- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT _PURPOSE_ To examine the lens epithelial cells obtained from the anterior lens capsules removed during cataract surgery and detect various subclasses of the cell surface adhesion
molecules known as integrins. _METHODS_ The circular sections of anterior capsules with attached lens epithelial cells (LECs) were obtained during cataract surgery from 28 patients. The lens
capsules were immunohistochemically stained. _RESULTS_ CD49b CD49c, CD49e, and CD18 were detected in varying degrees in specimens obtained from human cataractous lenses. The positive
percentages were 33, 75, 33, and 20%, respectively. The most striking feature was the increasing staining profiles towards the edges of the capsules (away from the central part of the lens
capsules) for CD49c, suggesting that the LECs showed higher immunoreactivity for this antibody. The immunoreactivity for CD49b and CD49e was weaker. This was absent for CD18 in the central
part of the lens capsules. _CONCLUSION_ The positive expression of antibodies suggests that specific integrin subunits were expressed in LECs of human cataracts. These results suggest that
lens epithelial cells expressing CD49b, CD49c, CD49e, and CD18 might be precursors in the process of anterior lens epithelial cell (A cell) adhesion, and hence play a role in anterior
capsule opacification or in subsequent migration and a possible role in posterior capsule opacification. SIMILAR CONTENT BEING VIEWED BY OTHERS CELLULAR FLICE-LIKE INHIBITORY PROTEIN (CFLIP)
CRITICALLY MAINTAINS APOPTOTIC RESISTANCE IN HUMAN LENS EPITHELIAL CELLS Article Open access 09 April 2021 ESSENTIAL FUNCTION OF ADAPTOR PROTEIN NCK1 IN PLATELET-DERIVED GROWTH FACTOR
RECEPTOR SIGNALING IN HUMAN LENS EPITHELIAL CELLS Article Open access 20 January 2022 FHL-1 INTERACTS WITH HUMAN RPE CELLS THROUGH THE Α5Β1 INTEGRIN AND CONFERS PROTECTION AGAINST OXIDATIVE
STRESS Article Open access 08 July 2021 INTRODUCTION Following cataract surgery posterior capsular opacification (PCO) is the most common complication, affecting up to 50% of all adult
patients after 2 years and virtually all paediatric cases.1,2 It is secondary to the migration of lens epithelial cells (LECs) from the lens equator onto the posterior lens capsule or lens
epithelial metaplasia into myofibroblasts (fibrosis).3 The blocking of _α_v _β_3 integrin expression was noted to inhibit specifically LEC migration and proliferation in an animal model.4
Various authors have also suggested the role of TGF-_β_ in accelerating transdifferentiation and contraction of the capsular bag.5,6 These results suggest that a common feature of anterior
subcapsular cataract (ASC) and PCO may be induction of an epithelial–mesenchymal transition by TGF-_β_. Epithelial cell proliferation, collagen deposition, basement membrane duplication, and
lens fibre regeneration subsequently result in capsular opacification and visual compromise.7,8 The anterior lens epithelial cells (A cells) are the source of any form of anterior
subcapsular cataract. The primary type of response of the A cells to any stimulus is to proliferate and form fibrous tissue by undergoing fibrous metaplasia, sometimes termed ‘pseudofibrous
metaplasia’.3 Several studies suggest that the residual LEC proliferation, differentiation, and migration onto the posterior capsule lead to PCO.3,7,8 Anterior capsular shrinkage and
ultimate constriction (capsulorrhexis contraction syndrome or capsular phimosis) of the anterior capsulectomy opening, excessive zonular traction and its sequelae, IOL decentration, and
retinal detachment can also occur.9,10,11 Anterior lens capsules discarded during cataract surgery contain a monolayer of epithelial cells. It has been reported that the migration of LECs in
the human eye is dependent on the extracellular matrix (ECM), which could promote the adhesion of LECs.3,4,5 Integrins are cell adhesion molecules that have been implicated in the migration
of cells to the posterior capsule of the lens. They are a family of heterodimeric membrane glycoproteins expressed on a diverse range of cell types. The glycoproteins function as the major
receptors for ECM proteins and as cell–cell adhesion molecules. All integrins consist of noncovalently associated _α_ and _β_ subunits. They bind to a variety of ECM proteins such as
collagen, fibronectin, von Willebrand factor and to members of the immunoglobulin superfamily. However, the various subtypes of lens adhesion molecules involved in anterior and posterior
capsular opacification have not been identified. As the knowledge of these processes increases, the possibility of developing a number of therapeutic agents will also increase. In this
article, we report the expression of integrins in anterior capsules removed during cataract surgery. An immunohistochemical technique was used to detect the presence of integrin receptors
for lens capsule components. PATIENTS AND METHODS Prior Ethics Committee approval from the Argyll and Clyde health board was obtained. The circular sections of 28 circular capsules with
attached LECs were obtained during surgery from 28 patients with age-related cataract. The average age of the patients was 75 years±2.49 (SD), and of the 28 patients 15 were women and 13
men. The type of cataract (six anterior subcapsular cataracts, five nuclear sclerosis, three posterior subcapsular cataracts, five cortical cataracts, and nine anterior and posterior
subcapsular changes with nuclear sclerosis) prior to surgery was also noted. In the present study, no information about the intraoperative CCC size or postoperative results were available.
Each capsule was immediately placed on a silane-coated glass slide. ANTIBODIES All anti-integrin antibodies used were monoclonal (mAb) (Novocastra lab, Newcastle upon Tyne, UK) and are
listed in Table 1. The site of primary antibody binding was detected using a peroxidase-based avidin–biotin complex (ABC) system (Vector labs, Peterborough, UK)—in which
biotinylated-immunoglobulin and peroxidase were utilized. IMMUNOSTAINING The specimens on the glass slide were fixed in acetone for 5 min, washed in phosphate-buffered saline (PBS), and
blocked with 3% aqueous hydrogen peroxide for 10 min at room temperature. After rinsing, the specimens were stained with primary antibodies _α_2, _α_3, _α_5, _β_1, and _β_2 for 60 min at
37°C. Primary antibody was removed (with Gielson) and diluted biotinylated antibody (Vectastain ABC kit, Vector labs, Burlingame, CA, USA) was added to each slide. The specimen was incubated
with Vectastain elite ABC reagent at room temperature for 30 min. Peroxidase activity was visualized by incubating with diaminobenzidine (DAB) for 10 min. The specimens were counterstained
with haematoxylin, mounted with DPX, and observed under light microscopy. Negative control staining was performed by omission of the primary antibodies. RESULTS A total of 28 human capsules
obtained from cataractous lenses were analysed for immunoreactivity for CD49b, CD49c, CD49e, and CD18 antibodies. The CD49b, CD49c, CD49e, and CD18 antibodies specifically test for the
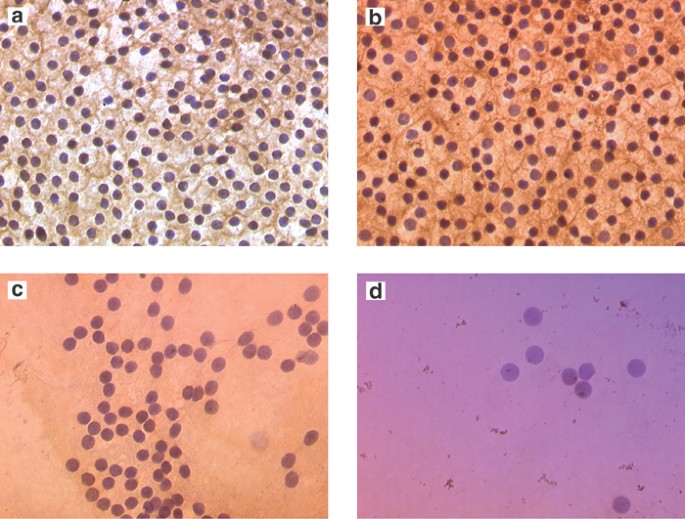
integrin subunits _α_2, _α_3, _α_5, and _β_2, respectively. The integrins were observed in some specimens and are portrayed in Figure 1. The individual subtypes of integrin antigens _α_2,
_α_3, _α_5, and _β_2 and their CD49b, CD49c, CD49e, and CD18 antibodies were analysed. The positive percentages were 2/6(33%), 6/8(75%), 3/9(33%) and 1/5(20%), respectively. There was no
staining in the negative control slides. Table 2 summarizes the immunostaining results obtained from the various subtypes of integrin dimers used. CD49c (_α_3_β_1 dimer) showed a
significantly higher proportion of positive results (χ2=4.72, df=1, _P_=0.03) in comparison to CD49b, CD49e, and CD18. The most striking staining profiles were obtained at the edges of the
capsules for CD49c. The edge staining was not an artefact, as indicated by the absence of staining when control immunoglobulin was substituted for anti-integrin antibodies and by the
specific staining pattern (brown colour) obtained with the anti-integrin antibodies. DISCUSSION Our results suggest that integrins of two different subfamilies are expressed in LECs.
Integrins are transmembrane heterodimers consisting of noncovalently associated _α_ and _β_ subunits. Colocalization of _α_ and _β_ subunits implies the presence of the corresponding
heterodimer.12 We report here that human cataract LECs express _α_2, _α_3, _α_5, _β_1 and _β_2 integrins in specimens removed during cataract surgery. There was a higher positive expression
of CD49c (_α_3β1 dimer) (75%) in comparison to _α_2_β_1, _α_5_β_1, and _α_1_β_2 subunits (33, 33, and 20%, respectively). In a previous report by Zhang _et al_13 the positive percentages of
_β_1, _β_2, _α_2, _α_3, and _α_5 integrins were 70, 65, 75, 70, and 80%, respectively. In the present study, the higher positive expression of _α_3 and _β_1 and a relatively poorer yield of
_α_2, _α_5, and _β_2 subunits may be secondary to the characteristics of the subtypes of integrins. The _α_3 and _β_1 subunits are considered to be responsible for the adherence of cells to
ECM components such as fibronectin, vitronectin, collagen type IV, and laminin. The _α_2, and _β_2 subunits are considered to be responsible for cell-to-cell adherence. During cataract
surgery, there is a breakdown of lens epithelial barrier representing altered cell surface receptor and cytoskeleton rearrangement similar to wound healing. In 1998, Saika _et al_14 reported
finding prolyl 4-hydroxylase subunits, which are needed for collagen production, in human capsules with IOLs showing that LECs are able to produce collagen. Recent studies indicate type IV
collagen, laminin, and fibronectin and vitronectin as important ECM proteins promoting lens epithelial cell adhesion.15,16 Integrins and other cell adhesion molecules may activate
intracellular signal pathways, altering the response of lens epithelial cell–cell adherence and cell–ECM interaction. Residual LECs come in contact with the lens capsule or with the IOL
after surgery. These cells undergo fibrous metaplasia and produce prostaglandin E2, resulting in capsular fibrosis and blood–aqueous barrier disruption.17,18 Continuous curvilinear
capsulorrhexis has been postulated to affect the degree of anterior capsular opacification leading to capsule contraction syndrome.19 After CCC and IOL implantation, the area of the anterior
capsule opening seems to decrease gradually for up to 3 months postoperatively.20,21,22,23,24 One possible explanation is the more important sphincter effect with a small capsulorrhexis. In
the present study, no information about the intraoperative CCC size or postoperative results was available. Therefore, we could not correlate with the immunohistochemical staining profile
at the edge of the capsulorrhexis and a subsequent role in capsule shrinkage leading to anterior capsular opacification. The sandwich theory of PCO15 states that an IOL made of a bioactive
material allows a single LEC to bond to the IOL and the posterior capsule at the same time, producing a sandwich pattern. This includes the IOL, cell monolayer, and posterior capsule. The
PCO rates would hence depend on the bioactivity of IOL, ECM properties, and LEC proliferation, differentiation and migration. A sealed sandwich may prevent the epithelial in growth through
this space. In the present study, the most striking feature was an increasing staining profile obtained towards the capsulorrhexis edges of the anterior capsule. Our results suggest numerous
integrin subunits being expressed prominently by proliferating cells, this being maximum in LECs towards the pre-equatorial zone. We suppose that the formation of PCO has a relationship
with differentiation that includes more than the proliferation of LECs. Cell adhesion molecules, especially integrins, play an important role in gene expression, signal transducers within
the cell, as well as receptors for ECM. In our study the positive percentages of integrin expression tested were not 100%, which may be the result of individual characteristics of each
patient. More studies are necessary to determine the presence of unique cellular receptors on human LECs for the ECM proteins, namely fibronectin, type IV collagen, and laminin. This would
therefore help us to understand the mechanisms by which these membrane proteins promote adhesion and migration of these cells. This would have clinical implications for the development of
new therapeutic approaches to inhibit PCO and postoperative inflammation caused specifically by residual LECs. REFERENCES * McDonnell PJ, Zarbin MA, Green WR . Posterior capsule
opacification in pseudophakic eyes. _Ophthalmology_ 1983; 90: 1548–1553. Article CAS PubMed Google Scholar * Moisseiev J, Bartov E, Schochat A, Blumenthal M . Long term study of the
prevalence of capsular opacification following extracapsular cataract extraction. _J Cataract Refract Surg_ 1989; 15: 531–533. Article CAS PubMed Google Scholar * Apple DJ, Solomon KD,
Tetz MR, Assia EL, Holland EY, Legler UF _et al_. Posterior capsule opacification. _Surv Ophthalmol_ 1992; 37(2): 73–116. Article CAS PubMed Google Scholar * Kim JT, Lee do H, Chung KH,
Kang IC, Kim DS, Joo CK . Inhibitory effects of salmosin a disintegrin on posterior capsular opacification _in vitro_ and _in vivo_. _Exp Eye Res_ 2002; 74(5): 585–594. Article CAS PubMed
Google Scholar * Wormstone IM, Tamiya S, Anderson I, Duncan G . TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. _Invest Ophthalmol Vis
Sci_ 2002; 43(7): 2301–2308. PubMed Google Scholar * Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG _et al_. TGF-beta induces morphological and molecular changes
similar to human anterior subcapsular cataract. _Br J Ophthalmol_ 2002; 86(2): 220–226. Article PubMed PubMed Central Google Scholar * Frezzotti R, Caporossi A, Mastrangelo D,
Hadjistilianou T, Tosi P, Cintorino M _et al_. Pathogenesis of posterior capsular opacification Part II: Histopathological and _in vitro_ culture findings. _J Cataract Refract Surg_ 1990;
16(3): 353–360. Article CAS PubMed Google Scholar * Blomstedt G, Fagerholm P, Gallo J, Philipson B . After-cataract in the rabbit eye following extracapsular cataract extraction—a wound
healing reaction. _Acta Ophthalmol Suppl_. 1987; 182 (Suppl): 93–99. CAS PubMed Google Scholar * Hansen SO, Crandall AS, Olson RJ . Progressive constriction of the anterior capsular
opening following intact capsulorrhexis. _J Cataract Refract Surg_ 1993; 19: 77–82. Article CAS PubMed Google Scholar * Nishi O, Nishi K . Intraocular lens encapsulation by shrinkage of
the capsulorrhexis opening. _J Cataract Refract Surg_ 1993; 19: 544–545. Article CAS PubMed Google Scholar * Nagamoto T, Hara E . Postoperative membranous proliferation from the anterior
capsulotomy margin onto the intraocular lens optic. _J Cataract Refract Surg_ 1995; 21: 208–211. Article CAS PubMed Google Scholar * Korhonen M, Ylanne J, Laitinen L, Cooper HM,
Quaranta V, Virtanen I . Distribution of the α1–_α_6 integrin subunits in human developing and term placenta. _Lab Invest_ 1991; 65(3): 347–356. CAS PubMed Google Scholar * Zhang HX, Jian
J, Zhang H, Tang X, Sun HM, Yuan JQ . Detection of integrins in cataract lens epithelial cells. _J Cataract Refract Surg_ 2000; 26: 287–291. Article CAS PubMed Google Scholar * Saika S,
Kawashima Y, Miyamito T _et al_. Immunolocalization of prolyl 4-hydroxylase subunits, α- smooth muscle actin, and extracellular matrix components in human lens capsules with lens implants.
_Exp Eye Res_ 1998; 66: 283–294. Article CAS PubMed Google Scholar * Linnola RJ, Werner L, Pandey SK, Escobar-Gomez M, Znoiko SL, Apple DJ . Adhesion of fibronectin, vitronectin,
laminin, and collagen type IV to intraocular lens materials in pseudophakic human autopsy eyes. _J Cataract Refract Surg_ 2000; 26(12): 1792–1806. Article CAS PubMed Google Scholar *
Olivero DK, Furcht LT . Type IV collagen, laminin, and fibronectin promote the adhesion and migration of rabbit lens epithelial cells _in vitro_. _Invest Ophthalmol Vis Sci_ 1993; 34:
2825–2834. CAS PubMed Google Scholar * Nishi O, Nishi K, Akaishi T, Shirasawa E . Detection of cell adhesion molecules in lens epithelial cells of human cataracts. _Invest Ophthalmol Vis
Sci_ 1997; 38: 579–585. CAS PubMed Google Scholar * Nishi O, Nishi K . Disruption of the blood–aqueous barrier by residual lens epithelial cells after intraocular lens implantation.
_Ophthalmic Surg_. 1992; 23: 325–329. CAS PubMed Google Scholar * Davison JA . Capsule contraction syndrome. _J Cataract Refract Surg_ 1993; 19: 582–589. CAS PubMed Google Scholar *
Dahlhauser KF, Wroblewski KJ, Mader TH . Anterior capsule contraction with foldable silicone intraocular lenses. _J Cataract Refract Surg_ 1998; 24: 1216–1219. Article CAS PubMed Google
Scholar * Hayashi K, Hayashi H, Nakao F, Hayashi F . Reduction in the area of the anterior capsule opening after polymethylmethacrylate, silicone, and soft acrylic intraocular lens
implantation. _Am J Ophthalmol_ 1997; 123: 441–447. Article CAS PubMed Google Scholar * Gonvers M, Sickenberg M, van Melle G . Change in capsulorrhexis size after implantation of three
types of intraocular lenses. _J Cataract Refract Surg_ 1997; 23: 231–238. Article CAS PubMed Google Scholar * Miyake K, Ota I, Miyake S, Maekubo K . Correlation between intraocular lens
hydrophilicity and anterior capsule opacification and aqueous flare. _J Cataract Refract Surg_ 1996; 22 (Suppl): 764–769. Article PubMed Google Scholar * Kimura W, Yamanishi S, Kimura T,
Sawada T, Ohte A . Measuring the anterior capsule opening after cataract surgery to assess capsule shrinkage. _J Cataract Refract Surg_ 1998; 24(9): 1235–1238. Article CAS PubMed Google
Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Ophthalmology Argyll and Clyde Acute Hospitals NHS Trust Paisley, Renfrewshire, PA2 9PN, UK M R K
Mathew, S B Murray, H G B Bennett, L A Webb & L Esakowitz * Department of Biological Sciences, University of Paisley, Paisley, UK S M McLean Authors * M R K Mathew View author
publications You can also search for this author inPubMed Google Scholar * S M McLean View author publications You can also search for this author inPubMed Google Scholar * S B Murray View
author publications You can also search for this author inPubMed Google Scholar * H G B Bennett View author publications You can also search for this author inPubMed Google Scholar * L A
Webb View author publications You can also search for this author inPubMed Google Scholar * L Esakowitz View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to M R K Mathew. ADDITIONAL INFORMATION Submitted in part at the Scottish Ophthalmological club meeting, Edinburgh, March 2001 RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mathew, M., McLean, S., Murray, S. _et al._ Expression of CD18, CD49b, CD49c and CD49e on lens anterior capsules in human
cataracts. _Eye_ 17, 473–477 (2003). https://doi.org/10.1038/sj.eye.6700380 Download citation * Received: 25 February 2002 * Accepted: 02 August 2002 * Published: 15 May 2003 * Issue Date:
01 May 2003 * DOI: https://doi.org/10.1038/sj.eye.6700380 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * anterior capsule opacification *
CD49b,CD49c,CD49e and CD18 * integrins * extracellular matrix proteins