- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT We report on the location of 283 miRNAs in the human genome in relation to copy number changes in three distinct types of tumours: prostate, bladder and colon. In prostate and colon
tumours, we find miRNAs over-represented in regions with copy number gain and under-represented in regions with copy number loss. Surprisingly this pattern appears to be reversed in bladder
cancer. We compared our miRNA copy number data to published miRNA expression data; unexpectedly, we did not find a statistically significant relationship between miRNA copy number and
expression level. This suggests that miRNA expression is regulated through different mechanisms than mRNA expression. SIMILAR CONTENT BEING VIEWED BY OTHERS MIRNA ACTIVITY INFERRED FROM
SINGLE CELL MRNA EXPRESSION Article Open access 28 April 2021 COMPREHENSIVE COMPUTATIONAL ANALYSIS OF DIFFERENTIALLY EXPRESSED MIRNAS AND THEIR INFLUENCE ON TRANSCRIPTOMIC SIGNATURES IN
PROSTATE CANCER Article Open access 29 January 2025 MICRORNA-184 IN THE LANDSCAPE OF HUMAN MALIGNANCIES: A REVIEW TO ROLES AND CLINICAL SIGNIFICANCE Article Open access 24 November 2023 MAIN
MicroRNAs (miRNAs) are small non-coding RNAs that are conserved in sequences between distantly related organisms. Mature miRNAs (∼22 nucleotides) regulate gene expression by targeting mRNAs
for cleavage or translational inhibition (Bartel, 2004). They are known to be differently expressed in different tissues. Moreover, several studies have suggested that miRNAs might be
involved in human tumorigenesis (Lu et al, 2005; Volinia et al, 2006). Calin et al (2004) showed that miRNAs are frequently located near genomic fragile sites as well as in cancer-associated
genomic regions. They screened PubMed for papers reporting any cancer-related abnormalities and found that 98 of 186 miRNAs were located in these regions. However, their study was not
cancer type specific and whether it generalises to specific cancers was not discussed. Lu et al (2005) reported on miRNAs expression profiles (217 miRNAs in 334 samples) and found that the
majority of the differentially expressed miRNAs were downregulated in tumour samples compared to normal samples. More recently, Volinia et al (2006) showed that miRNAs differently expressed
in solid cancer were mostly overexpressed. They evaluated the expression profiles of 228 human miRNAs in 540 samples from six solid tumour types. Based on these, they identified six
tissue-specific cancer signatures and proposed that the most common miRNA event in solid tumours is gain of expression, while loss of expression is more tissue-specific and less common. In
this study, we report on the location of miRNAs (_n_=283) in relation to copy number alterations in three specific cancer types, prostate, bladder and colon. We use Affymetrix 10 and 50 k
SNP arrays to identify genomic regions with abnormal copy numbers. Further, we discuss our results in relation to the findings of Volinia et al (2006) and Lu et al (2005). MATERIALS AND
METHODS The GeneChipMapping 10 k Early Access Array from Affymetrix was applied to a set of 128 samples (113 normal samples and 15 colon adenocarcinomas) and the GeneChipMapping 50 K array
was applied to 143 samples (72 normal samples, 41 prostate tumours and 30 bladder tumours). SNP intensities were extracted using the dChip software and subsequently all intensities were
normalised to facilitate comparisons between SNPs and arrays, as described in Supplementary Material. Using this procedure, the intensities in normal samples would be centred on zero with a
standard deviation (s.d.) of 1. We defined seven groups of tumours: all prostate tumours (P1), prostate tumours without metastasis (P2) or with metastasis (P3), all bladder tumours (B1),
bladder tumours in stage Ta (B2) or in stage T1 (B3) and all colon tumours (C1). For each group of tumours, an average intensity value for the group was calculated for each SNP. From these,
DNA copy numbers were estimated using a threshold of ±2 s.d. (above 2 s.d.: gain, below 2 s.d.: loss, and otherwise two copies, i.e. normal copy number). Subsequently, for each group of
tumours, the genome was divided into regions according to the DNA copy numbers (gain, loss, normal) of consecutive SNPs. Genomic regions without any SNP data were not considered (see
Supplementary Material for more information). Subsequently, it was investigated if the distribution of genomic copy number alterations correlated with the genomic distribution of 283 miRNAs
(identified in the miRNA registry (release 7.1), Griffiths-Jones, 2004; Griffiths-Jones et al, 2006). RESULTS Using the method and the predefined groups of tumours described in Materials and
Methods, we identified commonly occurring copy number alterations in the groups. Genomic alterations are generally coherent with previous findings (Zieger et al, 2005; Andersen et al, 2006;
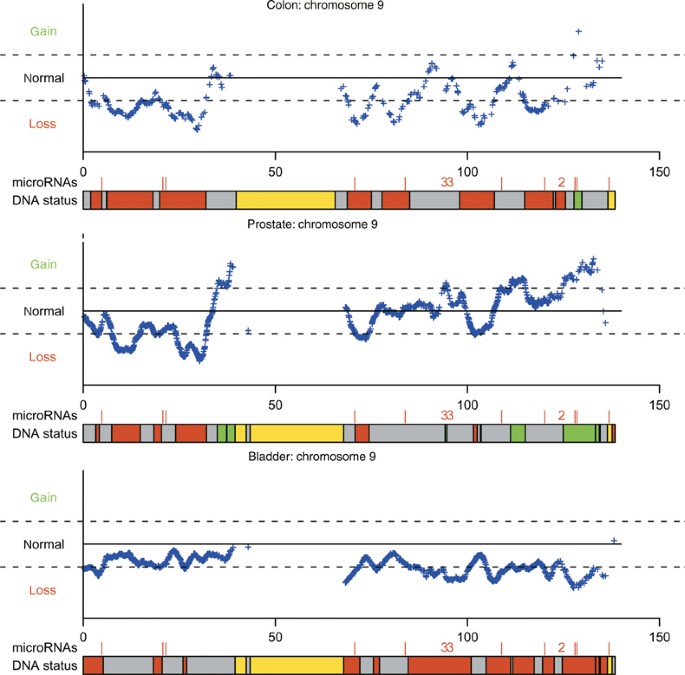
Tørring et al, submitted). LOCATION OF MIRNAS The number of miRNAs affected by the alterations was counted and it was calculated if these numbers were unexpectedly high or low (Table 1 and
Figure 1). For both the colon tumours (C1) and the prostate tumours (P1, P2 and P3), we observed an over-representation in gain regions and an under-representation of miRNAs in loss regions
(_P_<0.00001) (Table 1 and Figure 1). As regards the bladder tumours (B1 and B3), we observed a slight under-representation of miRNAs in the gain regions (_P_=0.0089; Table 1). Many
miRNAs are located in close vicinity of each other (distance <0.1 Mb). After grouping miRNAs with distance <0.1 Mb into clusters, we ended up with 152 clusters and did the analysis on
those. We found the same features as before (Table 1). COMPARISONS BETWEEN THE DIFFERENT TUMOURS We compared the different tumour groups in order to investigate whether the miRNAs were
concentrated in loss or gain regions that were shared between the groups. Tables S1 and S2 in Supplementary Material clearly indicate that regions are generally not shared and that the
findings above are not a consequence of shared alterations between tumour types. GENOMIC COPY NUMBER ALTERATIONS AND MIRNA EXPRESSION Volinia et al (2006) identified 39 overexpressed and six
under-expressed miRNAs (out of 228) in a sample of prostate tumours and 21 overexpressed and one underexpressed miRNAs in a sample of colon tumours. The differentially expressed miRNAs were
not statistically over-represented in the gain regions or loss regions identified in the corresponding tumours in our samples (_P_-values>0.18; see Table 2). DISCUSSION In several
papers, it has been shown that mRNA (or gene) expression and gene copy number correlate (e.g. Benetkiewicz et al, 2005; Tsafrir et al, 2006). It is natural to hypothesise that miRNA
expression also correlates with copy number. In this study, we have reported on the location of miRNAs in relation to copy number changes in three different cancers, prostate, colon and
bladder. In the prostate and colon tumours, we observed an over-representation in the gain regions and an under-representation of miRNA genes in the loss regions. Generally the gain and the
loss regions were not shared between the two cancer types and miRNAs were generally not located in shared regions. This pattern is consistent with the hypothesis stated above and the
findings in Volinia et al (2006) that many miRNA were overexpressed in prostate and colon tumours while few were underexpressed. However, when scrutinising the locations of the
differentially expressed miRNAs it appears that these are not more (less) frequently located in gain (loss) regions; contrary to what we would expect if miRNA expression correlated with copy
number. Importantly, we found a different pattern of copy number alterations in our set of bladder tumours, indicating that the relationship between cancer-related regions and miRNA
locations is not straight-forward and probably cancer type specific. Several explanations could accommodate for the discrepancies between expression and copy numbers. First of all, the copy
numbers and the miRNA expressions values are obtained from different samples and thus could show different conflicting features. However, our samples as well as the samples in Volinia et al
(2006) are not believed to represent special groups or subtypes of prostate and colon tumours, and general features should thus be preserved between the samples. Secondly, one could raise
doubt about the validity of the data. However, in a number of papers (Huang et al, 2004, 2006) it has been shown that copy numbers reliably can be derived using SNP arrays. The miRNA
expression data set is one of a few public available genome-wide data sets; in the only other data set that is known to us (Lu et al, 2005) it is found that miRNA expression consistently is
downregulated in prostate, while the pattern in colon is less clear. Finally, miRNA expression could be regulated by mechanisms very different from the mechanisms that regulate mRNA
expression, resulting in a less obvious pattern between expression and copy numbers. For example, miRNA expression could be correlated to the expression of their numerous mRNA targets
(Griffiths-Jones, 2004), which might be located in different parts of the genome and thus potentially have different copy-numbers. The data is available upon request. CHANGE HISTORY * _ 16
NOVEMBER 2011 This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Andersen CL, Wiuf C, Kruhøffer
M, Korsgaard M, Laurberg S, Ørntoft TF (2006) Frequent occurrence of uniparental disomy in colorectoral cancer. _Carcinogenisis_, advanced access, doi: 10.1093/carcin/bg1086 * Bartel DP
(2004) MicroRNAs: genomics, biogenesis, mechanism, and function. _Cell_ 116: 281–297 Article CAS PubMed Google Scholar * Benetkiewicz M, Wang Y, Schaner M, Wang P, Mantripragada KK,
Buckley PG, Kristensen G, Borresen-Dale AL, Dumanski JP (2005) High-resolution gene copy number and expression profiling of human chromosome 22 in ovarian carcinomas. _Genes Chromosomes
Cancer_ 42: 228–237, doi: 10.1002/gcc.20128 Article CAS PubMed Google Scholar * Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini
M, Croce CM (2004) Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. _Proc Natl Acad Sci USA_ 101: 2999–3004, doi: 10.1073/pnas.242606799
Article CAS PubMed PubMed Central Google Scholar * Griffiths-Jones S (2004) The microRNA registry. _Nucleic Acids Res_ 32: D109–D111 Article CAS PubMed PubMed Central Google
Scholar * Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ (2006) miRBase: MicroRNA sequences, targets and gene nomenclature. _Nucleic Acids Res_ 34: D140–D144, doi:
10.1093/nar/gkj112 Article CAS PubMed Google Scholar * Huang J, Wei W, Chen J, Zhang J, Liu G, Di X, Mei R, Ishikawa S, Aburatani H, Jones KW, Shapero MH (2006) CARAT: a novel method for
allelic detection of DNA copy number changes using high density oligonucleotide arrays. _BMC Bioinformat_ 7: 83 Article Google Scholar * Huang J, Wei W, Zhang J, Liu G, Bignell GR,
Stratton MR, Futreal PA, Wooster R, Jones KW, Shapero MH (2004) Whole genome dna copy number changes identified by high density oligonucleotide arrays. _Hum Genom_ 1: 287–299 Article CAS
Google Scholar * Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA
expression profiles classify human cancers. _Nature_ 435: 834–838, doi: 10.1038/nature03702 Article CAS PubMed Google Scholar * Tørring N, Borre M, Sørensen KD, Andersen CL, Wiuf C,
Ørntoft TF . Genome-wide analysis of allelic imbalance in prostate cancer using the Affymetrix 50 K SNP mapping array. Submitted * Tsafrir D, Bacolod M, Selvanayagam Z, Tsafrir I, Shia J,
Zeng Z, Liu H, Krier C, Stengel RF, Barany F, Gerald WL, Paty PB, Domany E, Notterman DA (2006) Relationship of gene expression and chromosomal abnormalities in colorectal cancer. _Cancer
Res_ 66: 2129–2137, doi: 10.1158/0008-5472.CAN-05-2569. Article CAS PubMed Google Scholar * Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C,
Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM (2006) A microRNA expression signature of human solid tumors defines cancer gene targets.
_Proc Natl Acad Sci USA_ 103: 2257–2261, doi: 10.1073/pnas.0510565103. Article CAS PubMed PubMed Central Google Scholar * Zieger K, Dyrskjot L, Wiuf C, Jensen JL, Andersen CL, Jensen
KM, Orntoft TF (2005) Role of activating fibroblast growth factor receptor 3 mutations in the development of bladder tumors. _Clin Cancer Res_ 11: 7709–7719, doi:
10.1158/1078-0432.CCR-05-1130. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We are grateful for financial support from the Danish Cancer Society, the University
and County of Aarhus, the Danish Research Council, the John and Birthe Meyer Foundation and the Fraenkel Foundation. We are also grateful for the thoughtful and helpful comments received
from the journal reviewers, which improved the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Bioinformatics Research Center (BiRC), University of Aarhus, Hoegh-Guldbergs Gade 10,
Bldg 1090, Aarhus C, 8000, Denmark P Lamy & C Wiuf * Molecular Diagnostic Laboratory, Aarhus University Hospital, Skejby, Brendstrupgaardsvej 100, Aarhus N, 8200, Denmark C L Andersen,
L Dyrskjøt, N Tørring, T Ørntoft & C Wiuf Authors * P Lamy View author publications You can also search for this author inPubMed Google Scholar * C L Andersen View author publications
You can also search for this author inPubMed Google Scholar * L Dyrskjøt View author publications You can also search for this author inPubMed Google Scholar * N Tørring View author
publications You can also search for this author inPubMed Google Scholar * T Ørntoft View author publications You can also search for this author inPubMed Google Scholar * C Wiuf View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to C Wiuf. ADDITIONAL INFORMATION Supplementary Information accompanies the
paper on British Journal of Cancer website (http://www.nature.com/bjc) SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIAL 1 (DOC 142 KB) RIGHTS AND PERMISSIONS From twelve months after its
original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lamy, P., Andersen, C., Dyrskjøt, L. _et al._ Are microRNAs located in genomic
regions associated with cancer?. _Br J Cancer_ 95, 1415–1418 (2006). https://doi.org/10.1038/sj.bjc.6603381 Download citation * Received: 07 August 2006 * Revised: 28 August 2006 *
Accepted: 30 August 2006 * Published: 26 September 2006 * Issue Date: 20 November 2006 * DOI: https://doi.org/10.1038/sj.bjc.6603381 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * microRNA * affymetrix SNP array * copy number * colon * prostate * bladder