- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Cellular structures are established and maintained through a dynamic interplay between assembly and regulatory processes. Self-organization of molecular components provides a
variety of possible spatial structures: the regulatory machinery chooses the most appropriate to express a given cellular function1. Here we study the extent and the characteristics of
self-organization using microtubules and molecular motors2 as a model system. These components are known to participate in the formation of many cellular structures, such as the dynamic
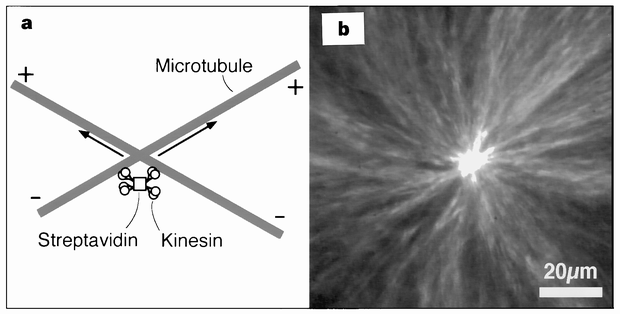
asters found in mitotic and meiotic spindles3,4. Purified motors and microtubules have previously been observed to form asters _in vitro_5. We have reproduced this result with a simple
system consisting solely of multi-headed constructs of the motor protein kinesin6 and stabilized microtubules. We show that dynamic asters can also be obtained from a homogeneous solution of
tubulin and motors. By varying the relative concentrations of the components, we obtain a variety of self-organized structures. Further, by studying this process in a constrained geometry
of micro-fabricated glass chambers7, we demonstrate that the same final structure can be reached through different assembly ‘pathways’. Access through your institution Buy or subscribe This
is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00
per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated
during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MECHANISMS OF
MICROTUBULE DYNAMICS AND FORCE GENERATION EXAMINED WITH COMPUTATIONAL MODELING AND ELECTRON CRYOTOMOGRAPHY Article Open access 28 July 2020 REGULATION OF MICROTUBULE DYNAMICS, MECHANICS AND
FUNCTION THROUGH THE GROWING TIP Article 18 August 2021 MECHANISMS OF MICROTUBULE ORGANIZATION IN DIFFERENTIATED ANIMAL CELLS Article 05 April 2022 REFERENCES * Kirschner, M. &
Mitchison, T. Beyond self-assembly: From microtubules to morphogenesis. _Cell_ 45, 329–342 (1986). Article CAS PubMed Google Scholar * Howard, J. The movement of kinesin along
microtubules. _Annu. Rev. Physiol._ 58, 703–729 (1996). Article CAS PubMed Google Scholar * Hyman, A. & Karsenti, E. Morphogenetic properties of microtubules and mitotic spindle
assembly. _Cell_ 84, 401–410 (1996). Article CAS PubMed Google Scholar * Barton, N. R. & Goldstein, L. S. B. Going mobile: Microtubule motors and chromosome segregation. _Proc. Natl
Acad. Sci. USA_ 93, 1735–1742 (1996). Article ADS CAS PubMed PubMed Central Google Scholar * Urrutia, R., McNiven, M., Albanesi, J., Murphy, D. & Kachar, B. Purified kinesin
promotes vesicle motility and induces active sliding between microtubules in vitro. _Proc. Natl Acad. Sci. USA_ 88, 6701–6705 (1991). Article ADS CAS PubMed PubMed Central Google
Scholar * Berliner, E. _et al_. Microtubule movement by a biotinilated kinesin bound to a streptavidin-coated surface. _J. Biol. Chem._ 269, 8610–8615 (1994). CAS PubMed Google Scholar *
Holy, T., Dogterom, M., Yurke, B. & Leibler, S. Assembly and positioning of microtubule asters in microfabricated chambers. _Proc. Natl Acad. Sci. USA_ 94, 6228–6233 (1997). Article
ADS CAS PubMed PubMed Central Google Scholar * Nicklas, R. B. & Ward, S. C. Elements of error correction in mitosis: microtubule capture, release, and tension. _J. Cell Biol._ 126,
1241–1253 (1994). Article CAS PubMed Google Scholar * Heald, R. _et al_. Self-organization of microtubules into bipolar spindles around artificial chromosomes in _Xenopus_ egg extracts.
_Nature_ 382, 420–425 (1996). Article ADS CAS PubMed Google Scholar * Sawin, K. E. & Mitchison, T. Mitotic spindle assembly by two different pathways in vitro. _J. Cell Biol._ 112,
925–940 (1991). Article CAS PubMed Google Scholar * Hunt, A. J., Gittes, F. & Howard, J. The force exerted by a single molecule against a viscous load. _Biophys. J._ 67, 766–781
(1994). Article ADS CAS PubMed PubMed Central Google Scholar * Svoboda, K. & Block, S. M. Force and velocity measured for single kinesin molecules. _Cell_ 77, 773–784 (1994).
Article CAS PubMed Google Scholar * Mayhofer, E. & Howard, J. The force generated by a single kinesin molecule against an elastic load. _Proc. Natl Acad. Sci. USA_ 92, 574–578
(1995). Article ADS Google Scholar * Kashina, A. S. _et al_. Abipolar kinesin. _Nature_ 379, 270–272 (1996). Article ADS CAS PubMed PubMed Central Google Scholar * Arnal, I. &
Wade, R. How does taxol stabilize microtubules? _Curr. Biol._ 5, 900–908 (1995). Article CAS PubMed Google Scholar * Verde, F., Berrez, J.-M., Antony, C. & Karsenti, E. Taxol-induced
microtubule asters in mitotic extracts of _Xenopus_ eggs: Requirement for phosphorylated factors and cytoplasmic dynein. _J. Cell Biol._ 112, 1177–1187 (1991). Article CAS PubMed Google
Scholar * Stearns, T. & Kirschner, M. In vitro reconstitution of centrosome assembly and function: The central role of gamma-tubulin. _Cell_ 76, 623–637 (1994). Article CAS PubMed
Google Scholar * Mitchison, T. & Kirschner, M. Microtubule assembly nucleated by isolated centrosomes and dynamic instability of microtubule growth. _Nature_ 312, 232–242 (1984).
Article ADS CAS PubMed Google Scholar * Theurkauf, W. E. Premature microtubule-dependent cytoplasmic streaming in cappucino and spire mutant oocytes. _Science_ 265, 2093–2096 (1994).
Article ADS CAS PubMed Google Scholar * Rodionov, V. & Borisy, G. G. Self-centring activity of cytoplasm. _Nature_ 386, 170–173 (1997). Article ADS CAS PubMed Google Scholar *
Vernos, I. & Karsenti, E. Motors involved in spindle assembly and chromosome segregation. _Curr. Opin. Cell Biol._ 8, 4–9 (1996). Article CAS PubMed Google Scholar * McNally, F. J.
Modulation of microtubule dynamics during the cell cycle. _Curr. Opin. Cell Biol._ 8, 23–29 (1996). Article CAS PubMed Google Scholar * Cross, M. C. & Hohenberg, P. C. Pattern
formation outside of equilibrium. _Rev. Mod. Phys._ 65(2)(1993). * Tabony, J. Morphological bifurcations involving reaction–diffusion processes during microtubule formation. _Science_ 264,
245–248 (1994). Article ADS CAS PubMed Google Scholar * Young, E. C., Berliner, E., Mahtani, H., Perez-Ramirez, B. & Gelles, J. Subunit interactions in dimeric kinesin heavy chain
derivatives that lack the kinesin rod. _J. Biol. Chem._ 270, 3926–3931 (1995). Article CAS PubMed Google Scholar * Bayer, E. & Wilchek, M. Avidin and streptavidin. _Methods Enzymol._
184, 51–67 (1990). Article Google Scholar * Dogterom, M., Leibler, S. Physical aspects of the growth and regulation of microtubule structures. _Phys. Rev. Lett._ 20, 1347–1350 (1993).
Article ADS Google Scholar * Erickson, H. & O'Brien, E. Microtubule dynamic instability and GTP hydrolysis. _Annu. Rev. Biophys. Biomol. Struct._ 21, 145–166 (1992). Article CAS
PubMed Google Scholar * Gittes, F., Mickey, B., Nettleton, J. & Howard, J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. _J. Cell
Biol._ 120, 923–934 (1993). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank E. Young and J. Gelles for kinesin plasmids; J. Johnson for taxol; T. Holy
for help with the preparation of glass chambers; and T. Holy, M. Elowitz, E. Wolf, E. Karsenti, T.Mitchison, J. Howard and S. Block for discussions. This work was supported by grants from
the NIH, the NSF and the HFSP, a fellowship from the Deutsche Forschungsgemeinschaft (T.S.) and the French Government (F.J.N.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Departments of
Molecular Biology and Physics, Princeton Unviersity, Princeton, 08544, New Jersey, USA F. J. Ndlec, T. Surrey & S. Leibler * Laboratoire de Physico-Chimie Thorique, Ecole Suprieure de
Physique et Chimie Industrielles, 10 rue Vauquelin, 75231, Paris, France F. J. Ndlec & A. C. Maggs Authors * F. J. Ndlec View author publications You can also search for this author
inPubMed Google Scholar * T. Surrey View author publications You can also search for this author inPubMed Google Scholar * A. C. Maggs View author publications You can also search for this
author inPubMed Google Scholar * S. Leibler View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to S. Leibler. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ndlec, F., Surrey, T., Maggs, A. _et al._ Self-organization of microtubules and motors. _Nature_ 389, 305–308
(1997). https://doi.org/10.1038/38532 Download citation * Received: 19 March 1997 * Accepted: 13 June 1997 * Issue Date: 18 September 1997 * DOI: https://doi.org/10.1038/38532 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative