- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Genetic services in Europe are based on world-leading scientific expertise. Furthermore, there has been rapid progress from research findings to the many diagnostic genetic tests currently
offered in clinics. However, for all these genetic tests scientists, counsellors and doctors have a special responsibility to provide services of the highest quality and to ensure that all
the citizens of Europe benefit from the same high standards of genetic care.1, 2 Poor testing and counselling can cause great anxiety among patients and their families. In addition, the
annual growth of testing within the EU continues to grow at a staggering rate – between 100 and 300%.3 An estimated 30 million people now suffer from a genetic disease within the enlarged
community. Both new and existing member states find genetics causing an increasing burden upon their healthcare systems, by the latest estimates 500 million Euros. EuroGentest is an EU
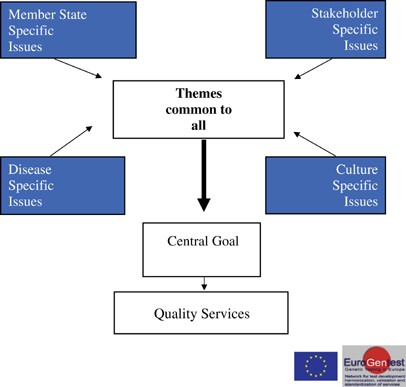
funded project over 5 years that aims to address these challenges through the creation of a European Network of Excellence (NoE) in genetic testing. The overall EuroGentest philosophy is
summarised in Figure 1. In effect a network of networks, this model works by encouraging a continuous cycle of critical self-examination among the genetic testing community in its widest
sense. By involving leading experts from across Europe, EuroGentest will develop the necessary infrastructure, tools, resources, guidelines and procedures that will structure, harmonize and
improve the overall quality of all EU genetic services – molecular, cytogenetic, biochemical and clinical. This wide-ranging project will also encompass all the relevant issues associated
with testing, including legal issues, health policies and health economic impact, Intellectual Property Rights ethical and social questions, such as confidentiality and informed consent.
From this overall aim, the following key objectives have already been identified: * Establish a network of quality across Europe. * Promote research, proper utilisation, quality control and
assurance and adequate management of genetic services. * Harmonize the accreditation of genetic testing laboratories and the certification of EQA schemes for cytogenetics, biochemical and
molecular genetics at a European, regional and national level throughout Europe. * Establish procedures and guidelines for the validation of methods and technologies. * Identify present and
future needs for Reference Measurement, Procedures and Materials for genetic testing. * Provide genetic healthcare workers, the end-users and healthcare authorities with a portfolio of
quality-assured information sources and informatic tools that are subject to validation and quality procedures. * Improve the quality of genetic testing counselling services in different
European countries. * Prepare a directory of organisations that provide and produce educational material for the public. * Define quality criteria for institutional courses and education in
genetic testing (eg Masters degree) and evaluate such courses. In addition, EuroGentest intends to become a model for similar initiatives in developing countries and will provide appropriate
support for their development. The EuroGentest project has been carefully constructed to ensure the above objectives are achieved. The overall coordination of the project receives the
assistance from a deputy and an operational management group. A steering committee is responsible for the operation and development of the project and consists of Unit leaders, their
coleaders and the coordinator of the project, while an advisory board composed of representatives of National Human Genetic societies, of the industry, of policy agencies and of
representatives of US organisations similarly involved in genetic testing, watches over the efficient progression of the NoE towards its goals. There are six units within the EuroGentest
network (Table 1). Each unit is subdivided into work groups and their respective work packages. The leaders of the units and coordinators of the work packages include representatives from
most European countries (the full list of participants is available on www.EuroGentest.org). There are 22 work packages within the EuroGentest project. Each work package has a coordinator
and consists of objectives, description of the work, milestones and deliverables. Within the context of EU policy, EuroGentest contributes to the goals included in the 6th Framework
Programme in thematic priority ‘Life Sciences, Genomics and Biotechnology for Health,’ specifically in Section 1, Advanced Genomics and its application for health, subsection b) Application
of knowledge and technologies in the field of genomics and biotechnology for health; Development of new diagnostics, LSH-2003-1.2.2.1: Development of genetic tests allowing for
harmonisation, validation and standardisation. The actual project was initiated by former commissioner and MEP Philippe Busquin who remains a staunch supporter. EuroGentest has also set-up
active collaboration with existing projects or networks such as Orphanet, EMQN, Crmgen, CF Network, ERNDIM, SAFE, as well as with the Spanish genetic networks INERGEN and RECGEN and is
supported by the ESHG and the ECA. Since commencing in January 2005, EuroGentest has made significant progress. Recruitment into the various units has been completed, work packages devised
and the first review meetings held. Different training sessions, expert meetings and international symposia have already taken place. More will come. The SMEs involved in the design of the
website, in technological aspects and in training aspects have become actively involved in the NoE. A comprehensive central European laboratory database is being developed in collaboration
with Orphanet and substantial progress has already been made on the identification of available educational materials for patients and their families. A meeting with the national
representatives of the Human Genetic societies in Europe will be the start of a more close involvement of the members of these societies in the Network. The challenge is the sheer diversity
of practice throughout the European Union. However, this is tempered by the enthusiasm being shown by the participants. Far too often at national level genetic testing has been low on the
list of political priorities compared to say cancer, STD and cardiovascular testing. For this as well as the obvious scientific reasons of volume of testing required for devising meaningful
standards, it is only possible to achieve results through a project of this scale and scope. Geneticists who wish to find out more about the project are invited in the first instance to
visit the website www.EuroGentest.org where they can register as professional users. This gives also access to information about the Units and their working parties. The coordinator also
welcomes enquiries from those centres or individuals wishing to become involved in EuroGentest at different levels▪ REFERENCES * Godard B, Kaariainen H, Kristoffersson U, Tranebjaerg L,
Coviello D, Ayme S : Provision of genetic services in Europe: current practices and issues. _Eur J Hum Genet_ 2003; 11 (Suppl 2): S13–S48. Article Google Scholar * McNally E _et al_:
Report on the ethical, legal and social aspects of genetic testing: research, development and clinical applications, 2004,
http://europa.eu.int/comm/research/conferences/2004/genetic/report_en.htm. * Ibarreta D, Elles R, Cassiman JJ, Rodriguez-Cerezo E, Dequeker E : Towards quality assurance and harmonization of
genetic testing services in the European Union. _Nat Biotechnol_ 2004; 22: 1230–1235. Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Center
for Human Genetics, University of Leuven, Campus Gasthuisberg, O&N 6, Herestraat 49, LEUVEN, B-3000, Belgium Jean-Jacques Cassiman Authors * Jean-Jacques Cassiman View author
publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Jean-Jacques Cassiman. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Cassiman, JJ. Research network: EuroGentest – a European Network of Excellence aimed at harmonizing genetic testing services. _Eur J Hum Genet_ 13, 1103–1105
(2005). https://doi.org/10.1038/sj.ejhg.5201484 Download citation * Published: 10 August 2005 * Issue Date: 01 October 2005 * DOI: https://doi.org/10.1038/sj.ejhg.5201484 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative







