- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
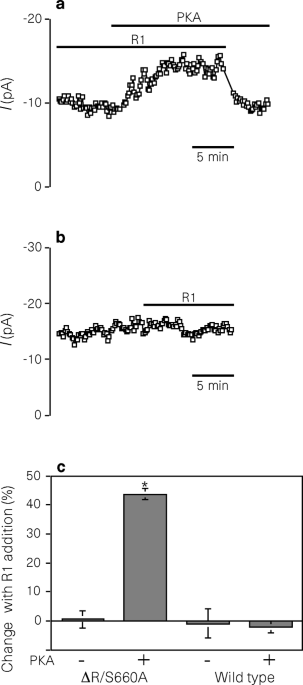
ABSTRACT Phosphorylation controls the activity of ion channels in many tissues. In epithelia, the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel is activated by
phosphorylation of serine residues in its regulatory (R) domain and then gated by binding and hydrolysis of ATP by the nucleotide-binding domains1,2,3. Current models propose that the
unphosphorylated R domain serves as an inhibitory particle that occludes the pore1,2,4,5,6, much like the inhibitory ‘ball’ in Shaker K+ channels7,8; presumably, phosphorylation relieves
this inhibition. Here we test this by adding an R-domain peptide to a CFTR variant in which much of the R domain had been deleted (CFTR-ΔR/S660A): in contrast to predictions, we found that
adding an unphosphorylated R domain to CFTR-ΔR/S660A did not inhibit activity, whereas a phosphorylated R-domain peptide stimulated activity. To investigate how phosphorylation controls
activity, we studied channel gating and found that phosphorylation of the R domain increases the rate of channel opening by enhancing the sensitivity to ATP. Our results indicate that CFTR
is regulated by a new mechanism in which phosphorylation of one domain stimulates the interaction of ATP with another domain, thereby increasing activity. Access through your institution Buy
or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and
online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes
which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY
OTHERS A TOPOLOGICAL SWITCH IN CFTR MODULATES CHANNEL ACTIVITY AND SENSITIVITY TO UNFOLDING Article 02 August 2021 CFTR FUNCTION, PATHOLOGY AND PHARMACOLOGY AT SINGLE-MOLECULE RESOLUTION
Article Open access 22 March 2023 PROLYL ISOMERIZATION CONTROLS ACTIVATION KINETICS OF A CYCLIC NUCLEOTIDE-GATED ION CHANNEL Article Open access 16 December 2020 REFERENCES * Welsh, M. J.,
Tsui, L.-C., Boat, T. F. & Beaudet, A. L. in _The Metabolic and Molecular Basis of Inherited Disease_ VOL. 7(eds Scriver, C. R., Beaudet, A. L., Sly, W. S.&Valle, D.) 3799–3876
(McGraw-Hill, New york, (1995). Google Scholar * Collins, F. S. Cystic fibrosis: molecular biology and therapeutic implications. _Science_ 256, 774–779 (1992). Article ADS CAS Google
Scholar * Riordan, J. R. The cystic fibrosis transmembrane conductance regulator. _Annu. Rev. Physiol._ 55, 609–630 (1993). Article CAS Google Scholar * Rich, D. P._et al_. Regulation of
the cystic fibrosis transmembrane conductance regulator Cl− channel by negative charge in the R domain. _J. Biol. Chem._ 268, 20259–20267 (1993). CAS PubMed Google Scholar * Ma, J. _et
al_. Phosphorylation-dependent block of cystic fibrosis transmembrane conductance regulator chloride channel by exogenous R domain protein. _J. Biol. Chem._ 271, 7351–7356 (1996). Article
CAS Google Scholar * Kartner, N. _et al_. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. _Cell_ 64, 681–691 (1991).
Article CAS Google Scholar * Zagotta, W. N., Hoshi, T. & Aldrich, R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. _Science_
250, 568–571 (1990). Article ADS CAS Google Scholar * Hoshi, T., Zagotta, W. N. & Aldrich, R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation.
_Science_ 250, 533–538 (1990). Article ADS CAS Google Scholar * Rich, D. P. _et al_. Effect of deleting the R domain on CFTR-generated chloride channels. _Science_ 253, 205–207 (1991).
Article ADS CAS Google Scholar * Picciotto, M. R., Cohn, J. A., Bertuzzi, G., Greengard, P. & Nairn, A. C. Phosphorylation of the cystic fibrosis transmembrane conductance regulator.
_J. Biol. Chem._ 267, 12742–12752 (1992). CAS PubMed Google Scholar * Anderson, M. P. _et al_. Nucleoside triphosphates are required to open the CFTR chloride channel. _Cell_ 67, 775–784
(1991). Article CAS Google Scholar * Maller, J. E., Kemp, B. E. & Krebs, E. G. _In vivo_ phosphorylation of a synthetic peptide substrate of cyclic AMP-dependent protein kinase.
_Proc. Natl Acad. Sci. USA_ 75, 248–251 (1978). Article ADS CAS Google Scholar * Winter, M. C., Sheppard, D. N., Carson, M. R. & Welsh, M. J. Effect of ATP concentration on CFTR Cl−
channels: a kinetic analysis of channel regulation. _Biophys. J._ 66, 1398–1403 (1994). Article CAS Google Scholar * Carson, M. R., Travis, S. M. & Welsh, M. J. The two
nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. _J. Biol. Chem._ 270, 1711–1717 (1995).
Article CAS Google Scholar * Carson, M. R., Winter, M. C., Travis, S. M. & Welsh, M. J. Pyrophosphate stimulates wild-type and mutant CFTR Cl− channels. _J. Biol. Chem._ 270,
20466–20472 (1995). Article CAS Google Scholar * Cheng, S. H. _et al_. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. _Cell_ 66,
1027–1036 (1991). Article CAS Google Scholar * Cohn, J. A., Nairn, A. C., Marino, C. R., Melhus, O. & Kole, J. Characterization of the cystic fibrosis transmembrane conductance
regulator in a colonocyte cell line. _Proc. Natl Acad. Sci. USA_ 89, 2340–2344 (1992). Article ADS CAS Google Scholar * Hwang, T. C., Nagel, G., Nairn, A. C. & Gadsby, D. C.
Regulation of the gating of cystic fibrosis transmembrane conductance regulator C1 channels by phosphorylation and ATP hydrolysis. _Proc. Natl Acad. Sci. USA_ 91, 4698–4702 (1994). Article
ADS CAS Google Scholar * Becq, F. _et al_. Phosphatase inhibitors activate normal and defective CFTR chloride channels. _Proc. Natl Acad. Sci. USA_ 91, 9160–9164 (1994). Article ADS CAS
Google Scholar * Li, C. _et al_. ATPase activity of the cystic fibrosis transmembrane conductance regulator. _J. Biol. Chem._ 45, 28463–28468 (1996). Article Google Scholar * Gunderson,
K. L. & Kopito, R. R. Effects of pyrophosphate and nucleotide analogs suggest a role for ATP hydrolysis in cystic fibrosis transmembrane regulator channel gating. _J. Biol. Chem._ 269,
19349–19353 (1994). CAS PubMed Google Scholar * Gründer, S., Thiemann, A., Pusch, M. & Jentsch, T. J. Regions involved in the opening of ClC-2 chloride channel by voltage and cell
volume. _Nature_ 360, 759–762 (1992). Article ADS Google Scholar * Lin, K., Rath, V. L., Dai, S. C., Fletterick, R. J. & Hwang, P. K. Aprotein phosphorylation switch at the conserved
allosteric site in GP. _Science_ 273, 1539–1541 (1996). Article ADS CAS Google Scholar * Lin, K., Hwang, P. K. & Fletterick, R. J. Mechanism of regulation in yeast glycogen
phosphorylase. _J. Biol. Chem._ 270, 26833–26839 (1995). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank P. Weber for preparing the cells, S. Travis for the R1
peptide, and G. Hill and D. Vermeer for assistance. This work was supported by the NHLBI and the HHMI. M.J.W. is an investigator of the HHMI. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Departments of Internal Medicine and Physiology and Biophysics, Howard Hughes Medical Institute, University of Iowa College of Medicine, Iowa City, 52242, Iowa, USA Michael C. Winter &
Michael J. Welsh Authors * Michael C. Winter View author publications You can also search for this author inPubMed Google Scholar * Michael J. Welsh View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Michael J. Welsh. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Winter, M., Welsh, M. Stimulation of CFTR activity by its phosphorylated R domain. _Nature_ 389, 294–296 (1997). https://doi.org/10.1038/38514 Download citation * Received: 06 March 1997 *
Accepted: 26 June 1997 * Issue Date: 18 September 1997 * DOI: https://doi.org/10.1038/38514 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative