987)]. POPULATION STRUCTURE The mean _F_ST value over all 13 samples was 0.220. This high value of
genetic differentiation was mainly caused by allele frequencies in Fium for _PEPD_* and _AAT-1_*, and ICan for _IDDH_* (see Table 2). When both samples were excluded from the analysis, the
mean _F_ST over all 11 Iberian samples was halved (_F_ST=0.100, Table 3). All the most polymorphic loci (_P_95) except _AAT-1_*, plus _ALPDH_* and _OPDH-2_*, exhibited significant
heterogeneity in allele frequencies among Iberian samples (Table 3). Genetic distances of Nei (1978) (_D_N) and Cavalli-Sforza & Edwards (1967) (_D_arc) are presented in Table 4. Both
distances showed relatively low values for all pairwise comparisons; _D_N ranges between 0.000 and 0.056, and _D_arc between 0.024 and 0.238. Maximum distances occur between the
geographically most distant samples (Fium vs. RoBo or Dono). The UPGMA trees (Fig. 2) showed that the non-Iberian samples (Fium and ICan) were clearly separated from the Iberian samples,
which fell into three groups. The most northern Iberian samples (RoBo and Dono) were clustered and separated from the east Iberian samples (CPla, VGel and Rosa), which constituted another
group. In the UPGMA based on _D_N(Fig. 2a) the six geographically intermediate Atlantic and Mediterranean samples were clustered together. However, in the UPGMA based on _D_arc (Fig. 2b),
these intermediate samples were split into western and southern clusters, except RMar, which was joined to the western group. When the neighbour-joining method was applied to both distance
matrices, trees with the same topology as those presented in Fig. 2 were obtained. Genetic distances were significantly correlated with geographical distances between samples (_r_=0.721, _P_
< 0.001; Fig. 3), but genetic distances were greater than expected for the 12 Fium comparisons. Separation of the 11 Iberian samples into Atlantic and Mediterranean regional groups is
further supported by consideration of variation over all loci. In the Atlantic Region (AR) were grouped RoBo, Dono, GalN, GalW and FFoz; and in the Mediterranean Region (MR) were included
SBar, CVel, RMar, CPla, VGel and Rosa (Table 3). SBar was considered as a Mediterranean sample on the basis of its geographical proximity to the Mediterranean Sea and because it is
genetically more similar to Mediterranean samples (Fig. 2b). The mean genetic differentiation (_F_ST, Table 3) decreased from 0.100 over all Iberian collections to 0.063 for AR, and to 0.061
for MR, the decrease being specially important for some of the polymorphic loci such as _ESTD_* and _MEP_*. Clearly significant geographical allozyme variation appeared for three loci
(after the application of the Bonferroni test) with a moderate genetic variability: _IDDH_*, _MEP_* and _OPDH-1_* (Fig. 4). In the case of _MEP_*, RMar behaved as an outlier for _MEP_*_75_,
showing an allele frequency of 0.094. Slight but significant allozyme variation was also observed at two loci with low levels of genetic variability: _ESTD_* and _OPDH-2_*. A V-shaped
pattern of variation was found at the _IDHP_* locus which showed significant clines on both sides of the Straits of Gibraltar, but not around the Iberian Peninsula as a whole. DISCUSSION The
study of 33 enzyme loci in 13 samples of _S. officinalis_ showed that this species has low levels of allozyme variability; the mean _H_e (0.057 ± 0.022) falls below the average for
invertebrates and molluscs (0.122 and 0.145, respectively; Ward et al., 1992), and also for marine molluscs (0.147; Fujio et al., 1983), but within the range for cephalopods (see Brierley et
al., 1996; Sanjuan et al., 1996b; and references therein). Despite the observed low genetic variability, extensive intersample variation (_F_ST=0.220) was observed over all the samples
studied. This genetic differentiation could result from the allozyme differences between the non-Iberian samples (Fium and ICan) and the Iberian samples, and the genetic differences between
the samples considered as Atlantic (AR) and Mediterranean (MR). The Tyrrhenian sample (Fium) was clearly separated from the other samples in the UPGMA trees (Fig. 2). The genetic
differentiation of this sample can be attributed mainly to the diagnostic locus _PEPD_* (_F_ST=1.000) and, to a lesser degree, to the locus _AAT-1_* (_F_ST=0.565). These results suggest that
the individuals from Fium are genetically distinct (no gene flow between these and the other samples), and may even comprise a separate and hitherto unrecognized subspecies or species of
_Sepia_. Both Nesis (1987) and Khromov (pers. comm.) have suggested that, based on morphological data, the notion of a subspecies of _S. officinalis_ in the Mediterranean Sea (_S.
officinalis mediterranea_ Nini 1884) is valid. Nevertheless, the unbiased genetic identity (_I_; Nei, 1978) between Fium and the other 12 samples (0.954–0.946) is of the order generally
considered to be indicative of conspecific populations (Thorpe, 1983). A more extensive study combining molecular (allozyme and DNA) markers with morphological characters, and more samples
between Rosa and Fium will be needed to clarify this hypothesis. The Canary Islands sample (ICan) is also distinctly divergent from the other samples in the UPGMA tree (Fig. 2), mainly
because of genetic variation at the loci _IDDH_*, _MEP_* and _OPDH-1_*. This sample is most closely related genetically (_D_N=0.008; _D_arc=0.106) to the SBar sample, which is also the
geographically most proximal (≈ 1000 km). Thus, isolation by distance could be responsible for the genetic divergence of this sample from the Iberian samples. The Iberian samples showed
moderate intersample differences overall (_F_ST=0.100; Table 3), although genetic distances for all pairwise comparisons were low (_D_N < 0.03; Table 4). Genetic differences are
significantly correlated with increasing geographical separation along the coastline between the Atlantic and Mediterranean (Fig. 3). These results are supported by the clustering of samples
(Fig. 2), which match rather well their geographical distribution. The UPGMA tree based on _D_arc (Fig. 2b) suggests a separation of the samples from the Atlantic Region (AR) from those of
the Mediterranean Region (MR). In the same way, the genetic differentiation between Atlantic and Mediterranean samples is also evident in the hierarchical analysis of _F_-statistics (Table
3), which indicates greater genetic heterogeneity between than within regions. Similarly, the estimate of number of effective migrants (_Nm_, where _Nm_=[1/_F_ST – 1]/4) over all 11 Iberian
samples (_Nm_=2.3) reflects a moderate amount of gene flow, and was lower than _Nm_ for each region separately (_Nm_=3.7 for AR; _Nm_=3.9 for MR). Samples from the MR possessed nine alleles
exclusive to this area, whereas only three alleles were exclusive to AR. This difference in number of exclusive alleles may be an indication of a real barrier to gene flow outwards from the
Mediterranean Sea, and suggests that alleles originating in Mediterranean populations are prevented from spreading to Atlantic populations, whereas those arising in Atlantic populations are
spreading into the Mediterranean Sea. This asymmetrical gene flow could be maintained by the unidirectional marine surface circulation southwards along the coast of the Iberian Peninsula and
into the Mediterranean Sea across the Straits of Gibraltar (Rodríguez, 1982). This study also showed that geographical allozyme variation around the Iberian Peninsula is a major feature of
allozyme intersample variability in _S. officinalis_ (Fig. 4). Such variation in allele frequencies may be the result of secondary contact of cuttlefish populations that were isolated in the
past and became differentiated in allopatry (i.e. secondary intergradation; Endler, 1977; Avise, 1994). In this sense, the main change for allele frequencies roughly coincides with the area
of transition from the Atlantic to the Mediterranean, the Straits of Gibraltar and the Alborán Sea, suggesting the position of the barrier that produced such isolation in the past. This
transition area is also suggested by the different clustering of the three southern Iberian samples (SBar, CVel and RMar) in the UPGMA trees (Fig. 2), indicating a genetic character
intermediate between the geographically distant Atlantic (RoBo–FFoz) and Mediterranean (Cpla–Rosa) samples. This is compatible with what is known of the historical hydrography of this area:
intermittent physical barriers, precluding colonization and migration, existed as recently as one million years ago (Maldonado, 1985 and references therein); recent lowering of sea level by
100–200 m during Quaternary glaciations resulted in the partial or total closure of the Straits (Bianco, 1990). One polymorphic locus (_IDHP_*), however, displayed a V-shaped pattern over
the Iberian Peninsula (Fig. 4). The coincidence of the V midpoint at this locus with the transition area described earlier, gives further support to the same geographical separation
hypothesis. However, V-shaped patterns cannot arise from the contact of two genetically differentiated populations, and clines implied in these patterns must have originated independently in
each sea basin. The results presented for _S. officinalis_ in this paper support the importance of the Atlantic–Mediterranean area in phylogeographical structuring within marine species
(Sanjuan et al., 1996a), as has been similarly suggested for the Gulf of Mexico/Atlantic coasts of North America and the Indian Ocean/Malayan Provinces (Avise, 1994; Palumbi, 1994).
REFERENCES * Avise, J. C. (1994) _Molecular Markers, Natural History and Evolution_. Chapman & Hall, New York. Book Google Scholar * Bianco, P. G. (1990). Potential role of the
palaeohistory of the Mediterranean and Paratethys basins on the early dispersal of Euro-Mediterranean freshwater fishes. _Ichthyol Expl Freshw_, 1: 167–184. Google Scholar * Brierley, A.
S., Allcock, A. L., Thorpe, J. P. and Clarke, M. R. (1996). Biochemical genetic evidence supporting the taxonomic separation of _Loligo edulis_ and _Loligo chinensis_ (Cephalopoda:
Teuthoidea) from the genus _Loligo_. _Mar Biol_, 127: 97–104. Article Google Scholar * Carvalho, G. R. and Hauser, L. (1994). Molecular genetics and the stock concept in fisheries. _Rev
Fish Biol Fish_, 4: 300–326. Article Google Scholar * Cavalli-Sforza, L. L. and Edwards, A. W. F. (1967). Phylogenetic analysis: models and estimation procedures. _Evolution_, 32: 550–570.
Article Google Scholar * Endler, J. A. (1977) _Geographic Variation, Speciation, and Clines_. Princeton University Press, Princeton, NJ. Google Scholar * FAO (FOOD AND AGRICULTURE
ORGANIZATION) (1994) _Yearbook Fishery Statistics_ _1992_ no. 74. FAO, Rome. * Fujio, Y., Yamanaka, R. and Smith, P. J. (1983). Genetic variation in marine molluscs. _Bull Jap Soc Sci Fish_,
49: 1809–1817. Article Google Scholar * Guerra, A. (1992). Mollusca, Cephalopoda. In: Ramos, M. A. _et al_(eds) _Fauna Ibérica_ vol. 1. Museo Nacional de Ciencias Naturales, CSIC, Madrid.
Google Scholar * Maldonado, A. (1985). Evolution of the Mediterranean basins and a reconstruction of the Cenozoic palaeoceanography. In: Margalef, R. (ed.) _Western Mediterranean_, pp.
18–61. Pergamon Press, London. Google Scholar * Murphy, R. W., Sites, C. W. Jr, Buth, D. G. and Haufler, C. H. (1996). Proteins: isozyme electrophoresis. In: Hillis, D. M. and Moritz, C.
(eds) _Molecular Systematics_. pp. 51–120. Sinauer Associates, Sunderland, MA. Google Scholar * Nei, M. (1978). Estimation of average heterozygosity and genetic distance from a small number
of individuals. _Genetics_, 89: 583–590. CAS PubMed PubMed Central Google Scholar * Nei, M. (1987) _Molecular Evolutionary Genetics_. Columbia University Press, New York. Google Scholar
* Nesis, K. N. (1987) _Cephalopods of the World_. T. H. F. Publications, Neptune City, NJ. Google Scholar * Palumbi, S. R. (1994). Genetic divergence, reproductive isolation, and marine
speciation. _Ann Rev Ecol Syst_, 25: 547–572. Article Google Scholar * Pérez-Losada, M., Guerra, A. and Sanjuan, A. (1996). Allozyme electrophoretic technique and phylogenetic
relationships in three species of _Sepia_ (Cephalopoda: Sepiidae). _Comp Biochem Physiol B_, 114: 11–18. Article Google Scholar * Rice, W. R. (1989). Analyzing tables of statistical tests.
_Evolution_, 43: 223–225. Article PubMed Google Scholar * Rodríguez, J. (1982) _Oceanografía Del Mar Mediterráneo_. Pirámide, Madrid. Google Scholar * Rohlf, F. J. (1994) Ntsys-Pc.
_Numerical Taxonomy and Multivariate Analysis System_ Version 1.80. Exeter Software, New York, NY. * Roldán, M. I., García-Marín, J. L., Utter, F. M. and Pla, C. (1998). Population genetic
structure of European hake, _Merluccius merluccius_. _Heredity_, 81: 327–334. Article Google Scholar * Saitou, N. and Nei, M. (1987). The neighbour-joining method: a new method for
reconstructing phylogenetic trees. _Mol Biol Evol_, 4: 406–425. CAS PubMed Google Scholar * Sanjuan, A., Comesaña, S. and De Carlos, A. (1996a). Macrogeographic differentiation by mtDNA
restriction site analysis in the SW European _Mytilus galloprovincialis_ Lmk. _J Exp Mar Biol Ecol_, 198: 89–100. Article Google Scholar * Sanjuan, A., Pérez-Losada, M. and Guerra, A.
(1996b). Genetic differentiation in three _Sepia_ species (Mollusca: Cephalopoda) from Galician waters (Northwest Iberian Peninsula). _Mar Biol_, 126: 253–259. Article Google Scholar *
Sanjuan, A., Zapata, C. and Álvarez, G. (1997). Genetic differentiation in _Mytilus galloprovincialis_ Lmk. throughout the world. _Ophelia_, 47: 13–31. Article Google Scholar * Sneath, P.
H. and Sokal, R. R. (1973) _Numerical Taxonomy_. W. H. Freeman, San Francisco. Google Scholar * Swofford, D. L. and Selander, R. B. (1981). Biosys-1: a Fortran program for the comprehensive
analysis of electrophoretic data in population genetics and systematics. _J Hered_, 72: 281–283. Article Google Scholar * Thorpe, J. P. (1983). Enzyme variation, genetic distance and
evolutionary divergence in relation to levels of taxonomic speciation. In: Oxford, G. S. and Rollinson, D. (eds) _Protein Polymorphism. Adaptive and Taxonomic Significance_. pp. 131–152.
Academic Press, London. Google Scholar * Ward, R. D., Skibinski, D. O. F. and Woodwark, M. (1992). Protein heterozygosity, protein structure and taxonomic differentiation. _Evol Biol_, 26:
73–159. Article CAS Google Scholar * Zaykin, D. V. and Pudovkin, A. I. (1993). Two programs to estimate significance of χ2values using pseudo-probability tests. _J Hered_, 84: 152–152.
Article Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to P. Shaw for his valuable suggestions on a draft of the manuscript and two anonymous referees for their
interesting comments. We thank F. Casas, J. M. Olveira, L. Pozzan, J. Ferrer, M. Bernal and A. Rodrigues for assistance in the collection of the samples. Thanks are also due to A. Lavalatina
for her MAC care. This research was supported by the AMB94–0371 project (CICYT, Spain) and its complementary one 64102C503 (University of Vigo, Spain) to A.S. This paper fulfils the Ph.D.
requirements for M.P.-L., who received a Scholarship from Xunta de Galicia (Spain). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Xenética Evolutiva Molecular, Facultade de
Ciencias-Bioloxía, Universidade de Vigo, Vigo, E-36200, Spain Marcos Pérez-Losada & Andrés Sanjuan * Instituto de Investigaciones Marinas (CSIC), Eduardo Cabello 6, Vigo, E-36208, Spain
Ángel Guerra Authors * Marcos Pérez-Losada View author publications You can also search for this author inPubMed Google Scholar * Ángel Guerra View author publications You can also search
for this author inPubMed Google Scholar * Andrés Sanjuan View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Andrés
Sanjuan. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Pérez-Losada, M., Guerra, Á. & Sanjuan, A. Allozyme differentiation in the cuttlefish _Sepia
officinalis_ (Mollusca: Cephalopoda) from the NE Atlantic and Mediterranean. _Heredity_ 83, 280–289 (1999). https://doi.org/10.1038/sj.hdy.6885520 Download citation * Received: 09 October
1998 * Accepted: 31 March 1999 * Published: 01 September 1999 * Issue Date: 01 September 1999 * DOI: https://doi.org/10.1038/sj.hdy.6885520 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative KEYWORDS * allozymes * genetic differentiation * phylogeography * _Sepia officinalis_








