- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT To compare immunophenotypic and molecular features between Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL) with c-_myc_ rearrangements (c-_myc_R DLBCL), we analyzed
18 cases of B-cell non-Hodgkin's lymphoma with c-_myc_R that were confirmed by chromosomal and/or Southern blotting analyses. The cases were histologically classified into 10 BLs and
five DLBCLs. The remaining three cases could not be classified because of suboptimal quality of the surgical materials. BLs were from five adults and five children, whereas all DLBCLs were
from adults. BLs were positive for CD20 (10/10 cases examined), CD10 (9/10), Bcl-2 (1/9), and Bcl-6 (10/10), whereas they were negative for CD3 (0/10) and EBV (0/8), by Epstein-Barr virus
(EBV) EBER-1 RNA _in situ_ hybridization. c-_Myc_R DLBCLs were positive for CD20 (5/5), CD10 (2/5), Bcl-2 (3/4), and Bcl-6 (4/4), whereas none of them were positive for CD3 and EBV. A mean
of MIB-1 index (MIB-1+ cells/neoplastic cells, %) of BLs (98.1%) was higher than that of c-_myc_R DLBCLs (66.3%; _P_ < .0001). Somatic mutation of immunoglobulin heavy-chain gene variable
region (VH gene) in BLs (four cases) ranged from 0.7 to 4.9% with an average value of 2.3%, whereas those in DLBCLs (three cases) from 8.2 to 32.0% with an average value of 17.0%. It is,
therefore, concluded that a growth fraction of nearly 100%, as well as a monotonous proliferation of medium-sized cells and c-_myc_R, should be of value in the diagnosis of BL, which is
probably different from c-_myc_R DLBCL. In addition, CD10+, Bcl-2−, and low frequency of mutation of the VH gene could be helpful for the histologic distinction of BL from (c-_myc_R) DLBCL.
SIMILAR CONTENT BEING VIEWED BY OTHERS _MYC_-REARRANGED MATURE B-CELL LYMPHOMAS IN CHILDREN AND YOUNG ADULTS ARE MOLECULARLY BURKITT LYMPHOMA Article Open access 07 October 2024 BURKITT
LYMPHOMA Article 15 December 2022 NON-IG::MYC IN DIFFUSE LARGE B-CELL LYMPHOMA CONFERS VARIABLE GENOMIC CONFIGURATIONS AND MYC TRANSACTIVATION POTENTIAL Article Open access 06 January 2024
INTRODUCTION Chromosomal translocations of 8q24, encoding an oncogene of c-_myc_, are considered to be associated with oncogenesis of non-Hodgkin's lymphoma (NHL) (1, 2, 3, 4). These
chromosomal translocations dysregulate the expression of c-_myc_, a gene encoding a basic helix-loop-helix (bHLH) transcription factor that binds to DNA in a sequence-specific fashion.
c-_Myc_ normally plays a central role in the transcriptional regulation of an emerging set of downstream genes that control diverse cellular processes, including cell cycle progression and
programmed cell death (apoptosis). Translocation of c-_myc_ and immunoglobulin gene, t(8;14)(q24;q32), t(2;8)(p13;q24), and t(8;22)(q24;q11) are observed in almost all Burkitt lymphomas
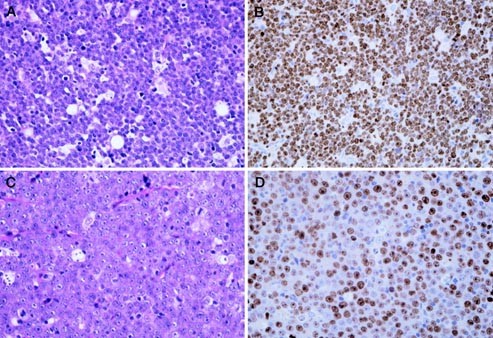
(BLs), highly aggressive B-cell lymphomas. The histologic hallmark of BL is a monomorphic proliferation of medium-sized lymphoma cells with several small nucleoli and a starry-sky appearance
(5, 6). The neoplastic cells usually express CD19, CD20, and CD10 (5, 6). Although identification of c-_myc_ rearrangement is prerequisite to a diagnosis of BL, it is occasionally seen in
other B-cell neoplasms, such as diffuse large B-cell lymphoma (DLBCL), lymphoblastic lymphoma (LBL), follicular lymphoma (FL), and multiple myeloma (MM) (7, 8, 9, 10, 11, 12, 13, 14). Most
cases of LBL, while showing a starry-sky appearance, can be easily distinguished from BL by their convoluted nuclei with fine chromatin and detection of antigens such as TdT. FL and MM are
different from BL in the cytologic features. However, DLBCL occasionally mimic BL. The neoplasms composed of medium-sized to large cells with a starry sky appearance and classified as _small
non-cleaved cell lymphoma, non-Burkitt_ in the Working Formulation (15) or as _high-grade B-cell lymphoma, Burkitt-like (provisional entity)_ in the REAL (5) are difficult to differentiate
from BL. In the new World Health Organization (WHO) classification, the gold standard for the diagnosis of BL should be the presence of t(8;14)(q24;q32) and its variants or c-_myc_
rearrangement (6). If cytogenetic or Southern blot analysis is not available, BL should not be diagnosed without a Ki-67 fraction of close to 100% (6). DLBCLs with c-_myc_ rearrangement,
however, are known to account for 5–7% of DLBCL (10, 11, 12). What are differences between BL and DLBCL with c-_myc_ rearrangement (c-_myc_R DLBCL)? In this article, we analyzed clinical,
histologic, immunohistochemical, and molecular features of 18 cases of B-cell NHL with c-_myc_ rearrangement, to examine 1) the difference between BL and c-_myc_R DLBCL and 2) whether MIB-1
fraction of almost 100% is feature specific to BL. We found that c-_myc_R DLBCL were clearly different from BL in term of immunoreactivity with CD10, Bcl-2, and MIB-1, as well as the
frequency of somatic mutation of the immunoglobulin heavy chain (IgH) gene variable region (VH gene). MATERIALS AND METHODS CASE SELECTION Eighteen cases of B-cell NHL with c-_myc_
rearrangement detected by a cytogenetic analysis or Southern blotting were retrieved from the files of malignant lymphoma in six pathology departments. No cases associated with AIDS were
included in this study. HISTOLOGIC AND IMMUNOHISTOCHEMICAL STUDIES A part of resected tissue was fixed in 20% buffered formalin and embedded in paraffin for the routine histologic and
immunohistochemical studies. All cases were re-evaluated by all authors, and the histologic diagnosis was established according to the new WHO classification (6). Immunohistochemistry was
performed on paraffin-embedded sections using the streptavidin-biotin complex technique (16). Monoclonal and polyclonal antibodies used in this study were as follows: CD3 (CD3ε,
DAKOPATTS[DA], DAKO, Glostrup, Denmark), CD10 (CD10, Novocastra Laboratories Ltd. [NC], Newcastle Upon Tyne, UK), CD20 (L26, DA), Bcl-6 (Santa Cruz, CA), Bcl-2 (Bcl-2, DA) and MIB-1 (MIB-1,
DA). A given lymphoma was judged positive when >20%-positive cells were immunostained by each antibody except MIB-1. The percentages of positive cells for MIB-1 were estimated. The
numbers of MIB-1 positive cells and all tumor cells were determined by an actual count in areas (2.5 mm2) counted at high-power field (400 ×). Background small lymphocytes and starry-sky
macrophages were excluded. A mean of three areas in each case was taken as the MIB-1 index. MOLECULAR STUDIES EPSTEIN-BARR VIRUS RNA _IN SITU_ HYBRIDIZATION Epstein-Barr (EB) virus RNA _in
situ_ hybridization (EBER-1 RNA-ISH) was performed as described elsewhere for detection of the genome of EBV (17). SOMATIC MUTATION OF THE IGH GENE VARIABLE REGION The IgH gene was amplified
by semi-nested PCR, according to a method described elsewhere (18), and variable region (CDR2 and FW3) was analyzed. RESULTS Clinical, histologic, immunohistochemical, and molecular data
are shown in Table 1. The patients were distributed in age from 4 to 81 years old, with a male to female ratio of 12:6. Eleven cases presented with extranodal lymphoma, whereas seven had
nodal lymphoma. Cytogenetic data was available in 13 cases. Twelve cases carried t(8;14)(q24;q32) and one case t(8;22)(q24;q11), and karyotypes of 10 cases are shown in Table 2. c-_Myc_
rearrangements were found in nine cases by Southern blotting analysis. A consensus diagnosis by re-evaluation of routine HE section of all 18 cases among all authors is shown in Table 1. Ten
cases were diagnosed as BL, whereas five cases were diagnosed as DLBCL. Because consensus diagnoses could not be obtained because of suboptimal quality of materials available, the remaining
three cases were put into an undetermined group. The BL group consisted of children and adults, whereas the DLBCL group consisted of adults only. Results of immunohistochemical study and
EBER-1 RNA-ISH are shown in Table 1. BLs represented 10 positive cases/10 examined cases for CD20, 0/10 for CD3, 9/10 for CD10, 10/10 for Bcl-6, 1/9 for Bcl-2, and 0/8 for EBV. DLBCLs
represented 5/5 for CD20, 0/5 for CD3, 2/5 for CD10, 4/4 for Bcl-6, 3/4 for Bcl-2, and 0/5 for EBV. The undetermined group represented 3/3 for CD20, 0/3 for CD3, 2/3 for CD10, 3/3 for Bcl-6,
0/3 for Bcl-2, and 1/3 for EBV. The Bcl-2 expression differed between BLs (1/9) and DLBCLs (3/4). There was no statistically significant difference between them (χ2-test). The MIB-1 index
of BLs and DLBCLs were distributed from 94.7 to 99.3%, with a mean of 98.1% and from 48.0 to 89.7% with a mean of 66.3%, respectively. There was a statistically significant difference
between them (_t_ test, _P_ < .0001). [Fig. 1] Frequency of somatic mutation of the VH gene in the BL group (four cases) ranged from 0.7 to 4.9%, with an average of 2.3%, whereas that in
the DLBCL group (three cases) ranged from 8.2 to 32.0%, with an average of 17.0%. (Table 1) The average of somatic mutation frequency in the DLBCL group was higher than that in the BL group,
but there was no statistical difference between them (_t_ test, _P_ = .0793). DISCUSSION To compare clinicopathologic features of BL with those of c-_myc_R DLBCL, we analyzed 18 cases of
B-cell lymphoma with c-_myc_ rearrangements. Except for three cases with suboptimal specimens, we reached a consensus diagnosis of either BL or DLBCL in all 15 cases. Because we failed to
obtain a consensus diagnosis in several cases using the REAL classification, the description in the new WHO classification can give more reproducible pathologic criteria for the
differentiation between BL and DLBCL. Although the translocation of c-_myc_ and Ig genes is the gold standard for BL, we demonstrated some differences between BLs and c-_myc_R DLBCL. CD10+,
Bcl-2−, and extremely high rate of MIB-1+ cells were characteristic for BL. The mean MIB-1 index in BLs was 98.1%, and the indices generally were >98%, except in two cases. A growth
fraction of nearly 100% can be expected for BLs. In c-_myc_R DLBCLs, on the contrary, MIB-1 index was distributed from 48.0 to 89.7%, and the mean (66.3%) was lower than that of BLs. More
than 95% of MIB-1 expression was not seen in any of the five cases of c-_myc_R DLBCL. Degree of the MIB-1 expression varied from case to case and were not clearly different from DLBCL
without c-_myc_ rearrangement (data not shown). As Bcl-2 was previously reported to be negative for BL (19), Bcl-2 did not react with all cases of BL but one. An absence of Bcl-2 protein, as
well as extremely high rate of the MIB-1 index is a good marker for BL. EBV was not detected by EBER-1 RNA-ISH in both BL and c-_myc_R DLBCL, but chromosomal abnormalities of c-_myc_R DLBCL
were more complex, with many losses and gains involving chromosomes other than those in BL. Because breakpoints at c-_myc_ and Ig genes have been reported to be similar in both BL and
c-_myc_R DLBCL by the long-distance PCR and cloning analyses (12), the hypothesis could be raised that both lymphomas are the same disease, showing different stages of tumor progression.
This hypothesis, however, seems unlikely because of the following reasons. Both of BL and DLBCL are considered to be derived from germinal center (GC) B cells because of the expression of
Bcl-6 and high frequency of somatic mutation of the rearranged VH genes (20, 21, 22, 23). Somatic mutation frequencies of the VH gene of the endemic and sporadic BLs are reported to have
ranges between 5–15% and 0–5%, respectively (24). In our series, the VH gene of BL was somatically mutated in a range of 0–5% with an average of 2.3%. On the other hand, two of three cases
of c-_myc_R DLBCL examined revealed >10% of mutation frequency in rearranged VH gene. We previously reported an average of 11.9% of mutation frequency, with a broad range, in 60 cases of
EBV− DLBCL (25), and this pattern is similar to that of c-_myc_R DLBCL. Moreover, using additional cases, we have found a statistical difference in somatic mutation rates between BLs and
c-_myc_R DLBCLs (unpublished data, in preparation). c-_Myc_R DLBCL is known to occasionally involve the coexistence of t(14;18) involving _bcl_-2 genes (26). Dual translocation of c-_myc_
and _bcl_-2 is characterized by a rapid clinical course and extremely poor outcome, and some cases with dual translocation of c-_myc_ and _bcl_-2 are regarded as instances of transformation
of follicular lymphoma. There were no cases with dual translocation of c-_myc_ and _bcl_-2 in our series. BL accounts for 1–2% of total NHLs (27, 28), and most of them are of a sporadic type
in non-African areas. Because c-_myc_ rearrangement is found in 5–7% of DLBCL, which account for 30% of total NHLs, c-_myc_R DLBCL is estimated to constitute 1.5–2.1% of total NHL. The
similar frequency of BL and c-_myc_R DLBCL indicates that the rearrangement may occur in various GC stages in secondary B-cell differentiation. When the neoplastic proliferation is initiated
by c-_myc_/Ig translocation at a given stage of the secondary B-cell differentiation in the GC, it is not likely that clinically and histologically distinct neoplasms, that is, BL and
DLBCL, arise. It is, therefore, indicated that the normal counterparts of BL and c-_myc_R DLBCL are different. Although BL has aggressive clinical course and results in poor outcome, this
tumor is highly sensitive to chemotherapeutic regimens, and the response is different from that of other aggressive B-cell lymphomas (3, 27). Five of eight patients with BL in this series
had a poor prognosis, and a majority of them died of disease within 1 year. Although the clinical outcome of two patients was unknown in our series, Vitolo _et al._ (10) analyzed 71 cases
with DLBCL including four cases of c-_myc_R DLBCL. All cases were treated with an anthracycline-containing chemotherapy regimen. These four patients with c-_myc_R DLBCL had an aggressive
disease with poor prognosis. Kramer _et al._ (11) reported that structural alterations of c-_myc_ were detected in 10 of 151 cases of DLBCL by Southern blot analysis (6.7%), and two of them
had both _bcl_-6 and c-_myc_ rearrangements. In their series, c-_myc_ rearrangements were found in 16% of primary extranodal lymphomas, whereas only 2% of primary nodal cases had these
abnormalities (_P_ = .02). In particular, 5 of 18 cases (28%) of gastrointestinal lymphomas had the c-_myc_ rearrangements. Seven of ten cases with c-_myc_ rearrangements achieved a complete
remission and six responders remained alive for >4 years, resulting in the trend of a better disease-free survival. Thus, our speculation that the normal counterparts of BL and c-_myc_R
DLBCL are different can be clinically supported. REFERENCES * Magrath IT . African Burkitt's lymphoma: history, biology, clinical features, and treatment. _Am J Pediatr Hematol Oncol_
1991; 13: 222–246. Article CAS PubMed Google Scholar * Hecht JL, Aster JC . Molecular biology of Burkitt's lymphoma. _J Clin Oncol_ 2000; 18: 3707–3721. Article CAS PubMed Google
Scholar * Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM . Human c-_myc_ oncogene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma
cells. _Proc Natl Acad Sci U S A_ 1982; 79: 7824–7827. Article CAS PubMed PubMed Central Google Scholar * Taub R, Kirsch I, Morton C, Lenoir G, Swan D, Tronick S, _et al_. Translocation
of the c-_myc_ gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. _Proc Natl Acad Sci U S A_ 1982; 79: 7837–7841. Article CAS PubMed
PubMed Central Google Scholar * Harris NL, Jaffe ES, Stein H, Banks PM, Chan J, Cleary ML, _et al_. A revised European-American classification of lymphoid neoplasms: a proposal from the
International Lymphoma Study Group. _Blood_ 1994; 84: 1361–1392. CAS PubMed Google Scholar * Diebold J, Jaffe ES, Raphael M, Warnke RA . Burkitt lymphoma.In: Jaffe ES, Harris NL, Stein H,
Vardiman JW, editors. _World Health Organization classification of tumours_. Tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2001:p. 181–184. Google Scholar *
Sigaux F, Berger R, Bernheim A, Valensi F, Daniel MT, Flandrin G . Malignant lymphomas with band 8q24 chromosome abnormality: a morphologic continuum extending from Burkitt's to
immunoblastic lymphoma. _Br J Haematol_ 1984; 57: 393–405. Article CAS PubMed Google Scholar * Slavutsky I, Andreoli G, Gutierrez M, Narbaitz M, Lucero G, Eppinger M . Variant (8;22)
translocation in lymphoblastic lymphoma. _Leuk Lymphoma_ 1996; 21: 169–172. Article CAS PubMed Google Scholar * Macpherson N, Lesack D, Klasa R, Horsman D, Connors JM, Barnett M, _et
al_. Small noncleaved, non-Burkitt's (Burkitt-Like) lymphoma: cytogenetics predict outcome and reflect clinical presentation. _J Clin Oncol_ 1999; 17: 1558–1567. Article CAS PubMed
Google Scholar * Vitolo U, Gaidano G, Botto B, Volpe G, Audisio E, Bertini M, _et al_. Rearrangements of bcl-6, bcl-2, c-_myc_ and 6q deletion in B-diffuse large-cell lymphoma: clinical
relevance in 71 patients. _Ann Oncol_ 1998; 9: 55–61. Article CAS PubMed Google Scholar * Kramer MH, Hermans J, Wijburg E, Philippo K, Geelen E, van Krieken JH, _et al_. Clinical
relevance of BCL2, BCL6, and _MYC_ rearrangements in diffuse large B-cell lymphoma. _Blood_ 1998; 92: 3152–3162. CAS PubMed Google Scholar * Akasaka T, Akasaka H, Ueda C, Yonetani N,
Maesako Y, Shimizu A, _et al_. Molecular and clinical features of non-Burkitt's, diffuse large-cell lymphoma of B-cell type associated with the c-_myc_/immunoglobulin heavy-chain fusion
gene. _J Clin Oncol_ 2000; 18: 510–518. Article CAS PubMed Google Scholar * Sawyer JR, Waldron JA, Jagannath S, Barlogie B . Cytogenetic findings in 200 patients with multiple myeloma.
_Cancer Genet Cytogenet_ 1995; 82: 41–49. Article CAS PubMed Google Scholar * Shou Y, Martelli ML, Gabrea A, Qi Y, Brents LA, Roschke A, _et al_. Diverse karyotypic abnormalities of the
c-_myc_ locus associated with c-_myc_ dysregulation and tumor progression in multiple myeloma. _Proc Natl Acad Sci U S A_ 2000; 97: 228–233. Article CAS PubMed PubMed Central Google
Scholar * National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a working formulation for clinical usage. The
Non-Hodgkin's Lymphoma Pathologic Classification Project. _Cancer_ 1982; 49: 2112–2135. * Hsu SM, Raine L, Fanger H . Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase
techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. _J Histochem Cytochem_ 1981; 29: 577–580. Article CAS PubMed Google Scholar * Kuze T, Nakamura N, Hashimoto
Y, Abe M, Wakasa H . Clinicopathological, immunological and genetic studies of CD30+ anaplastic large cell lymphoma of B-cell type; association with Epstein-Barr virus in a Japanese
population. _J Pathol_ 1996; 180: 236–242. Article CAS PubMed Google Scholar * Nakamura N, Hashimoto Y, Kuze T, Tasaki K, Sasaki Y, Sato M, _et al_. Analysis of the immunoglobulin heavy
chain gene variable region of CD5-positve diffuse large B-cell lymphoma. _Lab Invest_ 1999; 79: 925–933. CAS PubMed Google Scholar * Lai R, Arber DA, Chang KL, Wilson CS, Weiss LM .
Frequency of bcl-2 expression in non-Hodgkin's lymphoma: a study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. _Mod Pathol_ 1998; 11:
864–869. CAS PubMed Google Scholar * Tamaru J, Hummel M, Marafioti T, Kalvelage B, Leoncini L, Minacci C, _et al_. Burkitt's lymphomas express VH genes with a moderate number of
antigen-selected somatic mutations. _Am J Pathol_ 1995; 147: 1398–1407. CAS PubMed PubMed Central Google Scholar * Chapman CJ, Zhou JX, Gregory C, Rickinson AB, Stevenson FK . VH and VL
gene analysis in sporadic Burkitt's lymphoma shows somatic hypermutation, intraclonal heterogeneity, and a role for antigen selection. _Blood_ 1996; 88: 3562–3568. CAS PubMed Google
Scholar * Jain R, Roncella S, Hashimoto S, Carbone A, Francia di Celle P, Foa R, _et al_. A potential role for antigen selection in the clonal evolution of Burkitt's lymphoma. _J
Immunol_ 1994; 153: 45–52. CAS PubMed Google Scholar * Müller-Hermelink HK, Greiner A . Molecular analysis of human immunoglobulin heavy chain variable gene (IgVH) in normal and malignant
B cells. _Am J Pathol_ 1998; 153: 1341–1345. Article PubMed PubMed Central Google Scholar * Klein U, Goossens T, Fischer M, Kanzler H, Braeuninger A, Rajewsky K, _et al_. Somatic
hypermutation in normal and transformed human B cells. _Immunol Rev_ 1998; 162: 261–280. Article CAS PubMed Google Scholar * Kuze T, Nakamura N, Hashimoto Y, Sasaki Y, Abe M . The
characteristics of Epstein-Barr virus (EBV)- positive diffuse large B-cell lymphoma: comparison between EBV+ and EBV− cases in Japanese population. _Jpn J Cancer Res_ 2000; 91: 1233–1240.
Article CAS PubMed PubMed Central Google Scholar * Macpherson N, Lesack D, Klasa R, Horsman D, Connors JM, Barnett M, _et al_. Small noncleaved, non-Burkitt's (Burkitt-like)
lymphoma: cytogenetics predict outcome and reflect clinical presentation. _J Clin Oncol_ 1999; 17: 1558–1567. Article CAS PubMed Google Scholar * The Non-Hodgkin's Lymphoma
Classification Project. A clinical evaluation of the international lymphoma study group classification of non-Hodgkin's lymphoma. _Blood_ 1997; 89: 3909–3918. * Lymphoma Study Group of
Japanese Pathologists. The World Health Organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. _Pathol Int_ 2000; 50: 696–702. Article
Google Scholar Download references ACKNOWLEDGEMENTS The authors thank Prof. Dr. Masahiro Kikuchi, Fukuoka University, and Dr. Masao Seto, Aichi Cancer Center, for their excellent advice.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pathology, Fukushima Medical University School of Medicine, Japan Naoya Nakamura M.D. & Masafumi Abe M.D. * Department of
Clinical Laboratory Medicine, Wakayama Medical University School of Medicine, Wakayama Hirokazu Nakamine M.D. * Department of Pathology, Saitama Medical Center, Saitama Medical School,
Saitama Jun-ichi Tamaru M.D. * Department of Pathology and Genetics, Aichi Cancer Center Hospital, Nagoya Shigeo Nakamura M.D. * Department of Pathology, School of Medicine, Okayama
University, Okayama Tadashi Yoshino M.D. * Department of Pathology, School of Medicine, Fukuoka University, Fukuoka, Japan Kouichi Ohshima M.D. Authors * Naoya Nakamura M.D. View author
publications You can also search for this author inPubMed Google Scholar * Hirokazu Nakamine M.D. View author publications You can also search for this author inPubMed Google Scholar *
Jun-ichi Tamaru M.D. View author publications You can also search for this author inPubMed Google Scholar * Shigeo Nakamura M.D. View author publications You can also search for this author
inPubMed Google Scholar * Tadashi Yoshino M.D. View author publications You can also search for this author inPubMed Google Scholar * Kouichi Ohshima M.D. View author publications You can
also search for this author inPubMed Google Scholar * Masafumi Abe M.D. View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to Naoya Nakamura M.D.. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Nakamura, N., Nakamine, H., Tamaru, Ji. _et al._ The Distinction
between Burkitt Lymphoma and Diffuse Large B-Cell Lymphoma with c-_myc_ Rearrangement. _Mod Pathol_ 15, 771–776 (2002). https://doi.org/10.1097/01.MP.0000019577.73786.64 Download citation *
Accepted: 10 April 2002 * Published: 01 July 2002 * Issue Date: July 2002 * DOI: https://doi.org/10.1097/01.MP.0000019577.73786.64 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative KEYWORDS * Burkitt lymphoma * c-_myc_ rearrangement * Diffuse large B-cell lymphoma * Immunoglobulin heavy chain gene * Immunohistochemistry * MIB-1 * Somatic
mutation