- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT To evaluate the efficacy and safety of capecitabine and cisplatin in patients with recurrent gastric cancer after fluoropyrimidine-based adjuvant therapy. Patients with
histologically confirmed and measurable advanced gastric cancer that had relapsed after fluoropyrimidine-based adjuvant chemotherapy received oral capecitabine (1250 mg m−2 twice daily, days
1–14) and intravenous cisplatin (60 mg m−2 over 1 h, day 1) every 3 weeks. In total, 32 patients were enrolled, of whom 30 were evaluable for efficacy and 32 for safety. A median of 5
cycles (range 1–10) was administered. One patient achieved a complete response and eight had partial responses, giving an overall response rate of 28% (95% CI, 13–44%). The median time to
progression and median overall survival were 5.8 months (95% CI, 4.1–7.5 months) and 11.2 months (95% CI, 5.5–16.9 months), respectively. Grade 3 neutropenia and thrombocytopenia were
observed in 38 and 6% of patients, respectively. Grade 2/3 nonhaematological toxicities included diarrhoea (19%), stomatitis (19%) and hand-foot syndrome (31%). No grade 4 toxicity,
neutropenic fever or treatment-related deaths occurred. Capecitabine in combination with cisplatin was effective and well tolerated as first-line treatment in patients with recurrent gastric
cancer after fluoropyrimidine-based adjuvant chemotherapy. SIMILAR CONTENT BEING VIEWED BY OTHERS ALTERNATING MODIFIED CAPOX/CAPIRI PLUS BEVACIZUMAB IN UNTREATED UNRESECTABLE METASTATIC
COLORECTAL CANCER: A PHASE 2 TRIAL Article Open access 11 December 2024 TREATMENT OF PATIENTS WITH CARCINOMAS IN ADVANCED STAGES WITH 5-FLUOROURACIL, FOLINIC ACID AND PYRIDOXINE IN TANDEM
Article Open access 27 May 2024 A RANDOMIZED PHASE 3 TRIAL OF GEMCITABINE OR NAB-PACLITAXEL COMBINED WITH CISPLATIN AS FIRST-LINE TREATMENT IN PATIENTS WITH METASTATIC TRIPLE-NEGATIVE BREAST
CANCER Article Open access 12 July 2022 MAIN Gastric cancer is the second most common cancer worldwide (Parkin et al, 1999). While the incidence of gastric cancer has been declining for
several decades, it varies substantially between different racial and ethnic groups. For example, in South Korea, gastric cancer remains the most common and most fatal malignant neoplasm
(Bae et al, 2002). Surgical resection is the only curative treatment currently available for gastric cancer, but the role of adjuvant chemotherapy after curative resection is unclear. A
meta-analysis showed that adjuvant chemotherapy was associated with borderline statistically significant, but clinically insignificant, survival improvement (Earle and Maroun, 1999). A more
recent meta-analysis (Janunger et al, 2002) of 21 randomised studies found a significant survival benefit for patients treated with postoperative adjuvant chemotherapy compared with controls
(odds ratio (OR) 0.84, 95% confidence interval (CI) 0.74–0.96). However, when Western and Asian studies were analysed separately there was no benefit in the Western groups (OR 0.96, 95% CI
0.83–1.12). Recently, the North American Intergroup performed a prospective randomised study in 556 patients with advanced oesophagogastric cancer receiving postoperative adjuvant
5-FU/leucovorin plus radiotherapy (MacDonald et al, 2001). In this study, chemoradiation led to a significant improvement in median overall survival compared with surgery alone (36 _vs_ 27
months, _P_=0.005). However, this study has been criticised in light of the poor overall survival, probably because inadequate surgery (D0 lymph node dissection) was performed in over 50% of
the patients (Stahl, 2004). Overall, at the time of diagnosis, many patients have locally advanced unresectable or metastatic disease and, even after apparently complete resection, local
and distant relapses are common. The prognosis of patients with recurrent gastric cancer is very poor, with the median duration of survival ranging from 3 to 5 months in untreated patients
(Glimelius et al, 1997). In patients with unresectable/metastatic disease, first-line chemotherapy is superior to best supportive care in terms of quality of life and overall survival (Murad
et al, 1993; Pyrhonen et al, 1995; Glimelius et al, 1997). 5-FU is widely used for the treatment of gastric cancer and other gastrointestinal tumours. 5-FU in combination with cisplatin (FP
regimen) is commonly used in advanced disease because of the activity of both drugs when administered as single agents. In randomised phase III trials in advanced gastric cancer, FP led to
improved response rates compared with 5-FU, doxorubicin and mitomycin (FAM) or 5-FU single-agent therapy (Kim et al, 1993), and showed a trend towards improved response rates compared with
5-FU, doxorubicin and methotrexate (FAMTX) or etoposide, leucovorin and bolus 5-FU (ELF) (Vanhoefer et al, 2000). However, few studies have evaluated the efficacy of first-line treatment in
patients with gastric cancer that has relapsed after fluoropyrimidine-based adjuvant chemotherapy. Furthermore, first-line chemotherapy after relapse is often associated with pronounced
adverse effects and response rates rarely exceed 20% (Hill and Cunningham 1998). For these reasons, novel compounds with activity and less toxicity are needed in this setting. The oral
fluoropyrimidine capecitabine (Xeloda®) was designed to generate 5-FU preferentially in tumour tissue and to mimic a continuous infusion of 5-FU while minimising systemic 5-FU exposure.
Following absorption, capecitabine is metabolised in a three-step metabolic process, the final step being conversion to 5-FU by thymidine phosphorylase (TP): tumour selectivity results from
the significantly greater TP activity in tumour tissue compared with healthy tissue (Miwa et al, 1998; Schüller et al, 2000). In preclinical xenograft models, oral capecitabine has been
shown to be highly active against gastric cancer (Ishikawa et al, 1998; Ishitsuka, 2000). This finding was subsequently extended to the clinic in a phase II study of previously untreated
patients with advanced gastric cancer, in which capecitabine 1250 mg m−2 twice daily on days 1–14 every 3 weeks was both active (overall response rate 28%; stable disease 36%) and well
tolerated (Hong et al, 2004). In addition, capecitabine showed activity in a preclinical xenograft model of a 5-FU-resistant tumour (Cao et al, 1997) and some activity in patients with
metastatic colorectal cancer refractory to 5-FU/leucovorin chemotherapy (Lee et al, 2003). As oral capecitabine is a highly active single agent and its safety profile differs from that of
cisplatin with little overlap of key toxicities, capecitabine combined with cisplatin is an appealing and convenient alternative to 5-FU/cisplatin. In a recent phase II study (Kim et al,
2002), capecitabine plus cisplatin was active and well tolerated as first-line chemotherapy. In the present phase II study we evaluated the efficacy and safety of the same combination
regimen as first-line treatment in patients with gastric cancer recurring after fluoropyrimidine-based adjuvant chemotherapy. MATERIALS AND METHODS PATIENT SELECTION Patients were considered
eligible if they had histologically confirmed advanced gastric cancer with at least one measurable target lesion according to RECIST guidelines (Therasse et al, 2000) (diameter of ⩾2 cm)
that had relapsed after previous fluoropyrimidine-based adjuvant chemotherapy. In addition, patients had to be 18–75 years old, have an Eastern Cooperative Oncology Group (ECOG) performance
status of 0–2, have adequate liver, kidney, and bone marrow function, and to have received no prior treatment with capecitabine or platinum compounds. Patients with unresolved bowel
obstruction or malabsorption syndrome were excluded. The protocol was approved by the institutional review board of the Asan Medical Center, and all patients gave written informed consent
before enrolment. TREATMENT SCHEDULE Capecitabine was administered orally at a dose of 1250 mg m−2 twice daily according to the standard intermittent schedule (14 days of treatment followed
by a 7-day rest period, every 3 weeks). Cisplatin was administered intravenously on day 1 (before the first dose of capecitabine) at a dose of 60 mg m−2 for 1 h with hydration, and repeated
every 3 weeks. The hydration procedure consisted of 1 l of normal saline (containing 20 mEq of KCl and 8 mEq of MgSO4) infused intravenously for 2.5 h both before and after cisplatin
infusion, giving a total of 2 l of saline infused over a 5-h period. In addition, intravenous furosemide 20 mg was given 30 min before infusing cisplatin. Mannitol was not used. A serotonin
antagonist and dexamethasone were routinely given before cisplatin administration to prevent emesis. Treatment was given in the outpatient clinic and continued until disease progression,
unacceptable adverse events, or withdrawal by the patient. EVALUATION OF SAFETY AND DOSE MODIFICATION Safety was evaluated before each treatment cycle according to the National Cancer
Institute Common Toxicity Criteria (NCI-CTC), version 2.0. To begin the next treatment cycle, each patient was required to have a platelet count ⩾75 × 109 l−1, a neutrophil count ⩾1 × 109
l−1 and resolution or improvement of clinically significant nonhaematological adverse events (excluding alopecia) to grade 1 or 0. Dose adjustments (interruption and/or reduction) and
discontinuation in response to adverse events were made for each drug according to previous guidelines and depended on classification, grade and frequency of occurrence (Kim et al, 2002).
Dose adjustment criteria for cisplatin were based on serum creatinine levels immediately prior to each cycle: if serum creatinine was <1.5 mg dl−1, full-dose cisplatin was given; if serum
creatinine was 1.5–2.5 mg dl−1, the cisplatin dose was reduced by 50%; if serum creatinine was >2.5 mg dl−1, the patient was excluded from the study. Patients were withdrawn from study
treatment (but still followed-up) if treatment was delayed for more than 2 weeks. ASSESSMENT OF COMPLIANCE AND DOSE INTENSITY Compliance with capecitabine treatment was monitored by
questioning patients and counting their remaining pills at each outpatient visit. The ratio of the actual administered dose to the scheduled dose was then calculated. Dose intensity was
defined as the total amount of drug given (mg m−2) divided by the number of weeks. PRETREATMENT, FOLLOW-UP STUDIES AND RESPONSE EVALUATION Physical examination and chest X-rays were carried
out before each chemotherapy cycle, and complete blood counts and biochemical tests were performed before and on day 15 of each cycle. Response evaluation was performed by computed
tomography (CT) scan every 2–3 cycles until the tumour progressed. Tumour response was classified on the basis of the response evaluation criteria defined by RECIST guidelines (Therasse et
al, 2000), and responses were required to last longer than 4 weeks. STATISTICAL ANALYSIS All enrolled patients were included in the intention-to-treat (ITT) analysis of efficacy. The trial
was conducted according to the two-stage Gehan design (Simon 1987) with response rate as the primary end point. We planned to enrol at least 25 evaluable patients, with a target minimum
response rate of 20%. If no objective response was seen among the first 14 patients in the study, the probability of a response rate ⩾20% would be <5%, and the study was to be
discontinued. One or more responses would indicate that continuation was warranted, and at least 25 patients would be required to estimate a response rate with a standard error of
approximately 10%. The number of patients enrolled was increased to 32 patients to better estimate the response rate. The Fisher's exact test was used to compare late _vs_ early relapse
groups and the different adjuvant regimens. Time to progression (TTP), survival and duration of response were secondary end points and were estimated using the Kaplan–Meier method. The
duration of response was defined as the interval from the onset of complete response (CR) or partial response (PR) until first evidence of disease progression. If death occurred before
progression was documented, the date of death was assumed to be the date of progression. TTP was calculated from the date of entry into the study until the date of progression, and overall
survival was measured from the date of entry to the date of last follow-up or death. RESULTS PATIENT CHARACTERISTICS A total of 32 patients were enrolled between October 2000 and April 2003.
Baseline characteristics, which are shown in Table 1, show a relatively standard gastric cancer population (with more males than females). EFFICACY AND SURVIVAL A total of 30 patients were
evaluable for response (Table 2). One patient was not evaluable because of loss to follow-up after the first cycle of treatment, and the second patient withdrew consent. One CR and eight PRs
were observed, giving an overall response rate of 28% (95% confidence intervals (CI), 13–44%) in the ITT analysis (Table 2). There was a numerically superior overall response rate in
patients whose tumour relapsed more than 6 months (late relapse group) compared with those whose tumour relapsed within 6 months of completing adjuvant chemotherapy (early relapse group) (39
_vs_ 21%), although the difference did not reach statistical significance (_P_=0.427; Table 2). There was also no significant difference in overall response rate in patients who received
doxifluridine±mitomycin-C (40%) compared with 5-FU+doxorubicin+mitomycin-C (23%, _P_=0.407; Table 2). The median duration of response in the nine responding patients was 8.5 months (range
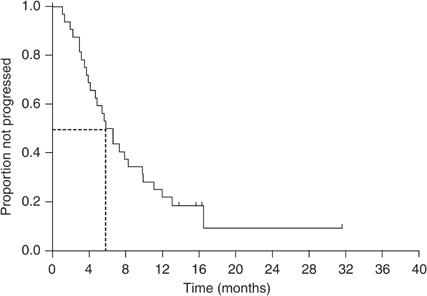
3.6–29.6 months). The median follow-up period was 19.4 months (range 9.2–39.8 months). The median TTP for all patients was 5.8 months (95% CI, 4.1–7.5 months; Figure 1). The median overall
survival was 11.2 months (95% CI, 5.5–16.9 months; Figure 2), with a 1-year survival rate of 49% (95% CI, 32–66%). Although there was a trend towards a more prolonged overall survival (14.1
_vs_ 9.3 months, _P_=0.075) and TTP (8.3 _vs_ 5.4 months, _P_=0.072) in the late relapse group compared with the early relapse group, the differences did not reach statistical significance.
ADVERSE EVENTS A total of 173 treatment cycles (median 5; range 1–10 cycles) were administered, of which there are no data for one cycle because one patient was lost to follow-up. The
frequencies of treatment-related haematological and nonhaematological adverse events are shown in Table 3. The most common treatment-related haematological adverse event was neutropenia,
which occurred at grade 3 intensity in 12 patients (38%). No patient experienced grade 4 neutropenia or febrile neutropenia. Grade 2 hand-foot syndrome was also relatively common, occurring
in 10 patients (31%). There were no treatment-related deaths. Treatment interruption or dose reduction was required in 71 cycles. In total, 20 patients (62.5%) required dose reductions,
which were due to haematological adverse events (12 of 20 patients; 60%), hand-foot syndrome (three patients; 15%), nausea/vomiting (two patients; 10%), stomatitis (one patient; 5%), and
diarrhoea (one patient; 5%). Treatment was delayed in 16 patients (50%) as a result of haematological adverse events (13 patients; 81.3%), hand-foot syndrome (two patients; 12.5%),
nausea/vomiting (one patient; 6.3%) and stomatitis (one patient; 6.3%). There was no treatment interruption or dose reduction with cisplatin. The median dose intensity for all treatment
cycles was 8902 mg m−2 week−1 (range 4298–15576 mg m−2 week−1) for capecitabine and 19.2 mg m−2 week−1 (range 15.0–27.5 mg m−2 week−1) for cisplatin, corresponding to 76 and 96%,
respectively, of the planned dose intensities. Although patient compliance with taking their prescribed number of capecitabine tablets was good (97% during the first six cycles), the dose
intensity of capecitabine decreased progressively during the first six cycles and fell below 80% of that planned after the second treatment cycle. Conversely, the dose intensity of cisplatin
was well maintained throughout the first 6 cycles. DISCUSSION The present study suggests that the combination of capecitabine and cisplatin is an effective and well-tolerated regimen for
the first-line treatment of patients with gastric cancer recurrent after fluoropyrimidine-based adjuvant chemotherapy. This combination regimen demonstrated promising efficacy, with a tumour
response rate of 28%, a median TTP of 5.8 months and a median overall survival of 11.2 months. The efficacy demonstrated in the present study may be a function of additive or synergistic
antitumour activity between the two agents also observed in other studies in a variety of gastrointestinal cancers (Evans et al, 2002; Kim et al, 2002, 2003; Pivot et al, 2003). The lack of
prior exposure to cisplatin may have also played a role in the response to the capecitabine–cisplatin combination. It is difficult to compare the results of the present study with other
studies, as there is little information in the literature on response to first-line chemotherapy after relapse of advanced gastric cancer following fluoropyrimidine-based adjuvant
chemotherapy. The results of this study seem to be similar or somewhat superior to those achieved with other regimens used in this setting, such as weekly high-dose infusional
5-FU/leucovorin, docetaxel or irinotecan (Futatsuki et al, 1994; Vanhoefer et al, 1994; Graziano et al, 2000; Giuliani et al, 2003). Weekly high-dose infusional 5-FU/leucovorin has been
reported to achieve a response rate of 18% and a median overall survival of 5 months (Vanhoefer et al, 1994), whereas docetaxel or irinotecan showed objective response rates in the range of
5–27% and overall survival times ranging from 3.5 to 10.2 months (Futatsuki et al, 1994; Graziano et al, 2000; Giuliani et al, 2003). However, since our study population was limited to
patients with gastric cancer recurrent after previous adjuvant chemotherapy, and excluded those with metastatic advanced gastric cancer who failed first-line chemotherapy, the results of
this study should be interpreted cautiously. Nevertheless, considering that single-agent cisplatin is associated with a response rate of approximately 19% when used as first-line therapy
(Aabo et al, 1985; Perry et al, 1986), we would not have expected to observe a response rate much greater than this by combining a fluoropyrimidine (i.e. capecitabine) with cisplatin in
patients who had recurrent disease after prior fluoropyrimidine-based adjuvant chemotherapy. Therefore, the current response rate of 28% is encouraging. The results of the present study are
similar to our previous study of the same regimen as a first-line treatment in previously untreated patients with advanced gastric cancer (Kim et al, 2002) with regard to both median overall
survival (11.2 _vs_ 10.1 months) and median TTP (5.8 _vs_ 6.3 months), although the objective response rate was lower (28 _vs_ 55%). It is logical that the same chemotherapeutic regimen
should have a lower response rate in patients who have previously received fluoropyrimidine-based adjuvant therapy than those who have not. The similar TTP and overall survival may have been
a result of differences in tumour burden between the two study populations at the start of chemotherapy; in the current study, 34% of patients had involvement of more than one organ
compared with 46% in our previous study. In addition, these similar survival results may be related to the second-line treatment received by patients; 47% of patients received second-line
treatment in the present study compared with 29% of patients in our previous study. Also in the current study, patients with early relapse, who may have developed fluoropyrimidine-resistant
tumours, showed a favourable response rate, TTP and overall survival, suggesting that the combination of capecitabine and cisplatin may overcome drug resistance to fluoropyrimidines.
However, further studies are required to verify this observation. Owing to the limited response duration, TTP and overall survival in patients with gastric cancer, safety and tolerability
are important considerations in the assessment of new treatment regimens. The combination of capecitabine and cisplatin has previously shown good antitumour efficacy with a favourable safety
profile as first-line chemotherapy in advanced gastric (Kim et al, 2002) and biliary cancer (Kim et al, 2003), and also as salvage treatment in previously treated head and neck cancer
patients (Pivot et al, 2003). In the present study, adverse events were generally mild and manageable without the need for hospitalisation. There were no treatment-related deaths or cases of
febrile neutropenia, despite 38% of patients developing grade 3 neutropenia. Hand-foot syndrome was common, but severe cases were successfully prevented through strict adherence to a
predefined dose modification schedule. These data suggest that capecitabine in combination with cisplatin can be administered safely in an outpatient clinic setting. Compliance with
capecitabine, which is very important for oral chemotherapeutic agents, was generally very good. However, the median dose intensity for capecitabine was 76% of that planned because of dose
reductions or delays primarily associated with neutropenia. In addition, the dose intensity of capecitabine gradually decreased over the first six treatment cycles, just as in other phase II
studies of capecitabine plus cisplatin as first-line chemotherapy (Kim et al, 2002, 2003). Therefore, we recommend that the starting dose of capecitabine be reduced to 1000 mg m−2 twice
daily on days 1–14 every 3 weeks in any future evaluations of this combination. It is interesting to note that triple-drug combinations, such as ECF (Webb et al, 1997), have shown
particularly favourable responses in the first-line treatment of previously untreated advanced gastric cancer. Recently, we conducted a phase I/II study of a new triple-drug combination of
docetaxel–capecitabine–cisplatin as first-line therapy in advanced gastric cancer and observed very promising efficacy and acceptable safety (Kang et al, 2004). Capecitabine/cisplatin in
combination with epirubicin (ECX) is also being evaluated in a randomised phase III study (REAL2 trial) as first-line therapy for previously untreated advanced gastro-oesophageal cancer
(Sumpter et al, 2003); another triple combination including capecitabine (epirubicin/oxaliplatin/capecitabine: EOX) is being examined in this trial together with the complimentary regimens
containing 5-FU (ECF and epirubicin/oxaliplatin/5-FU: EOF). An international phase III trial is also underway to evaluate replacing infusional 5-FU with capecitabine in 5-FU/cisplatin
combination chemotherapy in first-line advanced gastric cancer. In conclusion, the combination of capecitabine and cisplatin is effective and well tolerated as a first-line treatment for
gastric cancer recurrent after prior fluoropyrimidine-based adjuvant chemotherapy. It would be interesting to speculate that addition of a third agent (e.g. epirubicin, docetaxel,
paclitaxel, irinotecan) to capecitabine/cisplatin might improve this response in recurrent gastric cancer after prior fluoropyrimidine-based adjuvant chemotherapy. With the increasing
adoption of adjuvant chemotherapy and chemoradiotherapy after surgery for advanced gastric cancer, there will be an expansion of studies into the use of first-line chemotherapy regimens for
recurrence of gastric cancer following prior adjuvant therapy. CHANGE HISTORY * _ 16 NOVEMBER 2011 This paper was modified 12 months after initial publication to switch to Creative Commons
licence terms, as noted at publication _ REFERENCES * Aabo K, Pedersen H, Rorth M (1985) Cisplatin in the treatment of advanced gastric carcinoma: a phase II study. _Cancer Treat Rep_ 69:
449–450 CAS PubMed Google Scholar * Bae JM, Won WJ, Jung KW, Park JG (2002) Annual report of the Korea Central Cancer Registry Program 2000: based on registered data from 131 hospitals.
_Cancer Res Treat_ 34: 77–83 Article PubMed Google Scholar * Cao S, Lu K, Ishitsuka H, Rustum YM (1997) Antitumor efficacy of capecitabine against fluorouracil-sensitive and -resistant
tumours. _Proc Am Soc Clin Oncol_ 16: 226a (abstr 795) Google Scholar * Earle CC, Maroun JA (1999) Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients:
revisiting a meta-analysis of randomised trials. _Eur J Cancer_ 35: 1059–1064 Article CAS PubMed Google Scholar * Evans TR, Pentheroudakis G, Paul J, McInnes A, Blackie R, Raby N,
Morrison R, Fullarton GM, Soukop M, McDonald AC (2002) A phase I and pharmacokinetic study of capecitabine in combination with epirubicin and cisplatin in patients with inoperable
oesophago-gastric adenocarcinoma. _Ann Oncol_ 13: 1469–1478 Article CAS PubMed Google Scholar * Futatsuki K, Wakui A, Nakoa I, Sakata Y, Kambe M, Shimada Y, Yoshino M, Taguchi T, Ogawa N
(1994) Late phase II study of irinotecan hydrochloride (CPT-11) in advanced gastric cancer. CPT-11 Gastrointestinal Cancer Study Group. _Jpn J Cancer Chemother_ 21: 1033–1038 CAS Google
Scholar * Giuliani F, Gebbia V, De Vita F, Maiello E, Di Bisceglie M, Catalano G, Gebbia N, Colucci G (2003) Docetaxel as salvage therapy in advanced gastric cancer: a phase II study of the
Gruppo Oncologico Italia Meridionale (G.O.I.M.). _Anticancer Res_ 23: 4219–4222 CAS PubMed Google Scholar * Glimelius B, Ekstrom K, Hoffman K, Maiello E, Di Bisceglie M, Catalano G,
Gebbia N, Colucci G (1997) Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. _Ann Oncol_ 8: 163–168 Article CAS
PubMed Google Scholar * Graziano F, Catalano V, Baldelli AM, Graf W, Sjoden PO, Haglund U, Svensson C, Enander LK, Linne T, Sellstrom H, Heuman R (2000) A phase II study of weekly
docetaxel as salvage chemotherapy for advanced gastric cancer. _Ann Oncol_ 11: 1263–1266 Article CAS PubMed Google Scholar * Hill ME, Cunningham D (1998) Medical management of advanced
gastric cancer. _Cancer Treat Rev_ 24: 113–118 Article CAS PubMed Google Scholar * Hong YS, Song SY, Lee SI, Chung HC, Choi SH, Noh SH, Park JN, Han JY, Kang JH, Lee KS, Cho JY (2004) A
phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. _Ann Oncol_ 15: 1344–1347 Article CAS PubMed Google Scholar * Ishikawa T,
Sekiguchi F, Fukase Y, Sawada N, Ishitsuka H (1998) Positive correlation between the efficacy of capecitabine and doxifluridine and the ratio of thymidine phophorylase to dihydropyrimidine
dehydrogenase activities in tumours in human cancer xenografts. _Cancer Res_ 58: 685–690 CAS PubMed Google Scholar * Ishitsuka H (2000) Capecitabine: preclinical pharmacology studies.
_Invest New Drugs_ 18: 343–354 Article CAS PubMed Google Scholar * Janunger KG, Hafstrom L, Glimelius B (2002) Chemotherapy in gastric cancer: a review and updated meta-analysis. _Eur J
Surg_ 168: 597–608 Article CAS PubMed Google Scholar * Kang Y-K, Kim T-W, Chang H-M, Ryu M-H, Yook J-H, Oh S-T, Kim B-S, Lee J-S (2004) A phase I/II trial of docetaxel, capecitabine, and
cisplatin as a first line chemotherapy for advanced gastric cancer. _Proc Am Soc Clin Oncol_ 23: 328 (abstr 4066) Google Scholar * Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, Kang YK,
Shin DB, Kim HT, Kim HJ (1993) A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment
of advanced gastric cancer. _Cancer_ 71: 3813–3818 Article CAS PubMed Google Scholar * Kim TW, Chang HM, Kang HJ, Lee JR, Ryu MH, Ahn JH, Kim JH, Lee JS, Kang YK (2003) Phase II study of
capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. _Ann Oncol_ 14: 1115–1120 Article CAS PubMed Google Scholar * Kim TW, Kang YK, Ahn JH, Chang HM, Yook
JH, Oh ST, Kim BS, Lee JS (2002) Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced gastric cancer. _Ann Oncol_ 13: 1893–1898 Article CAS PubMed Google
Scholar * Lee JJ, Kim T, Kim D, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H (2003) Single-agent capecitabine in patients with metastatic colorectal cancer refractory to
5-fluorouracil/leucovorin chemotherapy. _Proc Am Soc Clin Oncol_ 22: 355a (abstr 1425) Google Scholar * MacDonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller
DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. _New
Engl J Med_ 345: 725–730 Article CAS PubMed Google Scholar * Miwa M, Ura M, Nishida M, Rocha PR, Rodrigues MA, Rausch M (1998) Design of a novel oral fluoropyrimidine carbamate,
capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. _Eur J Cancer_ 34: 1274–1281 Article CAS PubMed Google
Scholar * Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M (1993) Modified therapy with 5-fluorouracil, doxorubicin and methotrexate in advanced gastric cancer. _Cancer_
72: 37–41 Article CAS PubMed Google Scholar * Parkin DM, Pisani P, Ferlay J (1999) Global cancer statistics. _CA Cancer J Clin_ 49: 33–64 Article CAS PubMed Google Scholar * Perry
MC, Green MR, Mick R, Schein P (1986) Cisplatin in patients with gastric cancer: a cancer and leukemia group B phase II study. _Cancer Treat Rep_ 70: 415–416 CAS PubMed Google Scholar *
Pivot X, Chamorey E, Guardiola E, Magne N, Thyss A, Otto J, Giroux B, Mouri Z, Schneider M, Milano G (2003) Phase I and pharmacokinetic study of the association of capecitabine–cisplatin in
head and neck cancer patients. _Ann Oncol_ 14: 1578–1586 Article CAS PubMed Google Scholar * Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M (1995) Randomized comparison of fluorouracil,
epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. _Br J Cancer_ 71: 587–591 Article CAS PubMed PubMed
Central Google Scholar * Schüller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, Utoh M, Mori K, Weidekamm E, Reigner B (2000) Preferential activation of capecitabine in tumour
following oral administration in colorectal cancer patients. _Cancer Chemother Pharmacol_ 34: 291–297 Article Google Scholar * Simon R (1987) How large should a phase II trial of a new
drug be? _Cancer Treat Rep_ 71: 1079–1085 CAS PubMed Google Scholar * Stahl M (2004) Adjuvant chemoradiotherapy in gastric cancer and carcinoma of the oesophago-gastric junction.
_Onkologie_ 27: 33–36 CAS PubMed Google Scholar * Sumpter KA, Harper-Wynne C, Cunningham D, Oates J, Tebbutt N, Iveson T, Nicholson M, Hickish T, Hill M, Norman A (2003) Randomised,
multicenter phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer: confirmation of dose escalation. _Proc
Am Soc Clin Oncol_ 22: 257a (abstr 1031) Google Scholar * Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC,
Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumours. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United
States, National Cancer Institute of Canada. _J Natl Cancer Inst_ 92: 205–216 Article CAS PubMed Google Scholar * Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E,
Planker M, Santos JG, Piedbois P, Puillot B, Bodenstein H, Schmoll HJ, Bleiberg H, Nordlinger B, Couvreur ML, Bason B, Wils JA (2000) Final results of a randomized phase III trial of
sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a
trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. _J Clin Oncol_ 18: 2748–2757 Article Google Scholar * Vanhoefer U,
Wilke H, Weh HJ, Clemens M, Harstrick A, Stahl M, Hossfeld DK, Seeber S (1994) Weekly high-dose 5-fluorouracil and folinic acid as salvage treatment in advanced gastric cancer. _Ann Oncol_
5: 850–851 Article CAS PubMed Google Scholar * Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, Hughes M, Mansi J, Findlay M, Hill A, Oates J, Nicolson M, Hickish T,
O'Brien M, Iveson T, Watson M, Underhill C, Wardley A, Meehan M (1997) Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and
methotrexate in advanced espophagogastric cancer. _J Clin Oncol_ 15: 161–167 Article Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Oncology,
Department of Medicine, Asan Medical Center, 388-1 Pungnap-dong, Songpa-gu, Seoul, 138-736, South Korea H J Kang, H M Chang, T W Kim, M-H Ryu, H J Sohn, J-S Lee & Y-K Kang * Department
of Surgery, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea J H Yook, S T Oh & B S Kim Authors * H J Kang View author publications You can also search for this
author inPubMed Google Scholar * H M Chang View author publications You can also search for this author inPubMed Google Scholar * T W Kim View author publications You can also search for
this author inPubMed Google Scholar * M-H Ryu View author publications You can also search for this author inPubMed Google Scholar * H J Sohn View author publications You can also search for
this author inPubMed Google Scholar * J H Yook View author publications You can also search for this author inPubMed Google Scholar * S T Oh View author publications You can also search for
this author inPubMed Google Scholar * B S Kim View author publications You can also search for this author inPubMed Google Scholar * J-S Lee View author publications You can also search for
this author inPubMed Google Scholar * Y-K Kang View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Y-K Kang. RIGHTS
AND PERMISSIONS From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy
of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kang, H., Chang, H., Kim, T. _et al._ Phase II study
of capecitabine and cisplatin as first-line combination therapy in patients with gastric cancer recurrent after fluoropyrimidine-based adjuvant chemotherapy. _Br J Cancer_ 92, 246–251
(2005). https://doi.org/10.1038/sj.bjc.6602336 Download citation * Received: 22 July 2004 * Revised: 22 November 2004 * Accepted: 23 November 2004 * Published: 18 January 2005 * Issue Date:
31 January 2005 * DOI: https://doi.org/10.1038/sj.bjc.6602336 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * capecitabine * cisplatin *
first-line chemotherapy * advanced gastric cancer