- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The transmembrane receptor Notch1 plays a crucial role in differentiation and apoptosis of hematopoietic cells. To investigate the influence of Notch1 on apoptosis and cell growth
of mature murine B cells, we transduced the murine B-lymphoma line NYC 31.1 with a constitutively active, intracellular form of human Notch1 (Notch1-ICT). NYC cells represent mature
activated B cells that can be induced to undergo apoptosis by crosslinking of the B-cell receptor (BCR). In contrast to investigations in immature chicken B-cell lines, transduced Notch1-ICT
did not affect cell cycle progression, cell growth or surface IgM levels in NYC cells and resulted only in a slight induction of apoptosis. However, BCR-crosslinking enhanced apoptosis, but
did not influence cell cycle progression in Notch1-ICT-positive NYC cells. These data imply a distinct function of Notch1 in mature murine B-cells as compared to immature chicken B cells
and provide further evidence for Notch1's involvement in B-cell differentiation and development. SIMILAR CONTENT BEING VIEWED BY OTHERS AN INSTRUCTIVE ROLE FOR INTERLEUKIN-7 RECEPTOR Α
IN THE DEVELOPMENT OF HUMAN B-CELL PRECURSOR LEUKEMIA Article Open access 03 February 2022 VIRAL TRANSDUCTION OF PRIMARY HUMAN LYMPHOMA B CELLS REVEALS MECHANISMS OF NOTCH-MEDIATED IMMUNE
ESCAPE Article Open access 20 October 2022 A P38Α-BLIMP1 SIGNALLING PATHWAY IS ESSENTIAL FOR PLASMA CELL DIFFERENTIATION Article Open access 28 November 2022 INTRODUCTION In both murine and
human immune systems, B-cell development occurs in the bone marrow and originates from pluripotent hematopoietic stem cells. This development goes through several stages that are
characterized by DNA rearrangements, first at the immunoglobulin (Ig) heavy (H) and then at the Ig light (L) chain locus (reviewed in Melchers _et al._,1 Osmund,2 Löffert _et al._,3 Winkler
and Melchers,4 Rolink _et al._,5 Hardy and Hayakawa6). Productive and functional rearrangements of H- and L-chain genes result in the assembly of a membrane anchored IgM molecule that is
presented on the cell surface as an antigen or B-cell receptor (BCR). Immature B cells with a functional BCR develop subsequently into mature B cells (reviewed in Hardy and Hayakawa,6 Osmond
_et al._,7 Cornall _et al._8), which migrate to secondary peripheral lymphoid organs. Here, if activated by antigen, the B cells differentiate into antibody-secreting plasma cells, a
prerequisite for an effective humoral immune response against pathogens (reviewed in Lindhout _et al._9). The generation of B-lymphoid cells in the bone marrow depends on a delicate balance
between proliferation, differentiation and cell death. While proliferation and differentiation ensure the continuous production of B-lymphoid cells from hematopoietic precursors, cell death
processes are crucial to eliminate nonfunctional or potentially harmful B-cell clones.8,10,11 Cell death occurs at various times and stages during the maturation of B-lymphoid cells and one
of the best studied processes is the elimination of autoreactive immature B cells.10,11 Experiments with transgenic mice have shown that the engagement of multivalent autoantigens by
immature B cells in the bone marrow triggers their elimination via apoptotic cell death processes.12 Apoptosis in immature B cells can also experimentally be induced by crosslinking the BCR
with antibodies or F(ab)2 fragments against the H-chain of IgM-BCRs.12,13,14 The transmembrane receptor Notch1 and its specific ligands regulate proliferation, differentiation, cell fate
decisions and apoptosis of several cell lineages.15,16,17 Additionally, Notch1 has been shown to participate in developmental and apoptotic cell death processes during the generation of
lymphocytes and other hematopoietic cells (reviewed in Radke _et al._,18 Allman _et al._19). The Notch1 transmembrane receptor is synthesized as a large precursor and processed within the
Golgi apparatus.15,20 The mature form of the Notch1 receptor is a heterodimeric complex consisting of an extracellular ligand-accessible (Notch1-EC) and a membrane-anchored intracellular
chain (Notch1-IC). Thereafter, receptor–ligand interactions induce proteolytic processing and release of the intracellular portion of Notch1 into the cytoplasm.21,22,23,24,25 This so-called
activated form of Notch1 (Notch1-IC) translocates into the nucleus and, in conjunction with the DNA binding protein CBF-1 (core binding factor 1, also known as RBP-Jκ), modulates the
expression of target genes in many vertebrates (reviewed in Artavanis-Tsakonas _et al._,15,16). Experiments with transgenic mouse models have revealed that signals mediated by Notch1 control
the maturation and commitment of thymic T-lymphoid precursors to CD4 and CD8 as well as to _αβ_ and _γδ_ TCR lineage.26,27,28 Furthermore, the inducible deletion of the Notch1 gene in adult
mice results in a block in early T-cell development and in the appearance of a B-lymphoid subpopulation within the thymus of these animals.29,30 In contrast, reconstitution experiments
demonstrated that murine bone marrow cells transduced with an activated form of human Notch1 (Notch1-ICT) give rise to T cells, but are blocked at an early stage of B-cell maturation.31
Therefore, Notch1 seems to promote early T-cell development and to inhibit maturation of early B-lymphoid progenitor cells.31 Anti- as well as proapoptotic effects of Notch1 have been
demonstrated in cell lines of hematopoietic origin. For example, a constitutively active form of the intracellular portion of Notch1 (Notch1-ICT) reduces TCR-mediated apoptosis in T-cell
hybridoma cells transduced with a Notch1-ICT retrovirus.32,33 Also, inhibition of Notch1 expression by an antisense approach leads to an increased rate of apoptotic cell death in mouse
erythroleukemia (MEL) cells.34 In contrast, transfected Notch1-ICT induces apoptosis and cell cycle arrest in the chicken B-cell line DT40 in the absence of BCR-signals.35 In addition,
transfected Notch1-ICT downregulates surface IgM on the avian leukosis virus-transformed B-cell line 249L4.36 Therefore, Notch1 seems to exert different functions in different hematopoietic
lineages. To determine the effect of Notch1 on intracellular signal pathways in B cells representing a more mature stage of development, we stably transduced the murine B-cell line NYC 31.1
(NYC) with Notch1-ICT and investigated the effect of Notch1-ICT on apoptosis, cell growth and surface IgM expression. NYC cells represent mature activated B1-type like B cells37 that die
apoptotically in response to crosslinking of the BCR with anti-IgM antibodies (this study). In contrast to studies investigating the effect of a constitutively active form of Notch1 in
immature chicken B-cell lines,35,36 we found that a constitutive active form of human Notch1 did not affect cell cycle progression or surface IgM levels in NYC cells and resulted only in a
slight induction of apoptosis. However, BCR-crosslinking clearly enhanced apoptosis, but did not influence cell cycle progression or IgM expression in Notch1-ICT-positive NYC cells. Based on
these findings, we postulate that the effect of Notch1-signals on apoptosis and cell growth depends on the maturation and activation stage of a B cell. RESULTS The avian Bursa of
Fabricius-derived B-lymphoid cell line DT40 undergoes somatic gene conversion and represents cells at a developmental stage before the selection against autoreactive B cells occurs. Recent
studies showed that transfection of DT40 cells with Notch1-IC results in the enhancement of apoptotic cell death and in a block in cell cycle progression at the G0/G1 phase, even in the
absence of a BCR-mediated stimulus.35 To investigate the influence of activated Notch1 on B cells at a later stage of development, we have chosen the murine B-cell line NYC as a model
system. The NYC line was established from a clonal, spontaneous splenic B-cell tumor that originated in a (NZB × NZW) F1 mouse.37 NYC cells are characterized by surface expression of CD5, a
marker of B1 cells. In addition, NYC cells express surface IgM and secrete soluble IgM; therefore, NYC cells represent mature, activated B1-like B-cell blasts at the transition from the
surface IgM-positive B cell to the antibody-secreting plasma cell stage.37 EFFECT OF BCR-CROSSLINKING ON APOPTOSIS AND ENDOGENOUS MURINE NOTCH1 LEVELS IN NYC WILD-TYPE CELLS To determine
whether BCR signals can induce apoptosis in NYC cells, NYC cultures were incubated with crosslinking anti-BCR F(ab)2 fragments (i.e., anti-IgM antibodies) for 5 and 28 h; PBS as well as
isotype-matched control F(ab)2 fragments were used as negative controls. After each incubation period, cells were costained with AnnexinV-FITC (AnnexinV) and YOPRO-3-Iodide (YOPRO-3), and
analyzed by flow cytometry. AnnexinV-FITC staining represents a rather sensitive method to detect apoptotic cells as compared to YOPRO-3-iodide staining, in particular at very early time
points of induction of apoptosis. The frequencies of apoptotic and dead cells were determined from the relative numbers of AnnexinV- and YOPRO-3-positive cells, respectively. Representative
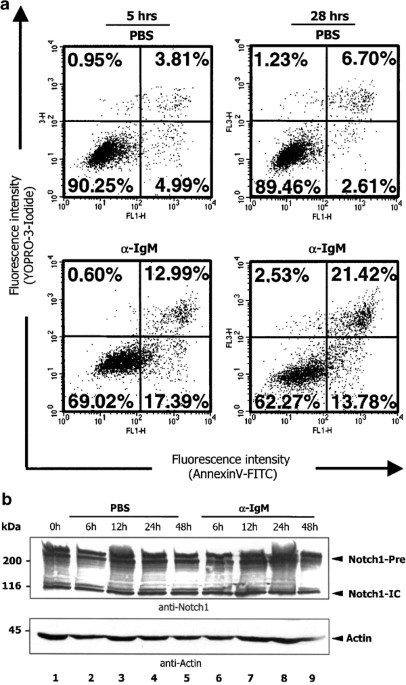
results from one of four independent experiments are shown in Figure 1a. Flow cytometric analysis revealed a significantly higher frequency of apoptotically dying cells after treatment of
NYC cells with crosslinking anti-IgM antibodies as compared to PBS- (Figure 1a) and isotype-matched antibody (data not shown) treated cells. Since Notch1 has been implicated in the control
of apoptosis in lymphocytes,32,33 we asked whether BCR-induced apoptosis correlates with a change in intracellular Notch1 levels in NYC cells. Western blot analysis of endogenous Notch1 over
a stimulation period of 48 h with either PBS or BCR-crosslinking anti-IgM antibodies revealed that NYC cells synthesize endogenous Notch1 at all analyzed time points (Figure 1b).
Furthermore, Notch1 levels remained unchanged after treatment of NYC cells with PBS (Figure 1b, lanes 2–5) or with the BCR-crosslinking antibody (Figure 1b, lanes 6–9). However, from this
experiment, it is not clear whether membrane-bound Notch1-IC was converted into the active form by proteolysis, since our Western blot analysis did not clearly resolve membrane-bound from
clipped, activated Notch-IC. However, even if we would detect the active form of endogenous Notch1 after BCR-crosslinking, we still could not conclude from this correlation that Notch1
participates in the control of BCR-mediated apoptosis. To address this question, we introduced a Notch1-ICT into NYC cells and examined its effect on apoptosis, cell cycle and growth in the
presence and absence of a BCR signal. GENERATION OF STABLE NYC INFECTANTS PRODUCING NOTCH1-ICT To directly determine the effect of an active form of Notch1 on cellular functions in mature
activated murine B cells, we used retrovirus-mediated gene transfer to generate pools and stable cell clones of NYC cells producing the Notch1-ICT. We then went on to analyze whether
constitutive expression of Notch1 modulates apoptosis and proliferation before and after crosslinking of surface IgM. To generate packaging cells capable of producing retroviral particles,
we transiently transfected the retroviral packaging cell line BOSC 2338 with Moloney Murine Leukemia Virus (MoMuLV)-based retroviral vectors (Figure 2a) without (MSCV 2.1=−Notch1) and with
the cDNA sequence encoding the complete intracellular portion of the human Notch1 gene (TAN1) including the glutamine-rich region (OPA) and proline(p), glutamine (E), serine (s) and
threonine (T)-rich region (PEST) sequence motifs (pGD-ICT=+Notch1). The efficiency of the transient transfection of BOSC 23 cells was verified by Western blot analysis (Figure 2b). As
expected, we obtained a Notch1-ICT-specific signal (110 kDa) only in +Notch1-transfected BOSC 23 cells, but not in BOSC 23 cells transfected with the control vector (−Notch1). Subsequently,
cell culture supernatants of BOSC 23 cells transfected with the Notch1 containing vector (+Notch1) or the control vector (−Notch1) were harvested and used for retroviral infection of NYC
cells. After selection of G418-resistant cell pools, single-cell clones were obtained by the limiting dilution method and tested for the expression and synthesis of ectopic Notch1-ICT. We
first analyzed pools as well as individual −Notch1- and +Notch1-infected NYC 31.1 clones for the presence of Notch1-ICT-specific mRNA. Human and murine Notch1 show a high degree of identity
at the protein as well as the nucleic acid level. However, subtle differences between the two sequences enabled us to design polymerase chain reaction (PCR) primers for the intracellular
portion of Notch1 that distinguished between endogenous murine and the transduced human Notch1 mRNA in a reverse transcription (RT)-PCR approach. As shown in Figure 3a, RT-PCR with primers
specific for human Notch1 detected signals in NYC pools and clones infected with +Notch1 virus, but not in noninfected NYC cells and in clones and pools infected with the control virus
(-Notch1). To verify the expression of transduced Notch1, a Western blot analysis was performed with a commercially available Notch1-specific antibody that reacts only with human but not
with murine Notch1. As expected, this antibody detected only a Notch1 signal in Notch1-transdcued NYC pools (Figure 3b, lane 1) and clones (lanes 6–9), but not in noninfected wild-type NYC
cells (lane 4) and in clones infected with the control virus (−Notch1, lanes 10–13). In addition, this analysis revealed clones with low (Clones (cls.) 1 and 9) and with high levels (Cls. 5
and 6) of human (ectopic) Notch1. For control reasons, the T-cell hybridoma DO11.10-ICT33 was used, which synthesizes high levels of ectopic Notch1-ICT (Figure 3b, lane 3). The presence and
levels of ectopic Notch1 was verified by a combined immunoprecipitation/Western blot analysis (Figure 3c). EFFECT OF ECTOPIC NOTCH1 ON APOPTOSIS IN NYC CELLS Initially, we investigated the
effect of ectopic Notch1-ICT on apoptotic cell death processes in NYC cells before and after induction of cell death with BCR-crosslinking IgM-specific antibodies. The frequencies of
apoptotic and dead cells were determined 5 h after the addition of PBS or anti-IgM antibodies by the AnnexinV/YOPRO-3 method as described earlier. Figure 4 shows the result of a typical flow
cytometric analysis of YOPRO-3- and AnnexinV-stained NYC wild-type cells as well as −Notch1- and +Notch1-infected NYC cells. In contrast to experiments with immature chicken B cell lines,35
+Notch1-infected NYC cells were viable and, when compared to wild-type and −Notch1-infected NYC cells, showed in this experiment a low level of spontaneous apoptosis in the absence of a
BCR-signal (Figure 4a). However, repeated experiments confirmed that this increase in the frequency of spontaneous apoptosis is not statistically relevant (Figure 4b). In contrast, in the
presence of anti-IgM-specific antibodies, +Notch1 NYC cells showed a significantly increased percentage of AnnexinV-positive, apoptotically dying cells as compared to uninfected wild-type or
−Notch1 NYC cells (Figure 4a, lower diagrams and Figure 4b, right panel). To verify the effect of BCR-crosslinking in the presence of Notch1 on cell death with a second approach, we
determined the frequency of cells with fragmented DNA by the Propidium-Iodide (PI)/Triton method. At 24 h after the addition of PBS (negative control) or anti-IgM antibodies to NYC cells and
NYC infectants, cells were first permeabilized with Triton X-100 and stained with PI. The percentage of cells with fragmented DNA was determined from relative cell numbers within the SubG1
gate. Figure 5a, which presents a typical result of an experiment performed 24 h after the addition of anti-IgM antibodies, illustrates a significantly increased number of PI-positive cells
in the SubG1 phase (dead cells) of the cell cycle in +Notch1 NYC clones (Cl. 5 and Cl. 9) as compared to a representative −Notch1 NYC clone (Cl. 2) or wild-type NYC cells (wt). This clear
increase in the frequency of dead cells occurs only in the presence of anti-IgM antibodies, that is, in the presence of a BCR-signal. PBS-treated +Notch1 NYC cells show only a slight
increase in SubG1 phase cells as compared to -Notch1 or wild-type NYC cells. In summary, enhancement of BCR-induced cell death could be demonstrated in +Notch1 NYC cells with both the
AnnexinV/YOPRO-3 and the PI/Triton method. INFLUENCE OF ECTOPIC NOTCH1 ON THE CELL CYCLE PROGRESSION OF NYC CELLS Morimura _et al._35 have recently demonstrated that inducible expression of
a constitutively active form of chicken Notch1 not only causes apoptosis but also a cell cycle arrest at the G1 phase in the chicken B-cell line DT40.35 To analyze the effect of Notch1 on
the cell cycle progression in NYC cells, wild-type NYC cells, a –Notch1 NYC clone and two +Notch1 NYC clones that differ in the level of human Notch1-ICT were stimulated for 24 h with PBS or
with BCR-crosslinking anti-IgM antibodies to undergo apoptosis. Subsequently, cells were permeabilized and stained with PI, after which they were subjected to flow cytometric analysis in
order to assess their DNA content. Figure 5a shows the PI-staining histograms of a typical experiment and Figure 5b summarizes the ModFit LT analyses of three independent experiments
performed in triplicates. Considering cells in the G0/1, S and G2/M phase, the results in Figure 5b revealed no differences in cell cycle distribution of all analyzed cell populations,
regardless of the presence and levels of Notch1-ICT. The slight increase in numbers of cells in the G1 phase (Figure 5b, compare panels b and f) as well as the slight decrease of cells in
the S phase (compare panels c and g) in a +Notch1/Cl. 9 culture treated with anti-IgM antibodies was not reproducible. Repetition of this experiment (+6 times) with +Notch1/Cl. 9 (low level
of ectopic Notch1-ICT) and comparison with +Notch1/Cl. 1 (also low level of ectopic Notch1–ICT) did not confirm the observations for Cl. 9 (data not shown); therefore, we conclude that these
variations are not statistically relevant and that a constitutively active Notch1 does not affect cell cycle progression. To determine the rate of cell death, wild-type and infected NYC
cells were cultured over a period from 12 to 96 h in the presence and absence of anti-IgM antibodies. Cells were removed at indicated time points and subjected to a PI/Triton staining. The
percentage of dead/apoptotic cells in the SubG1 phase was determined as described earlier by flow cytometry. Figure 5c exemplifies the ratios of the analyzed NYC cells in the apoptotic SubG1
phase. Most of cell death occurred within 24 h after the addition of anti-IgM antibodies, and prolonged stimulation periods of up to 96 h caused only a limited additional increase of
apoptosis. In addition, +Notch1 cells show a higher maximum in the frequency of dead cells than wild-type and –Notch1 NYC cells, suggesting that Notch1-ICT-positive NYC cells are more
susceptible to apoptosis. Finally, when compared to wild-type and −Notch1 NYC control cultures, we observed an increased rate of cell death in anti-IgM-treated NYC cells expressing human
Notch1-ICT; for example, 24 h after administration of the BCR-signal up to 70 % of +Notch1 NYC cells (Cls. 5 and 9) died, whereas 70% of cells were still viable in anti-IgM-treated control
NYC cultures. These data indicate that a constitutively active Notch1 not only increases the rate of but also the susceptibility to BCR-induced apoptosis. INFLUENCE OF ECTOPIC NOTCH1 ON THE
GROWTH OF NYC CELLS Apoptotic cell death is often associated with a cell cycle arrest and, thus, an inhibition of proliferation. In addition, the inducible expression of a constitutive
active form of chicken Notch1 in avian Bursa of Fabricius derived cell lines has been shown to cause a cell cycle inhibition at the G0/G1 boundary of the cell cycle, which is accompanied by
a significant growth suppression of the cells.35 Since we did not detect an arrest of the cell cycle and found only a slight increase of spontaneous apoptosis in the absence of a BCR-signals
in +Notch1 NYC cells, we would predict that −Notch1 and +Notch1 NYC cells show the same growth behavior. To determine the proliferation capacity of independent –Notch1 and +Notch1 NYC cell
clones, we measured the number of cells over a period of 4 days. Cells were plated initially at low density (1 × 104) in triplicate. Cells were mixed with a defined number of
fluorescence-labeled counting-beads, and absolute numbers were determined flow cytometrically. Figure 6a summarizes the cell counts of three independently performed experiments obtained at
day 4. Although the average growth rate of the individual +Notch1 clones appears to be different, after comparing the mean of the cell counts of −Notch1 and +Notch1 NYC cell clones, it is
apparent that there is no significant difference in the increase of cell numbers for all analyzed cell populations (Figure 6a). Similar results were obtained when we measured the
3H-thymidine incorporation rate of independent −Notch1 and +Notch1 NYC cell clones (Figure 6b). Again, no significant difference between the mean values of the 3H-thymidine incorporation
rate of the –Notch1 and +Notch1 NYC cell clones was observed. Curiously, the replication capacity of the individual clones does not correlate with their growth rate, regardless of ectopic
Notch1-ICT expression. Since BCR-crosslinking causes apoptosis in NYC cells, it was not feasible to measure cellular growth rates in NYC cells after administration of the BCR-signal.
Nevertheless, these data show that, in contrast to similar experiments performed in chicken DT40 cells, a constitutively active Notch1 does not affect cellular growth in the absence of a
BCR-signal. INFLUENCE OF ECTOPICALLY EXPRESSED NOTCH1-ICT ON INTRACELLULAR NOTCH SIGNALING PATHWAYS To investigate whether ectopically expressed Notch1-ICT activates Notch signaling pathways
in NYC cells, we continued our investigations with the analysis of the expression pattern of Hes1, a Notch1-specific target gene. Hes1 encodes a basic helix–loop–helix transcription factor
functioning downstream of the Notch1 receptor.39,40 Using a RT-PCR approach, we analyzed the expression profile of the Hes1 gene in wild-type, −Notch1 and +Notch1 NYC cells. Since parental
NYC cells synthesize endogenous Notch1 (Figure 1b), we expected to detect Hes1 gene expression in NYC cells, but only if the endogenous murine Notch1 is activated. As revealed by RT-PCR and
illustrated in Figure 7, a low signal of a Hes1-specific band with the expected size of 850 bp was detected in wild-type (lanes 2 and 6) and –Notch1 infected NYC cells (Cl. 2; lanes 3 and
7), indicating that some of the endogenous murine Notch1 is active. In contrast, a much stronger signal for Hes1 mRNA was detected in +Notch1 NYC cells (Cl. 5; lanes 4 and 8), indicating
that ectopically expressed Notch1-ICT is able to induce the expression of the Hes1 target gene and potentially activates intracellular Notch1 signaling pathways. INFLUENCE OF ECTOPIC NOTCH1
ON THE IGM SURFACE EXPRESSION OF NYC CELLS Recently, it has been shown that stable expression of a constitutively active form of chicken Notch1 or Notch2 in the avian leukosis virus
transformed B-cell line 249L4 resulted in a downregulation of surface IgM expression. This was also accompanied by the reduction of IgH transcripts, most probably because of a
Notch1-mediated inhibition of the IgH enhancer activity.36 Additionally, it was shown that Notch1–ICT negatively regulates IgM expression in Burkitt's lymphoma cell lines,41 indicating
that activated Notch1 is capable of participating in gene regulation in B-lymphoid cells. We therefore investigated if activated Notch1 participates in the regulation of the levels of
surface IgM on NYC cells; hence, wildtype, -Notch1 and +Notch1 NYC cells were analyzed for surface IgM levels. Flow cytometric analysis with a FITC-conjugated anti-IgM antibody revealed
equal surface IgM expression levels in NYC cells regardless whether activated Notch1-ICT was present (data not shown). This finding implies that an activated form of Notch1 is involved in
the regulation of neither the IgH gene expression nor the transport of IgM to the surface of NYC cells, which further strengthens the point for distinct functions of Notch1 during B-cell
differentiation. DISCUSSION Notch1 appears to be a key regulator of lineage determination in the hematopoietic system, particularly in regard to the T- and B-cell lineages. Several recent
publications have shown that Notch1 is involved in the regulation of cell fate decisions in the T-cell lineage,26,27,28,29,30,31,42 but only little is known about the role of Notch1 during
B-cell development as well as the differentiation processes and apoptotic cell death processes occurring therein. Interestingly, it was recently reported that in late B-cell stages and in
some B-cell lymphoma, such as Hodgkin's lymphoma, Notch1 might exert antiapoptotic and proproliferative functions.43 This survival promoting function of Notch1 appears to be also
important for specialized B-cell subsets, such as marginal zone B cells.44 However, in immature and autoreactive B cells, Notch1 seems to impose a proapoptotic function. Morimura _et al._35
have shown that conditional expression of a constitutive active form of chicken Notch1 in the immature avian Bursa of Fabricius derived cell line DT40 not only induces apoptosis in these
cells but also an arrest at the G0/G1 boundary of the cell cycle. Importantly, Notch1-induced apoptosis in DT40 cells is independent from any additional stimuli, such as BCR-crosslinking,
which explains why DT40 clones with a stably expressed active form of Notch1-IC could not be obtained. Therefore, depending on the cell line, the constitutive synthesis of activated Notch1
can be disadvantageous or even toxic to cells.35 In addition, Notch1 downregulates surface IgM expression, most probably because of a suppression of IgH gene expression.36 The toxic effects
of a constitutively active Notch1 in immature chicken B-cell lines is also in accordance with our observation that repeated attempts to generate stable Notch1-ICT-positive clones by
retroviral infection of the immature, murine B–lymphoid cell line WEHI 23145 failed (S Romer, H-M Jäck and BM Jehn, unpublished data). Ectopic Notch1-ICT appears to be lethal for immature
murine B–lymphoid WEHI 231 cells, most probably because of a Notch1-induced severe growth disadvantage or continuous spontaneous apoptosis. WEHI 231 and NYC 31.1 differ in the regard that
WEHI 231 cells represent a classical immature B-cell line characterized by surface IgM expression, whereas NYC 31.1 cells represent mature activated B1-type-like cells, characterized by
surface IgM and CD5 expression and IgM secretion.37 Furthermore, the BCR in WEHI 231 cells does not translocate into lipid rafts following BCR-crosslinking, whereas the BCR of mature B cells
can be found in these cholesterol- and sphingolipid-enriched membrane microdomains.46 Therefore, the BCR of immature B-lymphoid cells signals outside from rafts, which results in a dramatic
difference in the signaling in immature and mature B cells.46 These differences between WEHI 231 and NYC 31.1 may account for the differential responses to ectopic Notch1. To test the
hypothesis that Notch1 regulates apoptosis in mature activated murine B cells, we infected the murine B-cell line NYC 31.1 with Notch1-ICT. In contrast to the studies performed by Morimura
co-workers35,36 with the chicken DT40 line, we observed that activated Notch1 clearly increases the susceptibility to and enhances the rate of cell death in the mature activated murine
B-cell line NYC 31.1, but only after a BCR-signal has been initiated by crosslinking with anti-IgM antibodies. In the absence of BCR-crosslinking, only a low increase in spontaneous
apoptosis of +Notch1 NYC cells was detected. Furthermore, it was evident that the enhancement of cell death by activated Notch1 is not associated with either a cell cycle arrest, a block in
proliferation or a downregulation of surface IgM. Hence, it appears likely that the differential effects exerted by Notch1 in chicken and murine B cells are highly dependent on the cell type
and the cellular microenvironment in which Notch1 is functioning. Bursa of Fabricius derived B-lymphoid cells in birds undergo a distinct developmental pathway as compared to B-lymphoid
cells derived from the bone marrow of mice and man. The bone marrow of mammals represents a site of B-cell lymphopoiesis, which is free of exogenous antigens. Murine immature B cells are
eliminated in response to autoantigens to ensure the removal of potentially harmful autoreactive B-cell clones before migration to secondary peripheral lymphoid organs occurs.10,11 Avian
B–cell development proceeds differently: B-lymphoid precursor cells with a completely assembled BCR migrate into the Bursa of Fabricius, a gut-associated lymphoid tissue, where they are
exposed to gut-derived exogenous antigens and change their antigen specificity by gene conversion.47 B-lymphoid cells that either lose the expression of surface Ig complexes or create a
self-reactive BCR as a result of the gene conversion process are eliminated via apoptotic cell death in the bursa.48 It is believed that the surface Ig complex is responsible for basal
signaling levels in the absence of exogenous receptor ligation.47 Taking these differences in murine and avian B-lymphopoiesis into account, it is highly likely that Notch1 exerts different
functions in different hematopoietic lineages. Additional evidence that Notch1 functions in a cell type and developmental stage-specific way can be drawn from studies investigating the role
of Notch1 in apoptotic cell death processes in T-cell hybridoma lines. Constitutive active Notch1 was demonstrated to inhibit apoptotic cell death in T-lymphoid cell lines only in response
to TCR crosslinking.32,33 TCR-independent stimuli to induce apoptosis did not result in a Notch1-specific inhibition of apoptosis.33 For the T-cell hybridoma cell line DO11.10, activated
Notch1 was shown to inhibit specifically Nur77-dependent, TCR-mediated forms of cell death. Also, ectopic Notch1 results in reduced activity of a Nur77 reporter gene, raising the possibility
that Notch1 inhibits Nur77-dependent apoptosis through direct binding to Nur77 and a subsequent inhibition of Nur77 transcriptional activity.33 In the context of T-cell-specific apoptosis,
Notch1 exerts inhibitory effects most probably because of the interaction with Nur77, a transcription factor shown to be essential for thymocyte apoptosis.49,50,51 Nur77, however, could not
be detected in any B-lymphoid cell line or in _ex vivo_ isolated B-lymphoid cell populations (U Saunders, H-M Jäck and BM Jehn, unpublished data), implying that Notch1 may interact with
other and as yet unidentified B-lymphoid-specific transcription factors. Depending on the intra- as well as extracellular environment of a developing B cell, this hypothesized interaction
may cause either an enhancement or inhibition of death in B-lymphoid cells. The finding that Notch1-signaling is required for the generation of marginal zone B cells, another B-cell subset,
supports the contradicting actions of Notch1.44 It is tempting to speculate about the physiological role of Notch1 during B-cell development and the apoptotic cell death processes, which
occur naturally during B-cell development and antigen-driven differentiation into effector cells, such as memory B and plasma cells.10,11 Analysis of the mRNA and protein levels of Notch1 in
different B-cell lines, representing different developmental stages of B-lymphoid cells and different _ex vivo_ derived B-lymphoid cell populations, revealed that Notch1 mRNA and protein is
detectable in all, so far analyzed cell lines and cell populations; thus, indicating a role for Notch1 during B-lymphopoiesis (Bertrand and co-workers52,53 and U Saunders, H-M Jäck and BM
Jehn, unpublished data). However, the sole presence of Notch1 does not necessarily indicate that it is biologically active. Endogenous Notch1 might render cells susceptible to apoptotic
stimuli provided by surrounding cells and tissues in murine bone marrow and peripheral lymphoid organs. External signals from the microenvironment together with intracellular signals from
the BCR might also dictate the outcome of Notch1 signaling, that is, death or survival. We also can provide evidence that Notch1 is capable of inducing Notch1-specific target gene expression
in B cells. Analysis of the expression of Hes1, a basic helix–loop–helix transcription factor functioning downstream of the Notch1 receptor,39,40 revealed that ectopically expressed
Notch1-ICT is able to induce the expression of the Hes1 target gene and potentially activate intracellular Notch1 signaling pathways. In addition, low levels of Hes1 transcripts could be
detected in −Notch1 or wild-type NYC cells, indicating that a small amount of the endogenous murine Notch1 might be present in a active from. This further supports the idea that Notch1
signaling occurs in mature B cells. Further analysis will provide a detailed insight into Notch1-dependent signaling networks in B cells. Besides the direct influence of Notch1 on gene
transcription via Hes1, we also have to consider that some of our observation are mediated by an interaction of Notch1 with other intracellular factors like NF-_κ_B. Three recent
publications have studied the role of Notch1 and its regulatory capacity on the transcription factor NF-_κ_B. Wang _et al._54 demonstrated in transient transfections assays that
overexpressed Notch1 and NF-_κ_B are tightly interconnected, since an N-terminal portion of the intracellular domain of Notch1 is capable of inhibiting NF-_κ_B-dependent gene expression.
Furthermore, NF-_κ_B regulates Notch1 signaling through inducing the expression of the Notch1-specific ligand, Jagged1.55. In contrast, however, using a Notch1 antisense transgenic mouse
model (Notch1-AS-Tg), Cheng _et al._56 reported that DNA binding of NF-_κ_B and its ability to induce gene expression was strongly impaired in Notch1-AS-Tg mice as compared to wild-type
mice,56 indicating that physiological amounts of Notch1 have a positive effect on NF-_κ_B activity. Accordingly, precise physiological levels of active Notch1 are critical for exerting its
specific functional effects. Even subtle changes in the balance between Notch1 and cognate transcription factors seem to be sufficient to cause severe cellular responses, including impaired
proliferative capacity, diminished differentiation and also increased apoptotic cell death. Ectopic Notch1 might disturb a tightly regulated balance of endogenous Notch1 and associated pro-
and antiapoptotic factors, resulting finally in increased levels of cell death in B-lymphoid cells. However, the ability of Notch1 to render NYC cells more susceptible to apoptotic cell
death seems not to be dependent on levels of activated Notch1. For example, NYC cells with low levels of ectopic Notch1-ICT synthesis (Cls. 1 and 9) show similar enhanced rates of apoptotic
cell death as compared to NYC clones with high levels of ectopic Notch1-ICT synthesis (Cls. 5 and 6). Therefore, it seems that the effects of Notch1 we observed in NYC cells are a
consequence of rather the differentiation stage of NYC cells than that of Notch1 levels. Further support comes from the observation that endogenous Notch1 levels do not change in NYC cells
after a BCR signal has been delivered (Figure 1b) and that stable Notch1 infectants of WEHI 231 cells could never be obtained (data not shown). If the amount of Notch1 in WEHI cells would be
critical to induce ‘toxic’ effects, we would have expected to find at least some WEHI clones with a low expression of transduced Notch1. In this context, it will be of particular interest
to identify all relevant transcriptional regulator proteins mediating Notch1-specific effects in B-lymphoid cells. Investigations to identify these transcriptional partners of Notch1 in B
cells are currently underway. MATERIALS AND METHODS CELL CULTURE AND SUBCLONING The lymphoid cell line NYC 31.137 was cultured in RPMI 1640 medium (GIBCO) supplemented with 10% fetal calf
serum (Gibco), 2% L-glutamine (Gibco), 1% sodium-pyruvate (GIBCO), 2% penicillin/streptomycin (GIBCO) and 0.1% _β_-mercaptoethanol (GIBCO). Infected cells were maintained in medium
additionally supplemented with 1 mg/ml G418 (Pan Biotech). Single-cell clones were generated out of cell pools using the limiting dilution method. A total of 150 cells were diluted in 100 ml
medium and plated on 96-well plates. After 10 days in culture, the plates were screened for clones clearly deriving from single cells. These clones were marked and further expanded.
TRANSIENT TRANSFECTION OF BOSC 23 CELLS The BOSC 2338 cell line was cultured in DMEM medium (GIBCO) supplemented with 10% heat-inactivated fetal calf serum (GIBCO), 2% L-glutamine (GIBCO),
1% sodium-pyruvate (GIBCO) and 2% penicillin/streptomycin (GIBCO). The retroviral construct pGD-ICT33,38 and the control retroviral construct MSCV 2.133,38 were transiently transfected into
BOSC 23 packaging cells. The transfections were performed in six-well plates using the SuperFect reagent (Qiagen) with a total amount of 5 _μ_g DNA/well. Supernatants 24 h post-transfection
were discarded and cells were transferred to RPMI medium. The 48 and 72 h supernatants were finally collected for retroviral infection. At 72 h after transfection, whole-cell lysates were
prepared and analyzed on Western blots for Notch1-ICT synthesis. RETROVIRAL INFECTION OF NYC 31.1 CELLS Retroviral infections of NYC 31.1 cells were performed using the centrifugation
method. In brief, 1 × 106 cells were harvested and resuspended in 4 ml retroviral supernatant. After adding 1 ml fresh medium plus 10 _μ_g/ml heat-inactivated polybrene (Sigma), the mixture
was centrifuged for 3.5 h at 1671 × _g_ at 33°C in order to achieve close contact between the retroviral particles and the cells. Finally, cells were cultured in fresh medium and 24 h
postinfection, G418 selection (1 mg/ml) was initiated. WESTERN BLOT ANALYSIS AND IMMUNOPRECIPITATIONS For preparation of whole-cell lysates, 1 × 106 cells were lysed in NP-40 lysis buffer
containing 1 × PBS, 1% NP-40, 0.5% sodium-deoxycholate, 0.1% SDS and the protease inhibitors aprotinin (15 _μ_g/ml) (Sigma), leupeptin (5 _μ_g/ml) (Sigma), pepstatin (10 _μ_g/ml) (Sigma),
Pefabloc (500 _μ_g/ml) (Boehringer-Roche) and sodium-orthovanadate (1 mM) (Sigma). Lysates were separated on 8% denaturing SDS-PAGE gels and the proteins were transferred to PVDF membranes
(Schleicher & Schuell). To detect ectopically expressed or endogenous murine Notch1, the membranes were probed with a goat anti-human or –murine Notch1 antibody (C-20 and M-20) (Santa
Cruz), respectively. Signals were visualized by a horseradish-peroxidase (HRP) coupled donkey anti-goat secondary antibody (Santa Cruz) followed by enhanced chemiluminescence (ECL) and
autoradiography (Amersham Pharmacia). To assess loading, a rabbit anti-Actin antibody (Sigma) in combination with a HRP-coupled donkey anti-rabbit antibody (BioRad) were used. Again, signals
were visualized using an ECL detection system (Amersham Pharmacia). For immunoprecipitations, 1 × 107 cells were harvested and lysed in 750 _μ_l lysis buffer. Lysates were cleared by
centrifugation and mixed with 5 μg of an precipitating, polyclonal Notch1 antibody, generated by immunization of rabbits with a peptide corresponding to amino acids 1766–1780 of the mouse
Notch1 protein (Jehn _et al._, unpublished data). After incubation for 2 h on ice, 50 _μ_l of protein G sepharose beads (Pierce) were added to each sample and incubated over night at 4°C
with gentle agitation. Beads were washed four times, resuspended in 1 × SDS-loading buffer and loaded immediately on denaturing 8% SDS-PAGE gels. Proteins were transferred and specific
signals were visualized as described above. RT-PCR ANALYSIS Cells 1 × 107 were harvested and total cellular RNAs were isolated using the RNeasy-Mini protocol (Qiagen). RNAs were reverse
transcribed into cDNA with the SuperScriptII-Polymerase (GIBCO). Resulting cDNAs were amplified using the Deep-Vent DNA Polymerase (Biolabs) and specific Notch1-ICT primers
(5′-CTGAGGGCTTCAAAGTGTCTG-3′ and 5′-CATATCTTTGTTAGCCCCGTTC-3′) (MWG Biotech) or for control reasons, with specific hypoxanthine-guanine-phosphoribosyl-transferase (HPRT) primers
(5′-GCTGTTGAAAAGGACCTCT-3′ and 5′-CACAGGACTAGAACACCTGC-3′) (MWG Biotech). The Notch1-ICT primers specifically recognize the ectopically expressed form of human Notch1 and not the endogenous
murine form of Notch1. PCR products were separated on 0.8% 1X TAE agarose gels and visualized by ethidium bromide staining. To analyze Hes1 expression, cDNAs were amplified using the _Taq_
DNA Polymerase (Biolabs) along with Hes1 primers specific for the endogenous murine form (5′-CGAAAATGCCAGCTG-3′ and 5′-GATATCGAGGCTCTCAGTTCCG-3′) (MWG Biotech). PCR products were separated
on 1.0% 1X TAE agarose gels and visualized by ethidium bromide staining. INDUCTION AND ASSESSMENT OF APOPTOSIS In 1 ml R10 medium, 2 × 105 cells were plated on 24-well plates in triplicate
and induced to die apoptotically with 10 _μ_g anti-mouse-IgM F(ab)2-fragment (i.e., anti-IgM antibodies) (Jackson Dianova). PBS as well as isotype-matched control F(ab)2 fragments were used
as negative controls. Apoptosis was evaluated in the flow cytometer (EPICS XL3/ Coulter) 24 h postinduction by staining cellular DNA contents with hypotonic PI solution, containing 50
_μ_g/ml PI (Merck), 0.1 % sodium-citrate (Sigma) and 0.1% Triton X-100 (Sigma). To calculate the percentages of cells in the individual cell cycle phases, the results were analyzed with the
ModFit LT software (Becton-Dickinson). The cell death rate was determined as percentage of cells in the SubG1 phase. Alternatively, apoptosis was assessed 5 h postinduction by a combined
AnnexinV-FITC/YOPRO–3-Iodide staining according to the manufacturer's instructions (Becton-Dickinson). The measurement was performed in the flow cytometer (FACS-Calibur/
Becton-Dickinson) using the CellQuest software (Becton-Dickinson). ANALYSIS OF CELL GROWTH In 1 ml R10 medium, 1 × 104 cells were plated on 24-well plates in triplicate and cultured for 4
days. Every 24 h, cells were collected and resuspended in 200 _μ_l PBS. An equivalent amount of fluorescence-labeled beads (Coulter) was added and the mixture was analyzed in the flow
cytometer (EPICS XL3/ Coulter). The absolute number of cells was determined by calculating the number of cells in relation to the number of counted beads. 3H-THYMIDINE INCORPORATION ASSAY In
200 _μ_l R10 medium, 1 × 104 cells were plated in triplicate and were cultured for 4 days. On day 4, 1 _μ_Ci 3H-thymidine was added to each well and incubated for 6 h. Cells were finally
harvested and fixed on filters. The incorporation of 3H-thymidine was determined by measuring the filters with the fixed cells in a _β_-Counter in the presence of scintillation fluid.
ABBREVIATIONS * BCR: B-cell receptor * CBF1: core binding factor 1 * Ig: immunoglobulin * Notch1-ICT: constitutively active, intracellular domain of human Notch1 * Notch1-IC: constitutively
active, intracellular domain of Notch1 * NP-40: Nonidet P-40 * OPA: glutamine-rich region * PAGE: polyacrylamide gel electrophoresis * PEST: proline (P), glutamine (E), serine (S) and
threonine (T)-rich region * PCR: polymerase chain reaction * RBP-J: recombination signal binding protein Jkappa * RT: reverse transcription * TAN: translocation-associated human Notch1
homologue * TCR: T-cell receptor REFERENCES * Melchers F, Strasser A, Bauer SR, Kudo A, Thalmann P and Rolink A (1989) Cellular stages and molecular steps of murine B cell development _Cold
Spring Harbor Symp Quant Biol_ 1: 183–189 Article Google Scholar * Osmond DG . (1990) B cell development in the bone marrow. _Semin. Immunol._ 2: 173–180 CAS PubMed Google Scholar *
Löffert D, Schaal S, Ehrlich A, Hardy RR, Zou YR, Müller W, Rajewsky K (1994) Early B cell development in the mouse: insights from mutations introduced by gene targeting. _Immunol. Rev._
137: 135–153 Article Google Scholar * Winkler TH and Melchers F (1998) Structure and function of the antigen receptor on pro- and pre-B cells. In: _Molecular Biology of B-cell and T-cell
Development_, Monroe JG, Rothenberg EV, eds Totowa, NJ: Humana Press Inc. pp. 399–420 Chapter Google Scholar * Rolink AG, Brocker T, Bluethmann H, Kosco-Vilbois MH, Andersson J and
Melchers F (1999) Mutations affecting either generation or survival of cells influence the pool size of mature B cells. _Immunity_ 10: 619–628 Article CAS Google Scholar * Hardy RR and
Hayakawa K (2001) B cell development pathways. _Annu. Rev. Immunol._ 19: 595–621 Article CAS Google Scholar * Osmond DG, Rico-Vargas S, Valenzona H, Fauteux L, Liu L, Janani LU and
Jacobsen K (1994) Apoptosis and macrophage-mediated cell depletion in the regulation of B lymphopoiesis in mouse bone marrow. _Immunol. Rev._ 142: 209–230 Article CAS Google Scholar *
Cornall RJ, Goodnow CC, Cyster JG (1995) The regulation of self-reactive B cells. _Curr. Opin. Immunol._ 7: 804–811 Article CAS Google Scholar * Lindhout E, Koopman G, Pals ST and de
Groot C (1997) Triple check for antigen specificity of B cells during germinal center reactions. _Immunol. Today_ 18: 573–576 Article CAS Google Scholar * Lu L and Osmond DG (1997)
Apoptosis during B lymphopoiesis in mouse bone marrow. _J. Immunol._ 158: 5136–5145 CAS PubMed Google Scholar * Lu L and Osmond DG (2000) Apoptosis and its modulation during B lymphoiesis
in mouse bone marrow. _Immunol. Rev._ 175: 158–174 Article CAS Google Scholar * Nemazee D and Buerki K (1989) Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras.
_Proc. Natl. Acad. Sci. USA_ 86: 8039–8043 Article CAS Google Scholar * Nemazee D (2000) Role of B cell antigen receptor in regulation of V(D)J recombination and cell survival. _Immunol.
Res._ 21: 259–263 Article CAS Google Scholar * Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A and Goodnow CC (1993) Elimination of self-reactive B-lymphocytes proceeds in
two stages: arrested development and cell death. _Cell_ 3: 325–335 Article Google Scholar * Artavanis-Tsakonas S, Rand MD and Lake RJ (1999) Notch signaling: cell fate control and signal
integration in development. _Science_ 284: 770–776 Article CAS Google Scholar * Artavanis-Tsakonas S, Matsuno K and Fortini ME (1995) Notch signals. _Science_ 268: 225–232 Article CAS
Google Scholar * Milner LA and Bigas A (1999) Notch as a mediator of cell fate determination in hematopoiesis: evidence and speculation. _Blood_ 8: 2431–2448 Google Scholar * Radtke F,
Wilson A, Ernst B and MacDonald HR (2002) The role of Notch signaling during hematopoietic lineage commitment. _Immunol. Rev._ 187: 65–74 Article CAS Google Scholar * Allman D, Aster JC
and Pear WS (2002) Notch signaling in hematopoiesis and early lymphocyte development. _Immunol. Rev._ 187: 75–86 Article CAS Google Scholar * Blaumueller CM, Huilin Q, Zagouras P and
Artavanis-Tsakonas S (1997) Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. _Cell_ 90: 281–291 Article CAS Google Scholar * Schroeter EH,
Kisslinger JA and Kopan R (1998) Notch1 signaling requires ligand-induced proteolytic release of intracellular domain. _Nature_ 393: 382–386 Article CAS Google Scholar * De Strooper B,
Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A and Kopan R (1999) A presenilin-1-dependent _γ_-secretase-like protease mediates
release of Notch intracellular domain. _Nature_ 398: 518–522 Article CAS Google Scholar * Struhl G and Greenwald I (1999) Presenilin is required for activity and nuclear access of Notch
in Drosophila. _Nature_ 398: 522–525 Article CAS Google Scholar * Wolfe MS, Xia W, Ostazewski BL, Diehl TS, Kimberly WT and Selkoe DJ (1999) Two transmembrane aspartates in presenilin-1
required for presenilin endoproteolysis and _γ_-secretase activity. _Nature_ 398: 513–517 Article CAS Google Scholar * Ye Y, Lukinova N and Fortini ME (1999) Neurogenic phenotypes and
altered Notch processing in Drosophila presenilin mutants. _Nature_ 398: 525–529 Article CAS Google Scholar * Robey E, Chang D, Itano A, Cado D, Alexander H, Lans D, Weinmaster G and
Salmon P (1997) An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. _Cell_ 87: 483–492 Article Google Scholar * Washburn T, Schweighoffer E, Gridley T,
Chang D, Folkes BJ, Cado D and Robey E (1997) Notch activity influences the _αβ_ versus _γδ_ T cell lineage decision. _Cell_ 88: 833–843 Article CAS Google Scholar * Fowlkes BJ and Robey
EA (2002) A reassessment of the effect of activated Notch1 on CD4 and CD8 T cell development. _J. Immunol._ 169: 1817–1821 Article CAS Google Scholar * Radtke F, Wilson A, Stark G, Bauer
M, van Meerwijk J, MacDonald HR and Aguet M (1999) Deficient T cell fate specification in mice with an induced inactivation of Notch1. _Immunity_ 10: 547–558 Article CAS Google Scholar *
Wilson A, MacDonald HR and Radtke F (2001) Notch1 deficient common lymphoid precursors adopt a B cell fate in the thymus. _J. Exp. Med._ 194: 1003–10012 Article CAS Google Scholar * Pui
JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC and Pear WS (2000) Notch1 expression in early lymphopoiesis influences B _versus_ T lineage
determination. _Immunity_ 11: 299–308 Article Google Scholar * Deftos ML, He JY, Ojala EW and Bevan MJ (1998) Correlating Notch signaling with thymocyte maturation. _Immunity_ 9: 777–786
Article CAS Google Scholar * Jehn BM, Bielke W, Pear WS and Osborne BA (1999) Protective effects of Notch1 on TCR-induced apoptosis. _J. Immunol._ 162: 635–638 CAS PubMed Google Scholar
* Shelly LL, Fuchs C and Miele L (1999) Notch1 inhibits apoptosis in murine erythroleukemia cells and is necessary for differentiation induced by hybrid polar compounds. _J. Cell.
Biochem._ 73: 164–175 Article CAS Google Scholar * Morimura T, Goitsuka R, Zhang Y, Saito I, Reth M and Kitamura D (2000) Cell cycle arrest and apoptosis induced by Notch1 in B cells. _J.
Biol. Chem._ 275: 36523–36531 Article CAS Google Scholar * Morimura T, Miyatani S, Kitamura D and Goitsuka R (2001) Notch signaling suppresses IgH gene expression in chicken B cells:
implication in spatially restricted expression of Serrate2/Notch1 in the Bursa of Fabricius. _J. Immunol._ 166: 3277–3283 Article CAS Google Scholar * Jäck HM, Beck-Engeser G, Lee G,
Wofsy D and Wabl M (1992) Tumorigenesis mediated by an antigen receptor. _Proc. Natl. Acad. Sci. USA_ 89: 8482–8486 Article Google Scholar * Pear WS, Aster JC, Scott ML, Hasserjian RP,
Soffer B, Sklar JD and Baltimore D (1996) Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. _J. Exp. Med._ 83: 2283–2291
Article Google Scholar * Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R and Israel A (1995) Signaling downstream of activated mammalian Notch. _Nature_ 377: 355–358 Article CAS
Google Scholar * Jarriault S, Le Bail O, Hirsinger E, Pourquie O, Logeat F, Strong CF, Brou C, Seidah NG and Israel A (1998) Delta-1 activation of Notch1 signaling results in Hes1
transactivation. _Mol. Cell Biol._ 18: 7423–7431 Article CAS Google Scholar * Strobl LJ, Hofelmayr H, Marschall G, Brielmeier M, Bornkamm GW and Zimber-Strobl U (2000) Activated Notch1
modulates gene expression in B cells similarly to Epstein–Barr viral nuclear antigen 2. _J. Virol._ 74: 1727–1735 Article CAS Google Scholar * Izon DJ, Punt JA, Xu L, Karnell FG, Allman
D, Myung PS, Boerth NS, Pui JC, Koretzky GA and Pear WS (2001) Notch1 regulates maturation of CD4+ and CD8+ thymocytes by modulating TCR signal strength. _Immunity_ 14: 253–264 Article CAS
Google Scholar * Jundt F, Anagnostopoulos I, Forster R, Mathas S, Stein H and Dörken B (2002) Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and
anaplastic large cell lymphoma. _Blood_ 99:3398–3403 Article CAS Google Scholar * Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T and Honjo T (2002)
Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. _Nat. Immunol._ 3: 443–450 Article CAS Google Scholar * Boyd AW and Schrader JW (1981) The
regulation of growth and differentiation of a murine cell lymphoma. II. The inhibition of WEHI 231 by anti-immunoglobulin antibodies. _J. Immunol._ 126: 2466–2469 CAS PubMed Google Scholar
* Sproul TW, Malapati S, Kim J and Pierce SK (2000) B cell antigen receptor signaling occurs outside lipid rafts in immature B cells. _J. Immunol_. 165: 6020–6023 Article CAS Google
Scholar * Sayegh CE, Demaries SL, Pike KA, Friedman JE and Ratcliffe MJH (2000) The chicken B cell receptor complex and its role in avian B cell development. _Immunol. Rev._ 175: 187–200
Article CAS Google Scholar * Paramithiotis E, Jacobsen KA and Ratcliffe MJH (1995) Loss of surface immunoglobulin expression precedes B cell death by apoptosis in the Bursa of Fabricius.
_J. Exp. Med._ 181: 105–113 Article CAS Google Scholar * Liu ZG, Smith SW, McLaughlin KA, Schwartz LM and Osborne BA (1994) Apoptotic signals delivered through the T cell receptor require
the immediate-early gene nur77. _Nature_ 367: 281–284 Article CAS Google Scholar * Woronicz JD, Calnan B, Ngo V and Winoto A (1994) Requirement for the orphan receptor Nur77 in apoptosis
of T cell hybridomas. _Nature_ 367: 277–281 Article CAS Google Scholar * Jehn BM and Osborne BA (1996) Gene regulation associated with apoptosis. _Crit. Rev. Eukaryot. Gene Exp._ 7:
179–193 Article Google Scholar * Bertrand FE, Eckfeldt CE, Lysholm AS and LeBien TW (2000) Notch1 and Notch2 exhibit unique patterns of expression in human B-lineage cells. _Leukemia_ 14:
2095–2102 Article CAS Google Scholar * Bertrand FE, Eckfeldt CE, Fink JR, Lysholm AS, Pribyl JA, Shah N and LeBien TW (2000) Microenvironmental influences on human B-cell development.
_Immunol. Rev._ 175: 175–186 Article CAS Google Scholar * Wang J, Shelly L, Miele L, Boykins R, Norcoss MA and Guan E (2001) Human Notch1 inhibits NF-_κ_B activity in the nucleus through
a direct interaction involving a novel domain. _J. Immunol._ 167: 289–295 Article CAS Google Scholar * Bash J, Zong WX, Banga S, Rivera A, Ballard DW, Ron Y, Gelinas C (1999) Rel/NF-_κ_B
can trigger the Notch signaling pathway by inducing the expression of Jagged1, a ligand for Notch receptors. _EMBO J._ 18: 2803–2811 Article CAS Google Scholar * Cheng P, Zlobin A,
Volgina V, Gottipati S, Osborne B, Simel EJ, Miele L and Gabrilovich DI (2001) Notch1 regulates NF-_κ_B activity in hematopoietic progenitor cells. _J. Immunol._ 167: 4458–4467 Article CAS
Google Scholar Download references ACKNOWLEDGEMENTS We thank Christine Matzner for excellent technical assistance and W Bielke (Erlangen) for advice, support and critical reading of the
manuscript. The authors are grateful to Warren Pear for providing the retroviral vectors MSCV 2.1 and pGD-ICT. This work was supported in part by the research training grant GRK 592 from the
German Research Society (DFG) to SR, the project grant SFB 263 from the DFG to US, BMJ and H-MJ, and intramural funding through the ELAN program of the University of Erlangen to H-MJ BMJ
was supported by a fellowship from the German Cancer Research Center, Heidelberg (DKFZ), Germany. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Molecular Immunology, Department
of Internal Medicine III, Nikolaus-Fiebiger Center, Friedrich-Alexander-University Erlangen-Nürnberg, Glückstrasse 6, 91054, Erlangen, Germany S Romer, U Saunders, H-M Jäck & B M Jehn
Authors * S Romer View author publications You can also search for this author inPubMed Google Scholar * U Saunders View author publications You can also search for this author inPubMed
Google Scholar * H-M Jäck View author publications You can also search for this author inPubMed Google Scholar * B M Jehn View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to H-M Jäck. ADDITIONAL INFORMATION Edited by B Osborne RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE
THIS ARTICLE Romer, S., Saunders, U., Jäck, HM. _et al._ Notch1 enhances B-cell receptor-induced apoptosis in mature activated B cells without affecting cell cycle progression and surface
IgM expression. _Cell Death Differ_ 10, 833–844 (2003). https://doi.org/10.1038/sj.cdd.4401253 Download citation * Received: 14 February 2003 * Accepted: 04 March 2003 * Published: 18 June
2003 * Issue Date: 01 July 2003 * DOI: https://doi.org/10.1038/sj.cdd.4401253 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * B1 cells * B
lymphocytes * hematopoiesis * NYC 31.1 * B-cell maturation