- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
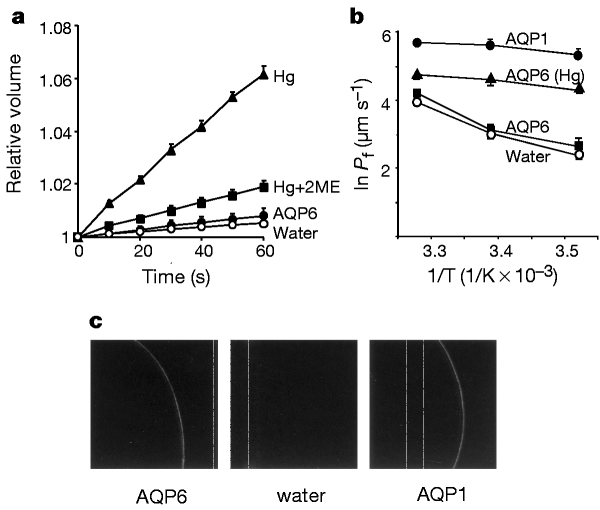
ABSTRACT Aquaporin (AQP) water-channel proteins are freely permeated by water but not by ions or charged solutes1. Although mammalian aquaporins were believed to be located in plasma
membranes, rat AQP6 is restricted to intracellular vesicles in renal epithelia2. Here we show that AQP6 is functionally distinct from other known aquaporins. When expressed in _Xenopus
laevis_ oocytes, AQP6 exhibits low basal water permeability; however, when treated with the known water channel inhibitor, Hg2+, the water permeability of AQP6 oocytes rapidly rises up to
tenfold and is accompanied by ion conductance. AQP6 colocalizes with H+-ATPase in intracellular vesicles of acid-secreting α-intercalated cells in renal collecting duct. At pH less than 5.5,
anion conductance is rapidly and reversibly activated in AQP6 oocytes. Site-directed mutation of lysine to glutamate at position 72 in the cytoplasmic mouth of the pore changes the
cation/anion selectivity, but leaves low pH activation intact. Our results demonstrate unusual biophysical properties of an aquaporin, and indicate that anion-channel function may now be
explored in a protein with known structure. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink *
Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional
subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS NARROWED PORE CONFORMATIONS OF AQUAGLYCEROPORINS AQP3 AND GLPF Article Open access 20 March
2025 AQUAPORIN 2 REGULATION: IMPLICATIONS FOR WATER BALANCE AND POLYCYSTIC KIDNEY DISEASES Article 01 July 2021 PROTON-DRIVEN SODIUM SECRETION IN A SALINE WATER ANIMAL Article Open access 03
June 2024 REFERENCES * Agre,P., Bonhivers,M. & Borgnia,M. J. The aquaporins, blueprints for cellular plumbing systems. _J. Biol. Chem._ 273, 14659–14662 (1998). Article CAS Google
Scholar * Yasui,M., Kwon,T.-H., Knepper,M. A., Nielsen,S. & Agre,P. Aquaporin-6: an intracellular vesicle water channel protein in renal epithelia. _Proc. Natl Acad. Sci. USA_ 96,
5808–5813 (1999). Article ADS CAS Google Scholar * Ma,T., Frigeri,A., Skach,W. & Verkman,A. S. Cloning of a novel rat kidney cDNA homologous to CHIP28 and WCH-CD water channels.
_Biochem. Biophys. Res. Commun._ 197, 654–659 (1993). Article CAS Google Scholar * Fushimi,K. et al. Cloning and expression of apical membrane water channel of rat kidney collecting
tubule. _Nature_ 361, 549–552 (1993). Article ADS CAS Google Scholar * Gorin,M. B., Yancey,S. B., Cline,J., Revel,J. P. & Horwitz,J. The major intrinsic protein (MIP) on the bovine
lens fiber membrane. _Cell_ 39, 49–59 (1984). Article CAS Google Scholar * Nielsen,S. et al. Vasopressin increases water permeability of kidney collecting duct by inducing translocation
of aquaporin-CD water channels to plasma membrane. _Proc. Natl Acad. Sci. USA_ 92, 1013–1017 (1995). Article ADS CAS Google Scholar * Mulders,S. M. et al. Water channel properties of
major intrinsic protein of lens. _J. Biol. Chem._ 270, 9010–9016 (1995). Article CAS Google Scholar * Ma,T., Yang,B., Kuo,W. L. & Verkman,A. S. cDNA cloning and gene structure of a
novel water channel expressed exclusively in human kidney: evidence for a gene cluster of aquaporins at chromosome locus 12q13. _Genomics_ 35, 543–550 (1996). Article CAS Google Scholar *
Preston,G. M., Carroll,T. P., Guggino,W. B. & Agre,P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. _Science_ 256, 385–387 (1992). Article ADS
CAS Google Scholar * Fahlke,C., Yu,H. T., Beck,C. L., Rhodes,T. H. & George,A. L. Jr Pore-forming segments in voltage-gated chloride channels. _Nature_ 390, 529–532 (1997). Article
ADS CAS Google Scholar * Roos,N., Benz,R. & Brdiczka,D. Identification and characterization of the pore-forming protein in the outer membrane of rat liver mitochondria. _Biochem.
Biophys. Acta_ 686, 204–214 (1982). Article CAS Google Scholar * Friedrich,T., Breiderhoff,T. & Jentsch,T. J. Mutational analysis demonstrates that CIC-4 and CIC-5 directly mediate
plasma membrane currents. _J. Biol. Chem._ 274, 896–902 (1999). Article CAS Google Scholar * Blachly-Dyson,E. et al. Selectivity changes in site-directed mutants of the VDAC ion channel:
structural implications. _Science_ 247, 1233–1236 (1990). Article ADS CAS Google Scholar * Jung,J. S., Preston,G. M., Smith,B. L., Guggino,W. B. & Agre,P. Molecular structure of the
water channel through aquaporin CHIP. The hourglass model. _J. Biol. Chem._ 269, 14648–14654 (1994). CAS PubMed Google Scholar * Walz,T. et al. The three-dimensional structure of
aquaporin-1. _Nature_ 387, 624–627 (1997). Article ADS CAS Google Scholar * Mitsuoka,K. et al. Short α-helices in hourglass pore-forming domains of aquaporin-1, a water channel protein,
visualized at 4.5 Å resolution. _J. struct. Biol._ (in the press). * Schwartz,G. J. & Al-Awqati,Q. Regulation of transepithelial H+ transport by exocytosis and endocytosis. _Annu. Rev.
Physiol._ 48, 153–161 (1986). Article CAS Google Scholar * Glickman,J., Croen,K., Kelly,S. & Al-Awqati,Q. Golgi membranes contain an electrogenic H+ pump in parallel to a chloride
conductance. _J. Cell Biol._ 97, 1303–1308 (1983). Article CAS Google Scholar * Günther,W., Luchow,A., Cluzeaud,F., Vandewalle,A. & Jentsch,T. J. CIC-5, the chloride channel mutated
in Dent's disease, colocalizes with the proton pump in endocytotically active kidney cells. _Proc. Natl Acad. Sci. USA_ 95, 8075–8080 (1998). Article ADS Google Scholar * Jena,B. P.
et al. Gi regulation of secretory visicle swelling examined by atomic force microscopy. _Proc. Natl Acad. Sci. USA_ 94, 13317–13322 (1997). Article ADS CAS Google Scholar * Hirsch,S. et
al. Isolation and sequence of a cDNA clone encoding the 31-kDa subunit of bovine kidney vacuolar H+-ATPase. _Proc. Natl Acad. Sci. USA_ 85, 3004–3008 (1988). Article ADS CAS Google
Scholar * Yool,A. J., Stamer,W. D. & Regan,J. W. Forskolin stimulation of water and cation permeability in aquaporin-1 water channels. _Science_ 273, 1216–1218 (1996). Article ADS CAS
Google Scholar * Agre,P. et al. Aquaporins and ion conductance. _Science_ 275, 1490–1492 (1997). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank M. Lu for the
AQP6 plasmid, M. A. Knepper for the anti-H+-ATPase, and M. R. T. Hall for confocal microscopy. Support was provided by grants from the NIH and the Cystic Fibrosis Foundation (P.A. and
W.B.G.), the Human Frontier Science Program (M.Y.), and the Novo Nordic Foundation, the Karen Elise Jensen Foundation, the Danish Medical Research Council, the Biomembrane Research Center at
Unviersity of Aarhus and the EU Commission (EU-Biotech and TMR Programmes) (S.N.). AUTHOR INFORMATION Author notes * Masato Yasui, Akihiro Hazama, Tae-Hwan Kwon and Søren Nielsen: These
authors contributed equally to this work AUTHORS AND AFFILIATIONS * Departments of Biological Chemistry, Medicine, Johns Hopkins University School of Medicine, MD 21205, Baltimore, USA
Masato Yasui & Peter Agre * Physiology, Johns Hopkins University School of Medicine, MD 21205, Baltimore, USA Akihiro Hazama & Wm. B. Guggino * Department of Cell Biology, Institute
of Anatomy, University of Aarhus, DK-8000, Denmark Tae-Hwan Kwon & Søren Nielsen Authors * Masato Yasui View author publications You can also search for this author inPubMed Google
Scholar * Akihiro Hazama View author publications You can also search for this author inPubMed Google Scholar * Tae-Hwan Kwon View author publications You can also search for this author
inPubMed Google Scholar * Søren Nielsen View author publications You can also search for this author inPubMed Google Scholar * Wm. B. Guggino View author publications You can also search for
this author inPubMed Google Scholar * Peter Agre View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Peter Agre.
RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yasui, M., Hazama, A., Kwon, TH. _et al._ Rapid gating and anion permeability of an intracellular
aquaporin. _Nature_ 402, 184–187 (1999). https://doi.org/10.1038/46045 Download citation * Received: 28 July 1999 * Accepted: 21 September 1999 * Issue Date: 11 November 1999 * DOI:
https://doi.org/10.1038/46045 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently
available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative