- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
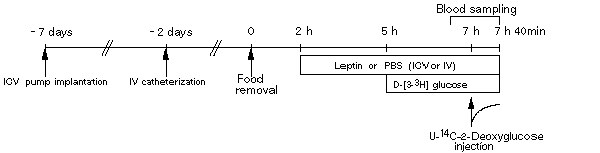
ABSTRACT Leptin is an adipocyte hormone that functions as an afferent signal in a negative feedback loop regulating body weight1,2,3,4, and acts by interacting with a receptor in the
hypothalamus and other tissues5,6. Leptin treatment has potent effects on lipid metabolism, and leads to a large, specific reduction of adipose tissue mass after several days1,4. Here we
show that leptin also acts acutely to increase glucose metabolism, although studies of leptin's effect on glucose metabolism have typically been confounded by the weight-reducing
actions of leptin treatment, which by itself could affect glucose homoeostasis1,2,3. We have demonstrated acute _in vivo_ effects of intravenous and intracerebroventricular administrations
of leptin on glucose metabolism. A five-hour intravenous infusion of leptin into wild-type mice increased glucose turnover and glucose uptake, but decreased hepatic glycogen content. The
plasma levels of insulin and glucose did not change. Similar effects were observed after both intravenous and intracerebroventricular infusion of leptin, suggesting that effects of leptin on
glucose metabolism are mediated by the central nervous system (CNS). These data indicate that leptin induces a complex metabolic response with effects on glucose as well as lipid
metabolism. This response is unique to leptin, which suggests that new efferent signals emanate from the CNS after leptin treatment. Access through your institution Buy or subscribe This is
a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per
year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during
checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS SYMPATHETIC NERVOUS
SYSTEM INHIBITION ENHANCES CARDIAC METABOLISM AND IMPROVES HEMODYNAMICS AND GLUCOSE-INSULIN DYNAMICS IN OBESE AND LEAN RAT MODELS Article Open access 02 January 2025 CENTRAL NERVOUS SYSTEM
REGULATION OF ORGANISMAL ENERGY AND GLUCOSE HOMEOSTASIS Article 21 June 2021 LEPTIN ALTERS ENERGY INTAKE AND FAT MASS BUT NOT ENERGY EXPENDITURE IN LEAN SUBJECTS Article Open access 13
October 2020 REFERENCES * Halaas, J. L. et al. Weight-reducing effects of the plasma protein encoded by the _obese_ gene. _Science_ 269, 543–546 (1995). Article ADS CAS Google Scholar *
Campfield, L. A., Smith, F. J., Guisez, Y., Devos, R. & Burn, P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. _Science_
269, 546–549 (1995). Article ADS CAS Google Scholar * Pelleymounter, M. A. et al. Effects of the _obese_ gene product on body weight regulation in _ob_/_ob_ mice. _Science_ 269, 540–543
(1995). Article ADS CAS Google Scholar * Halaas, J. L. et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. _Proc. Natl Acad. Sci.
USA_ 94, 8878–8883 (1997). Article ADS CAS Google Scholar * Tartaglia, L. A.. et al. Identification and expression cloning of a leptin receptor, OB-R. _Cell_ 83, 1263–1271 (1995).
Article CAS Google Scholar * Lee, G. H. et al. Abnormal splicing of the leptin receptor in _diabetic_ mice. _Nature_ 379, 632–635 (1996). Article ADS CAS Google Scholar * Levin, N.,
Nelson, C., Gurney, A., Vanelen, R. & De Sauvage, F. Decreased food intake does not completely account for adiposity reduction after ob protein infusion. _Proc. Natl Acad. Sci. USA_ 93,
1726–1730 (1996). Article ADS CAS Google Scholar * Cohen, B., Novick, D. & Rubinstein, M. Modulation of insulin activities by leptin. _Science_ 274, 1185–1188 (1996). Article ADS
CAS Google Scholar * Saladin, T. et al. Transient increase in _obese_ gene expression after food intake or insulin administration. _Science_ 377, 527–529 (1995). CAS Google Scholar *
Mercer, J. G. et al. Localization of leptin receptor mRNA and the long form splice variant (Ob-R) in mouse hypothalamus and adjacent brain regions by in situ hybridization. _FEBS Lett._ 387,
113–116 (1996). Article CAS Google Scholar * Golden, P. L., Maccagnan, T. J. & Pardridge, W. M. Human blood-brain barrier leptin receptor. _J. Clin. Invest._ 99, 14–18 (1997).
Article CAS Google Scholar * Maffei, M. et al. Increased expression in adipocytes of _ob_ RNA in mice with lesions of the hypothalamus and with mutations at the _db_ locus. _Proc. Natl
Acad. Sci. USA_ 92, 6957–6960 (1995). Article ADS CAS Google Scholar * Satoh, N. et al. Pathophysiological significance of the obese gene product, leptin, in ventromedial hypothalamus
(VMH)-lesioned rats: evidence for loss of its satiety effect in VMH-lesioned rats. _Endocrinology_ 138, 947–954 (1997). Article CAS Google Scholar * Fei, H. et al. Anatomic localization
of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. _Proc. Natl Acad. Sci. USA_ 94, 7001–7005 (1997). Article ADS CAS Google Scholar * Vaisse, C. et al.
Leptin activation of Stat3 in the hypothalamus of wild type and _ob_/_ob_ mice but not _db_/_db_ mice. _Nature Genet._ 14, 95–97 (1996). Article CAS Google Scholar * Schwartz, M. W.,
Seeley, R. J., Campfield, L. A., Burn, P. & Baskin, D. G. Identification of targets of leptin action in rat hypothalamus. _J. Clin. Invest._ 98, 1101–1106 (1996). Article CAS Google
Scholar * Caro, J. F. et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. _Lancet_ 348, 159–161 (1996). Article CAS Google
Scholar * Miles, P. D. G., Yamatani, K., Lickley, H. L. A. & Vranic, M. Mechanism of glucoregulatory responses to stress and their deficiency in diabetes. _Proc. Natl Acad. Sci. USA_
88, 1296–1300 (1991). Article ADS CAS Google Scholar * Leong, S. F. & Clark, J. B. Regional enzyme development in rat brain: Enzymes associated with glucose utilization.
_Biochemistry_ 218, 131–138 (1984). Article CAS Google Scholar * Lautala, P. & Martin, J. M. Glucose metabolism in rat hypothalamus. _Acta Endocrinol._ 98, 481–487 (1981). Article
CAS Google Scholar * Nagai, K., Fujii, T., Inoue, S., Takamura, Y. & Nakagawa, H. Electrical stimulation of the suprachiasmatic nucleus of the hypothalamus causes hyperglycemia.
_Hormone Metab. Res._ 20, 37–39 (1988). Article CAS Google Scholar * Collins, S. et al. Role of Leptin in fat regulation. _Nature_ 380, 677 (1996). Article ADS CAS Google Scholar *
Vranic, M., Kawamori, R., Pek, S., Kovacevic, N. & Wrenshall, G. A. The essentiality of insulin and the role of glucagon in regulating glucose utilization and production during strenuous
exercise in dogs. _J. Clin. Invest._ 57, 245–255 (1976). Article CAS Google Scholar * Gosteli-Peter, M. A., Schmid, C. & Zapf, J. Triiodothyronine increases glucose transporter
isotype 4 mRNA expression, glucose transport, and glycogen synthesis in adult rat cardiomyocytes in long-term culture. _Biochem. Biophys. Res. Commun._ 221, 521–524 (1996). Article CAS
Google Scholar * Weinstein, S. P., O'Boyle, E. & Haber, R. S. Thyroid hormone increases basal and insulin-stimulated glucose transport in skeletal muscle. The role of GLUT4 glucose
transporter expression. _Diabetes_ 43, 1185–1189 (1994). Article CAS Google Scholar * Ahima, R. S. et al. Role of leptin in the neuroendocrine response to fasting. _Nature_ 382, 250–252
(1996). Article ADS CAS Google Scholar * Somogyi, M. Determination of blood sugar. _J. Biol. Chem._ 160, 69–73 (1945). CAS Google Scholar * Tsao, T. S., Burcelin, R., Katz, E. B.,
Huang, L. & Charron, M. J. Enhanced insulin action due to targeted GLUT4 overexpression exclusively in muscle. _Diabetes_ 45, 28–36 (1996). Article CAS Google Scholar * Massillon, D.,
Barzilai, N., Hawkins, M., Prus-Wertherimer, D. & Rossetti, L. Quantitation of hepatic glucose fluxes and pathways of hepatic glycogen synthesis in conscious mice. _Am. J. Physiol._
269, E1037–E1043 (1995). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank E. B. Katz, C. Vaisse, J. Li and T. S. Tsao for discussions; J. Blaire-West and D. A.
Denton for help with the ICV surgery; S. Korres for helping to prepare the manuscript; and Amgen for recombinant leptin. This work was supported by grants from the NIH (J.M.F. and M.J.C.),
Pew Charitable Trust (M.J.C.), Juvenile Diabetes Foundation International (R.B.), the Philippe Foundation (R.B.) and the Manpei Suzuki Diabetes Foundation (S.K.). AUTHOR INFORMATION Author
notes * Seika Kamohara and Rémy Burcelin: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Laboratory of Molecular Genetics, the Rockefeller University, 1230 York
Avenue, New York, 10021, New York, USA Seika Kamohara, Jeffrey L. Halaas & Jeffrey M. Friedman * Howard Hughes Medical Institute, the Rockefeller University, 1230 York Avenue, New York,
10021, New York, USA Jeffrey M. Friedman * Department of Biochemistry, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, 10461, New York, USA Rémy Burcelin & Maureen
J. Charron Authors * Seika Kamohara View author publications You can also search for this author inPubMed Google Scholar * Rémy Burcelin View author publications You can also search for this
author inPubMed Google Scholar * Jeffrey L. Halaas View author publications You can also search for this author inPubMed Google Scholar * Jeffrey M. Friedman View author publications You
can also search for this author inPubMed Google Scholar * Maureen J. Charron View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR
Correspondence to Maureen J. Charron. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kamohara, S., Burcelin, R., Halaas, J. _et al._ Acute stimulation
of glucose metabolism in mice by leptin treatment. _Nature_ 389, 374–377 (1997). https://doi.org/10.1038/38717 Download citation * Received: 02 June 1997 * Accepted: 18 July 1997 * Issue
Date: 25 September 1997 * DOI: https://doi.org/10.1038/38717 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative



:max_bytes(150000):strip_icc():focal(319x0:321x2)/people_social_image-60e0c8af9eb14624a5b55f2c29dbe25b.png)
